Abstract
Objective:
To evaluate the effect of ischemic preconditioning (IPC) in an experimental setting of extended liver resection with 30 minutes of inflow occlusion in rats.
Summary Background Data:
IPC has been proven an effective strategy against hepatic ischemia-reperfusion injury in both animal and human studies. However, decreased protective effects in terms of transaminase levels were found in patients with larger resection volume, questioning the benefit of IPC in case of small liver remnants.
Methods:
Rats undergoing 90% hepatectomy under strict inflow occlusion for 30 minutes were subjected to either receive or not receive an IPC period (5 minutes of ischemia followed by 30 minutes of reperfusion). In addition to 10-day survival rate, laser Doppler flowmetry of hepatic blood flow and fluorescence microscopic analysis of the hepatic microcirculation were performed to assess the effect of IPC on initial microvascular reperfusion of liver remnants after 90% resection. Moreover, regeneration capacity of livers undergoing IPC and 70% resection was studied over 7 days by means of histology and immunohistochemistry.
Results:
Ten-day survival of rats which underwent IPC and 90% hepatectomy was 0 out of 10 animals versus 1 out of 10 animals without IPC. Hemodynamic and microcirculatory analysis revealed signs of hyperperfusion during initial reperfusion of preconditioned liver remnants in 90% hepatectomized animals. In addition to increased transaminase levels, IPC impaired hepatic proliferative response after 70% organ resection, as indicated by both a significant reduction in mitotic figures and Ki-67 nuclear staining of hepatocytes, as well as a decrease in restitution of liver mass.
Conclusions:
Though portal hypertension reflecting shear stress has been reported to trigger liver regeneration, remnant liver tissue after major hepatectomy may not benefit from hyperperfusion-induced trigger for cell cycle entry but is rather dominated from hyperperfusion-induced local organ injury. Further studies are required to finally judge on the harmfulness of IPC in extended liver resection.
Ischemic preconditioning (IPC) has been shown to lose its benefit in patients undergoing major liver resection. We now demonstrate in a rat model using laser Doppler flowmetry and in vivo fluorescence microscopy that IPC causes microvascular hyperperfusion within the remnant liver and high transaminase release. IPC further impairs regenerative capacity upon extended hepatectomy, as assessed by histology and immunohistochemistry. These injurious effects of IPC may oppose its application in extended liver resection.
Major liver surgery, including partial hepatectomy and liver transplantation, is frequently associated with an episode of ischemia-reperfusion (I/R) and, as its consequence, parenchymal cell injury and organ dysfunction.1,2 Hepatic I/R injury affects the recovery of patients after major surgery and bears a risk of poor postoperative outcome.3–5 Based on the increase in understanding of mechanisms underlying hepatic reperfusion injury, a variety of strategies has been developed to counteract I/R injury.6,7 However, postischemic reperfusion injury still significantly contributes to morbidity and mortality after liver surgery, in particular, in patients with liver disease.8–10
Ischemic preconditioning (IPC) has been considered a promising approach to effectively minimize hepatic I/R injury.11–13 The term IPC is used for the process in which a short period of ischemia, separated by intermittent reperfusion, renders a solid organ more tolerant to subsequent longer episodes of ischemia, as initially described for the canine myocardium by Murry et al14 in 1986. In numerous experimental settings, IPC has been shown to confer protection by an immediate phase, involving the direct modulation of energy supplies, pH regulation, Na+ and Ca2+ homeostasis, and caspase activation, as well as by a later phase, which includes the synthesis of multiple stress-response proteins,15 finally leading to an amelioration of hepatic microcirculation,16 a reduction of hepatic tissue apoptosis and necrosis,17–19 and an improvement of survival.18
Most recently, a prospective randomized study in 100 consecutive patients undergoing major liver resection established IPC as a protective strategy against hepatic ischemia also in humans.20 However, in this study by Clavien and coworkers,20 regression multivariate analysis revealed only an increased benefit of IPC in cases of lower resected liver volume. The protective effects of IPC inversely correlated with the percentage of resected tissue and, of utmost importance, was lost in patients undergoing major tissue loss, ie, >50% liver resection.20 Thus, IPC seems not unequivocally be associated with a benefit of tissue in resistance against the ischemic insult.
Though the liver is unique with its ability to regenerate and to revert to its original function after resection, there is a limitation of the extent of liver resection. The drastic reduction of the microvascular bed upon major hepatectomy is associated with remnant liver exposure to excessive portal perfusion, known to induce irreversible sinusoidal endothelial injury as described in small-for-size liver grafts.21–23 With the fact in mind that IPC increases hepatic microvascular perfusion upon I/R,16 IPC might lose its benefit in extended liver resection due to a hyperperfusion-induced derangement in hepatic microcirculation. To test this hypothesis, we performed IPC in a rat model of extended hepatic resection and studied hepatic microvascular and proliferative response.
MATERIALS AND METHODS
Animals
Male Wistar rats (body weight 250–300 g; Charles River Laboratories, Sulzfeld, Germany) were used for the experiments. Animals were housed in standard animal laboratories with a 12-hour light-dark cycle and had free access to water and standard laboratory chow ad libitum. The experiments were conducted in accordance with the German legislation on protection of animals and the NIH Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council).
Experimental Procedures
The experiments included 2 groups of animals: an IPC group (IPC) and a control group (control). After induction of isoflurane/oxygen inhalative anesthesia, animals were laparotomized, and hepatic ischemia was accomplished using a microvascular clip placed on the hepatoduodenal ligament. Reflow was initiated after 30 minutes of hepatic ischemia by removal of the clamp. In the IPC groups, animals were subjected to 5 minutes of hepatic ischemia followed by 30 minutes of reperfusion16,24 prior to the sustained 30-minute period of ischemia. IPC combination, ie, 5 minutes of ischemia followed by 30 minutes of reperfusion, was chosen in accordance to results of a recent study of our group16 demonstrating 5 minutes/30 minutes IPC as the most effective strategy to reduce tissue injury upon sustained ischemia. During ischemia, the animals underwent partial hepatectomy according to the method of Higgins and Anderson.25 Briefly, the right median, the left median, and the left lateral lobes were ligated and removed, resulting in 70% hepatectomy. For 90% hepatectomy, the right lateral lobe was additionally excised. Postoperatively, animals were allowed to recover from surgery with access to food and water ad libitum.
Survival Study
Ten rats in each group with a 90% hepatectomy were used for the survival study. Rats that lived more than 10 days after hepatectomy were considered survivors.
Regeneration Study
Animals with 70% hepatectomy were killed at postoperative days 1, 4, and 7 (n = 5 animals per group and time point). The remnant livers were harvested, weighed, and processed for histologic analysis. Arterial blood samples were withdrawn for subsequent analysis of serum transaminase activities.
Liver Mass Regenerated
Weight of remnant liver mass was used to calculate growth of residual liver lobes as weight of remnant liver mass/body weight × 100 (%).
Histology and Immunohistochemistry
In 70% hepatectomized animals, remnant liver tissue at days 1, 4, and 7 after resection was excised, fixed in 4% phosphate-buffered formalin for 2 to 3 days, and then embedded in paraffin. From the paraffin-embedded tissue blocks, 5-μm sections were cut and stained with hematoxylin-eosin (HE). To evaluate hepatocyte replication, mitotic figures were counted in 1000 hepatocytes (200-fold magnification) and given as mitotic index (number of mitotic figures per 1000 hepatocytes). Resected liver tissue was processed for HE staining as described above and served for comparison as normal tissue with regular morphology.
The monoclonal antibody MIB-5, that has been described to be reactive with the rat equivalent Ki-67 protein and useful to monitor proliferation in rat liver,26 was used for immunohistochemical analysis of hepatocellular regeneration, using an indirect enzyme-linked antibody method. The proliferative capacity was quantitatively assessed by counting the number of labeled hepatocytes per 1000 hepatocellular nuclei (200-fold magnification) and given as proliferative index (number of Ki-67 positive cells per 1000 hepatocytes).
Hepatocellular Damage
The extent of hepatocellular damage was assessed at days 1, 4, and 7 after 70% hepatectomy by spectrophotometric determination of alanine transaminase (ALT), using a commercially available reaction kit (Roche Diagnostics, Mannheim, Germany).
Hemodynamic and Microcirculatory Study
Laser Doppler Flowmetry
Six rats with 90% hepatectomy per group were used for monitoring of hepatic blood flow by means of laser Doppler flowmetry (Cliniflow II, Model FM 701D; Carolina Medical Electronics, King, NC). Blood flow (arbitrary unit, aU) was measured by placing the probe (SF105) on the hepatoduodenal ligament, mainly reflecting portal venous blood flow.27,28 After baseline recording prior to IPC, data were collected immediately before and during the sustained ischemic period, as well as 1, 3, 5, and 10 minutes after reperfusion. Blood flow was recorded for at least 30 seconds after obtaining a stable signal. The mean value of blood flow at respective time points was calculated and expressed as percent of the initial blood flow values.
Intravital Fluorescence Microscopy
Eight animals with 90% hepatectomy per group were used to assess hepatic microcirculation. For this purpose, spontaneously breathing anesthetized animals were placed in supine position on a heating pad for maintenance of body temperature at 36°C to 37°C. Polyethylene catheters (PE 50, ID 0.58 mm; Portex, Hythe, UK) in the right carotid artery and jugular vein allowed for assessment of systemic hemodynamics and injection of fluorescent dyes. After laparotomy, hepatic ischemia and partial hepatectomy (90%) were accomplished, as described above. After 10 minutes of reperfusion, animals were positioned on their right side, and the residual caudate lobe was exteriorized and covered with a glass slide for microscopy. Microcirculation of the remnant liver was assessed during the first 30 to 60 minutes upon reperfusion. At the end of the microscopic procedure, liver tissue was excised and processed for HE staining as described above.
Using a fluorescence microscope equipped with a 100-W mercury lamp (Axiotech Vario; Zeiss, Jena, Germany) and different filter sets for blue (excitation/emission wavelength: 450–490 nm/>520 nm) and green (530–560/>580 nm) light epi-illumination, microscopic images were taken by a water immersion objective (×20/0.50 W; Zeiss), recorded by a CCD video camera (FK 6990A-IQ; Pieper, Berlin, Germany) and transferred to a video system (S-VHS Panasonic AG 7350-E; Matsushita, Tokyo, Japan). Sodium fluorescein (2 μmol/kg iv; Merck, Darmstadt, Germany) served for tissue contrast enhancement with assessment of sinusoidal perfusion and red blood cell (RBC) velocity within the individual microvessels.29,30 Rhodamine-6G (1 μmol/kg iv; Merck) allowed for in vivo staining of leukocytes with assessment of their flow behavior within the individual microvascular segments.29,30
Quantitative Video Analysis
Assessment of hepatic microcirculatory parameters was performed off line by frame-to-frame analysis of the videotaped images at a magnification of 424-fold, using a computer-assisted image analysis system with a 19-inch monitor (CapImage; Zeintl, Heidelberg, Germany). Within 10 lobules per animal, sinusoidal perfusion failure was determined by counting the number of nonperfused sinusoids (given in percent of all sinusoids crossing a 200-μm raster line).29,30 Leukocyte-endothelial cell interaction was analyzed within 10 hepatic lobules and 8 to 10 postsinusoidal venules per animal, including (i) the number of stagnant leukocytes located within sinusoids (given as cells/mm2 observation field) and not moving during an observation period of 20 seconds; and (ii) the number of adherent leukocytes located within postsinusoidal venules (given as cells/mm2 endothelial surface, calculated from diameter and length of the vessel segment studied, assuming cylindrical geometry) and not moving or detaching from the endothelial lining during an observation period of 20 seconds.29,30 For estimation of volumetric blood flow (VQ), RBC velocity and the respective diameter were measured within 8 to 10 individual sinusoids in midzonal regions (classification according to Rappaport31) and 5 postsinusoidal venules per animal.32 VQ in the individual microvessels was estimated from RBC velocity and microvascular cross-sectional area (π × r2) according to the equation of Gross and Aroesty,33 ie, VQ = RBC velocity × π × r2, and given in picoliter per second.
Statistics
All data are expressed as means ± standard error (SE). After testing for normality and equal variance across groups, intergroup differences were assessed using the appropriate pairwise comparison test (Student t test, Mann-Whitney rank sum test). For statistical analysis of repeated measures, ie, laser Doppler flowmetry data, analysis of variance followed by the Dunn test was performed. Statistical significance was set at P < 0.05. Statistics were performed using the software package SigmaStat (Jandel Corporation, San Rafael, CA).
RESULTS
Survival Data
After 90% hepatectomy, none of the IPC animals and 1 of 10 control animals survived the full observation time of 10 days. Animals died within the first 3 days after liver resection.
Hemodynamic and Microcirculatory Data
Data of hepatic blood flow, as assessed by laser Doppler flowmetry in 90% hepatectomized animals, are given in Figure 1. During hepatic ischemia induced by clamping of the hepatoduodenal ligament, hepatic blood flow dropped to zero, indicating complete standstill of perfusion (Fig. 1). Upon removal of the microsurgical clip, blood flow gradually increased and returned to about 30% of preischemic values in control animals, while in IPC animals hepatic blood flow revealed a faster recovery, reaching almost 50% of baseline values within 10 to 15 minutes of reperfusion (Fig. 1).
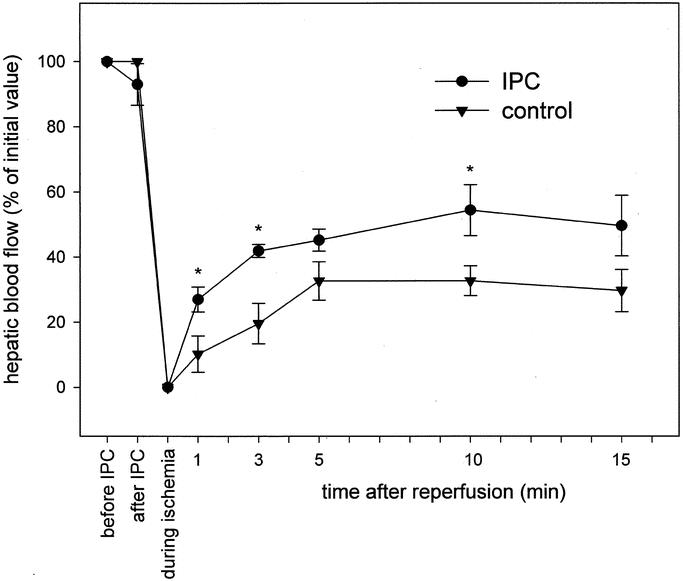
FIGURE 1. Hepatic blood flow in 90% hepatectomized animals without (control, triangle) and with ischemic preconditioning (IPC, circle), as assessed by laser Doppler flowmetry on the hepatoduodenal ligament. Hepatectomy was performed during a 30-minute of inflow occlusion of the liver. Values are expressed as percentage of initial blood flow and are given as means ± SE, n = 6 animals per group; * P < 0.05 versus control, ANOVA followed by Dunn test.
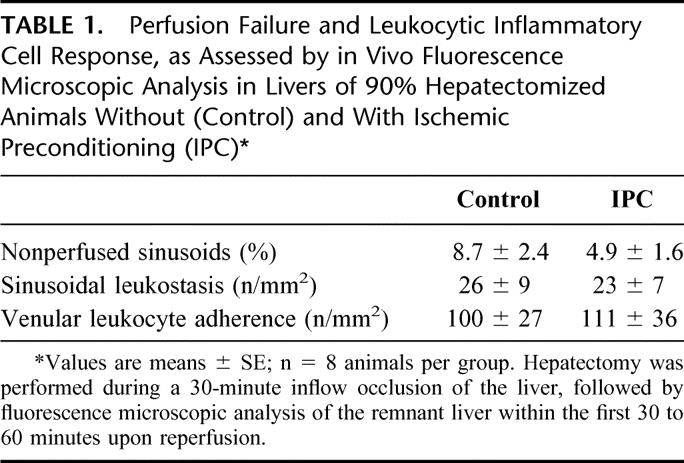
In line with global hepatic blood flow, detailed quantitative analysis of the hepatic microcirculation revealed higher values of RBC velocity in sinusoids (Fig. 2A) and, in particular, in postsinusoidal venules of IPC animals when compared with controls (Fig. 2B). Moreover, values of both sinusoidal and venular VQ in livers of IPC animals exceeded those found in livers of control animals (Fig. 3A, B). Hepatectomy was associated with a moderate deterioration of sinusoidal perfusion and a slight increase of the number of leukocytes located within sinusoids and postsinusoidal venules (Table 1). Notably, control and IPC animals did not significantly differ with respect to inflammatory cell response and perfusion failure, though IPC animals tended to show lower numbers of nonperfused sinusoids (Table 1).

FIGURE 2. RBC velocity in sinusoids (A) and postsinusoidal venules (B), as assessed by in vivo fluorescence microscopic analysis in 90% hepatectomized animals without (control, closed bars) and with ischemic preconditioning (IPC; open bars). Hepatectomy was performed during a 30-minute inflow occlusion of the liver, followed by fluorescence microscopic analysis of the remnant liver within the first 30 to 60 minutes upon reperfusion. Values are means ± SE, n = 8 animals per group; * P < 0.05 versus control, Student t test.

FIGURE 3. Volumetric blood flow in sinusoids (A) and postsinusoidal venules (B), as assessed by in vivo fluorescence microscopic analysis in 90% hepatectomized animals without (control, closed bars) and with ischemic preconditioning (IPC; open bars). Hepatectomy was performed during a 30-minute inflow occlusion of the liver, followed by fluorescence microscopic analysis of the remnant liver within the first 30 to 60 minutes upon reperfusion. Values are means ± SE, n = 8 animals per group.
TABLE 1. Perfusion Failure and Leukocytic Inflammatory Cell Response, as Assessed by in Vivo Fluorescence Microscopic Analysis in Livers of 90% Hepatectomized Animals Without (Control) and With Ischemic Preconditioning (IPC)
Regeneration Data
To evaluate the influence of IPC on regenerative capacity upon partial hepatectomy, 70% of liver mass was removed to guarantee 100% animal survival, with harvesting of liver tissue and blood samples at postoperative days 1, 4, and 7. Indices of hepatocellular regenerative response, ie, number of mitotic figures and Ki-67 positive cells, revealed markedly higher values in control animals without IPC when compared with IPC animals, with most pronounced differences at day 1 after hepatectomy (Fig. 4A, B). Whereas mitotic index was almost similar in both groups on postoperative days 4 and 7, percentage of Ki-67-positive cells in control animals was found still markedly above the corresponding value of IPC animals at day 4 (Fig. 4A, B). Concomitantly, ratios of remnant liver mass to body weight of controls significantly exceeded those of IPC animals, indicating impaired liver regeneration upon IPC (Fig. 5). Serving as parameter of hepatocellular injury, IPC animals exhibited 2-fold higher transaminase levels when compared with controls at day 1 posthepatectomy (Fig. 6).
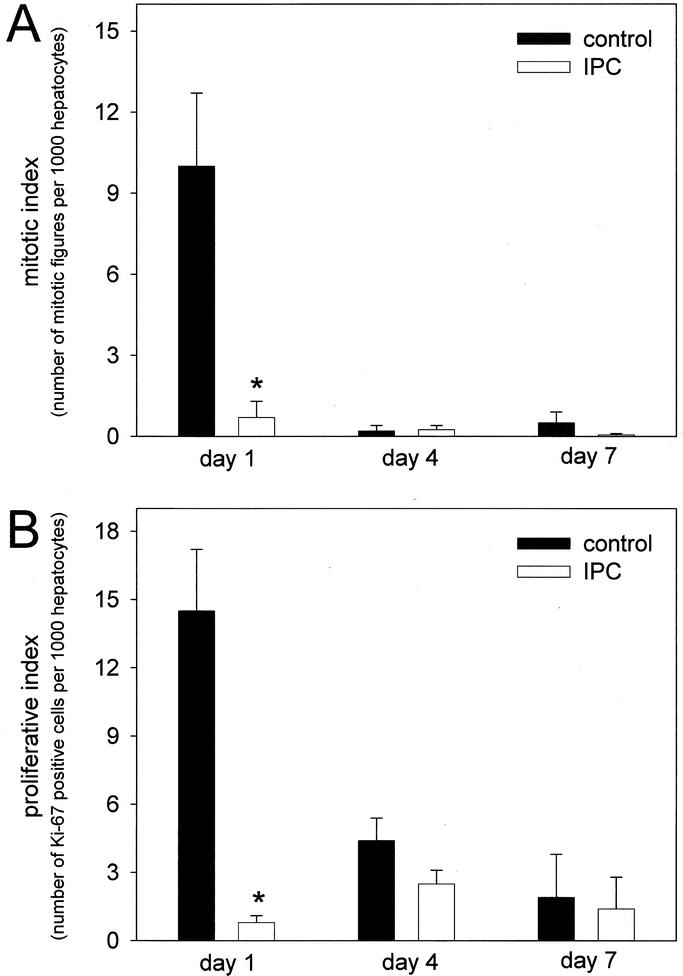
FIGURE 4. Mitotic index, ie, number of mitotic figures per 1000 hepatocytes (A) and proliferative index, ie, number of Ki-67 positive cells per 1000 hepatocytes (B) in regenerating liver of animals without (control, closed bars) and with ischemic preconditioning (IPC; open bars) at days 1, 4, and 7 after 70% hepatectomy. Hepatectomy was performed during a 30-minute inflow occlusion of the liver. Values are means ± SE, n = 5 animals per group and time point; * P < 0.05 versus control, Student t test (A) and Mann-Whitney rank sum test (B).
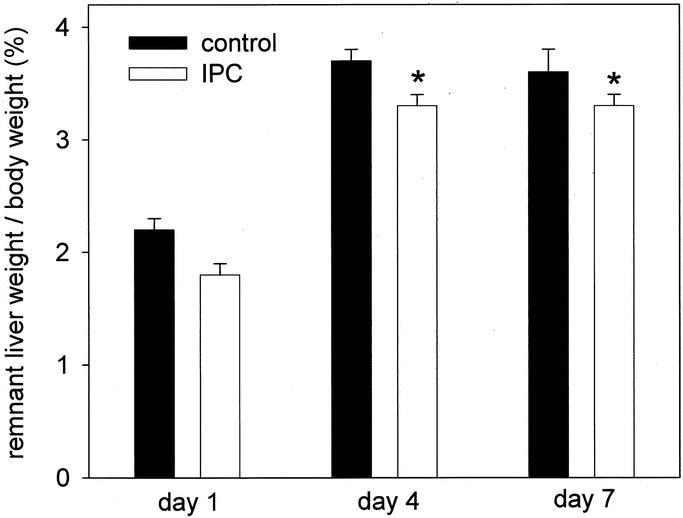
FIGURE 5. Ratio of remnant liver to body weight after 70% hepatectomy in animals without (control, closed bars) and with ischemic preconditioning (IPC; open bars). Hepatectomy was performed during a 30-minute inflow occlusion of the liver. Values are means ± SE, n = 5 animals per group and time point; * P < 0.05 versus control, Student t test.

FIGURE 6. Serum ALT activities in regenerating liver of animals without (control, closed bars) and with ischemic preconditioning (IPC; open bars) at days 1, 4, and 7 after 70% hepatectomy. Hepatectomy was performed during a 30-minute inflow occlusion of the liver. Values are means ± SE, n = 5 animals per group and time point; * P < 0.05 versus control, Student t test.
Histology
In contrast to regular histologic appearance of normal liver tissue, HE staining of liver tissue after IPC in 90% hepatectomized animals revealed marked vacuolization of hepatocytes, bleb formation, and disintegration and detachment of sinusoidal lining cells with direct exposure of hepatocellular microvilli into the sinusoidal lumen (Fig. 7A, B). Histology of liver remnants in non-IPC-treated control animals did not markedly differ, though signs of sinusoidal disintegration were slightly less pronounced.
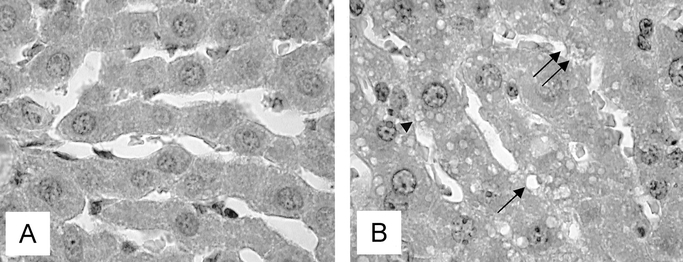
FIGURE 7. Light microscopic images of HE-stained liver tissues. In contrast to regular histologic appearance of normal liver tissue (A), liver tissue after IPC in 90% hepatectomized animals (B) presents with marked vacuolization of hepatocytes (arrow), bleb formation (arrowhead) and disintegration and detachment of sinusoidal lining cells with direct exposure of hepatocellular microvilli into the sinusoidal lumen (double arrow). Original magnification × 1000.
DISCUSSION
Both animal experiments and clinical experience have shown that IPC triggers a protective response to subsequent I/R injury in several organ systems, including the liver.34 The IPC procedure includes 2 temporally and mechanistically distinct types of protection with an acute, protein synthesis–independent phase and a second phase of late protection, based on the de novo synthesis of antioxidant enzymes, heat shock proteins, and NO synthase.15 Experimental evidence suggests that intra- and extracellular generation of adenosine, as well as activation of adenosine A2 receptors with subsequent generation of NO, is involved in mediating protection by IPC.15 Moreover, IPC-induced NO release prevented I/R-induced increase in endothelins,35 favoring local vasodilation and reduction of flow hindrance. In line with this, IPC treatment has been demonstrated to increase overall liver blood flow and to improve nutritive perfusion and tissue oxygenation.16,36,37 As a consequence, IPC averted postischemic hepatic excretory dysfunction and the increase in enzyme release.16 In the present study, we now confirm and extend our previous experience in that IPC improves not only the number of perfused sinusoids but causes also increased flow velocities and VQ values in sinusoidal and postsinusoidal microvessels, reflecting high shear-stress conditions.
Mechanical forces that arise from changes in portal flow are well defined and discussed as putative physiologic trigger mechanisms for liver regeneration.38–40 Accordingly, a positive correlation between portal venous velocities at postoperative day 7 and regeneration of partial-liver allografts at 1 month has recently been reported.41 Sato et al39 proposed that shear stress due to portal flow, which appears to be a simple mechanical force, may trigger liver regeneration and controls the volume of the liver after partial hepatectomy.
On the other hand, portal hyperperfusion has been considered responsible for organ damage frequently observed after reperfusion of small-for-size liver grafts,22,42,43 resulting in hepatocyte ballooning with tremendous mitochondrial swelling, irregular large gaps between sinusoidal lining cells, and collapse of the space of Disse.22 This small-for-size syndrome has been recognized as the main factor leading to graft dysfunction and poor survival in adult living donor liver transplantation with extreme size mismatch.43,44 Thereby, safety limit is thought to be 40% of the ideal liver weight or 1% of the recipient body weight.45,46 The mechanism of injury, including both enhanced injury and reduced metabolic and synthetic capacity of parenchymal cells,47 has been postulated to be comparable to the one that resulted in progressive necrosis of the liver remnant after 85% hepatectomy in the rat.48
As demonstrated in the present study by detailed in vivo analysis of the hepatic microcirculation, IPC is capable of even increasing the obligatory rise in blood flow through the small remnant to pathologically high flow rates in individual sinusoidal and postsinusoidal vascular segments. Thus, we like to state that IPC-induced hyperperfusion may aggravate microvascular injury, with the consequence of accelerated death in 90% hepatectomized animals. Though IPC-induced disturbance of the microcirculatory homeostasis seems to be not harmful enough to lower survival in rats with 70% hepatectomy, from which complete recovery is the rule, IPC caused significantly higher ALT levels in those animals. These results further underscore our view that in small liver remnants of about 30%, the detrimental effects of IPC might still outweigh its effects known to render tissue more resistant against subsequent injury. In line with this, we first demonstrate that IPC caused a transient delay in liver regeneration after hepatectomy, as given by the striking reduction of the proliferation indices, namely, Ki67 and mitotic figures. Of interest, in models of prolonged I/R injury, protective effects of IPC have been shown to be associated with a reduced labeling index for proliferating cell nuclear antigen and a significant decrease in the transcription levels of immediate early genes (IEGs).49 While decreased IEG expression might confer protection in liver tissue upon I/R,50 this has not necessarily been valid for remnant liver tissue after resection. Proliferation of hepatocytes encompasses a multistep process with the transition of quiescent hepatocytes into the cell cycle (priming) and the progression beyond the restriction point in the G1 phase of the cycle.51 Priming is controlled by IEGs, in particular, by those encoding transcription factors and protooncogenes.51 Due to the fact that hepatocytes do not react to replication-involved humoral factors in the circulation unless they are primed, priming and its control by IEGs represents a key step in proliferation. Thus, it is reasonable to speculate that the regeneration potential in IPC-treated animals may suffer from interference of IPC with expression of IEGs.
In summary, an important, although most likely not the only, cause for failure of IPC to improve regeneration seems to rely on the fact that reduced liver mass does not benefit from IPC-induced hyperperfusion as a trigger for proliferation but rather suffers from shear stress–associated microvascular injury and, thus, reduced regenerative capacity. This may explain the clinical observation that the effect of preconditioning was lost in patients undergoing major tissue loss, ie, above 50%.20 Therefore, in case of extended liver resection, other strategies than IPC should be used to induce protection. The identification of pharmacologic agents, which specifically interfere with the injurious effects of IPC, might be of particular interest in the design of new protective strategies.
ACKNOWLEDGMENTS
The authors kindly thank Berit Blendow, Department of Experimental Surgery, University of Rostock, and Anja Schirmeier, Department of General, Visceral and Transplantation Surgery, Charité, Humboldt University Berlin, for their excellent technical assistance.
Footnotes
This work is supported by a grant of the Deutsche Forschungsgemeinschaft, Bonn-Bad Godesberg, Germany (Vo 450/7-1 and 7-2) and by a grant from the Charité Research Program (2001-730/GLA).
Christian Eipel and Matthias Glanemann contributed equally to this work.
Reprints: B. Vollmar, MD, Department of Experimental Surgery, University of Rostock, D-18055 Rostock, Germany. E-mail: brigitte.vollmar@med.uni-rostock.de.
REFERENCES
- 1.Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508–515. [DOI] [PubMed] [Google Scholar]
- 2.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. [DOI] [PubMed] [Google Scholar]
- 3.Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454–458. [PubMed] [Google Scholar]
- 5.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–338. [DOI] [PubMed] [Google Scholar]
- 6.Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003;10:189–194. [DOI] [PubMed] [Google Scholar]
- 7.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. [DOI] [PubMed] [Google Scholar]
- 8.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. [DOI] [PubMed] [Google Scholar]
- 9.Ezaki T, Seo Y, Tomoda H, et al. Partial hepatic resection under intermittent hepatic inflow occlusion in patients with chronic liver disease. Br J Surg. 1992;79:224–226. [DOI] [PubMed] [Google Scholar]
- 10.Glanemann M, Langrehr JM, Stange BJ, et al. Clinical implications of hepatic preservation injury after adult liver transplantation. Am J Transplant. 2003;3:1003–1009. [DOI] [PubMed] [Google Scholar]
- 11.Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480–1491. [DOI] [PubMed] [Google Scholar]
- 12.Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Dig Surg. 2003;20:383–396. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. [DOI] [PubMed] [Google Scholar]
- 14.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 15.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. [DOI] [PubMed] [Google Scholar]
- 16.Glanemann M, Vollmar B, Nussler AK, et al. Ischemic preconditioning protects from hepatic ischemia/reperfusion-injury by preservation of microcirculation and mitochondrial redox-state. J Hepatol. 2003;38:59–66. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizumi T, Yanaga K, Soejima Y, et al. Amelioration of liver injury by ischaemic preconditioning. Br J Surg. 1998;85:1636–1640. [DOI] [PubMed] [Google Scholar]
- 18.Yadav SS, Sindram D, Perry DK, et al. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223–1231. [DOI] [PubMed] [Google Scholar]
- 19.Teoh N, Dela Pena A, Farrell G. Hepatic ischemic preconditioning in mice is associated with activation of NF-kappaB, p38 kinase, and cell cycle entry. Hepatology. 2002;36:94–102. [DOI] [PubMed] [Google Scholar]
- 20.Clavien PA, Selzner M, Rudiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troisi R, Cammu G, Militerno G, et al. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Man K, Fan ST, Lo CM, et al. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003;237:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Man K, Lo CM, Ng IO, et al. Liver transplantation in rats using small-for-size grafts: a study of hemodynamic and morphological changes. Arch Surg. 2001;136:280–285. [DOI] [PubMed] [Google Scholar]
- 24.Glanemann M, Strenziok R, Kuntze R, et al. Ischemic preconditioning and methylprednisolone both equally reduce hepatic ischemia/reperfusion injury. Surgery. 2004;135:203–214. [DOI] [PubMed] [Google Scholar]
- 25.Higgins GM, Anderson RM. Experimental pathology of the liver. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 26.Gerlach C, Sakkab DY, Scholzen T, et al. Ki-67 expression during rat liver regeneration after partial hepatectomy. Hepatology. 1997;26:573–578. [DOI] [PubMed] [Google Scholar]
- 27.Kazuo H, Nishida T, Seiyama A, et al. Recovery of blood flow and oxygen transport after temporary ischemia of rat liver. Am J Physiol. 1998;275:H243–249. [DOI] [PubMed] [Google Scholar]
- 28.Nishida T, Ueshima S, Kazuo H, et al. Vagus nerve is involved in lack of blood reflow into sinusoids after rat hepatic ischemia. Am J Physiol Heart Circ Physiol. 2000;278:H1565–1570. [DOI] [PubMed] [Google Scholar]
- 29.Eipel C, Bordel R, Nickels RM, et al. Impact of leukocytes and platelets in mediating hepatocyte apoptosis in a rat model of systemic endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004;286:G769–776. [DOI] [PubMed] [Google Scholar]
- 30.Schäfer T, Scheuer C, Roemer K, et al. Inhibition of p53 protects liver tissue against endotoxin-induced apoptotic and necrotic cell death. FASEB J. 2003;17:660–667. [DOI] [PubMed] [Google Scholar]
- 31.Rappaport AM. The microcirculatory hepatic unit. Microvasc Res. 1973;6:212–228. [DOI] [PubMed] [Google Scholar]
- 32.Richter S, Vollmar B, Mucke I, et al. Hepatic arteriolo-portal venular shunting guarantees maintenance of nutritional microvascular supply in hepatic arterial buffer response of rat livers. J Physiol. 2001;531:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross JF, Aroesty J. Mathematical models of capillary flow: a critical review. Biorheology. 1972;9:225–264. [DOI] [PubMed] [Google Scholar]
- 34.Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. [DOI] [PubMed] [Google Scholar]
- 35.Peralta C, Closa D, Hotter G, et al. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Commun. 1996;229:264–270. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson B, Friman S, Gustafsson BI, et al. Preconditioning protects against ischemia/reperfusion injury of the liver. J Gastrointest Surg. 2000;4:44–49. [DOI] [PubMed] [Google Scholar]
- 37.Zapletal C, Maksan SM, Lehmann T, et al. Ischemic preconditioning improves liver microcirculation after ischemia/reperfusion. Transplant Proc. 1999;31:3260–3262. [DOI] [PubMed] [Google Scholar]
- 38.Wang HH, Lautt WW. Evidence of nitric oxide, a flow-dependent factor, being a trigger of liver regeneration in rats. Can J Physiol Pharmacol. 1998;76:1072–1079. [DOI] [PubMed] [Google Scholar]
- 39.Sato Y, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Surg Today. 1999;29:1–9. [DOI] [PubMed] [Google Scholar]
- 40.Niiya T, Murakami M, Aoki T, et al. Immediate increase of portal pressure, reflecting sinusoidal shear stress, induced liver regeneration after partial hepatectomy. J Hepatobiliary Pancreat Surg. 1999;6:275–280. [DOI] [PubMed] [Google Scholar]
- 41.Eguchi S, Yanaga K, Sugiyama N, et al. Relationship between portal venous flow and liver regeneration in patients after living donor right-lobe liver transplantation. Liver Transpl. 2003;9:547–551. [DOI] [PubMed] [Google Scholar]
- 42.Marcos A, Olzinski AT, Ham JM, et al. The interrelationship between portal and arterial blood flow after adult to adult living donor liver transplantation. Transplantation. 2000;70:1697–1703. [DOI] [PubMed] [Google Scholar]
- 43.Troisi R, de Hemptinne B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients. Liver Transpl. 2003;9:S36–41. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein MJ, Salame E, Kapur S, et al. Analysis of failure in living donor liver transplantation: differential outcomes in children and adults. World J Surg. 2003;27:356–364. [DOI] [PubMed] [Google Scholar]
- 45.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. [DOI] [PubMed] [Google Scholar]
- 46.Lo CM, Fan ST, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112–1116. [DOI] [PubMed] [Google Scholar]
- 47.Kiuchi T, Tanaka K, Ito T, et al. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29–35. [DOI] [PubMed] [Google Scholar]
- 48.Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142–149. [DOI] [PubMed] [Google Scholar]
- 49.Yamada F, Abe T, Saito T, et al. Ischemic preconditioning enhances regenerative capacity of hepatocytes after prolonged ischemia. Transplant Proc. 2001;33:956. [DOI] [PubMed] [Google Scholar]
- 50.Ishii S, Abe T, Saito T, et al. Effects of preconditioning on ischemia/reperfusion injury of hepatocytes determined by immediate early gene transcription. J Hepatobiliary Pancreat Surg. 2001;8:461–468. [DOI] [PubMed] [Google Scholar]
- 51.Kountouras J, Boura P, Lygidakis NJ. Liver regeneration after hepatectomy. Hepatogastroenterology. 2001;48:556–562. [PubMed] [Google Scholar]


