Abstract
Objective:
To determine whether first-line treatment with percutaneous or surgical drainage of liver abscesses larger than 5 cm results in better clinical outcome.
Summary Background Data:
Pyogenic liver abscesses larger than 5 cm are currently treated by intravenous antibiotics and either percutaneous (PD) or surgical drainage (SD). Percutaneous techniques have been increasingly performed in place of open drainage as first-line treatment. This paradigm shift has been fueled by the drive for low-risk and less-invasive procedures and the surgical option being reserved for percutaneous failures. Yet there is a lack of data to support percutaneous drainage over open surgical drainage as first-line treatment.
Methods:
Over a 3-year period, 80 patients with liver abscesses larger than 5 cm amenable to PD and SD were included in the study. This situation was possible as 1 team of surgeons favored the use of PD and 1 team favored the use of SD as first-line treatment. The treatment outcomes in both groups were compared, and clinical end-points included time to defervescence of fever, failure of treatment, secondary procedures, hospital stay, morbidity, and mortality.
Results:
PD was performed in 36 patients and SD in 44 patients as first-line treatment. Clinical, laboratory, and abscess parameters were comparable in both groups. Sixty-four of 80 patients (80%) had multiloculated abscess. The time to defervescence of fever was not statistically significant (PD versus SD, 4.85 versus 4.38 days; P = 0.09). However, SD had less treatment failures (3 versus 10, P = 0.013), less requirement for secondary procedures (5 versus 13, P = 0.01), and shorter length of hospital stay (8 versus 11 days, P = 0.03). There was no difference in morbidity or mortality rates.
Conclusions:
The results of our study show that for large liver abscesses more than 5 cm, SD provides better clinical outcomes than PD in terms of treatment success, number of secondary procedures, and hospital stay with comparable morbidity and mortality rates. SD should be considered as first-line treatment of large liver abscesses.
A comparison of surgical versus percutaneous drainage of pyogenic liver abscesses larger than 5 cm as first-line treatment suggests better clinical outcomes for surgical drainage.
Historically, morbidity and mortality rates associated with the treatment of pyogenic liver abscess has been high.1–3 This has improved significantly with the introduction of ultrasound (US) and computed tomography (CT).4 When detected early, effective treatment of small abscesses involves the use of diagnostic percutaneous aspiration and appropriate intravenous antibiotics.5 Liver abscesses larger than 5 cm in size often require prompt drainage for resolution of sepsis. Currently, there are 2 alternative methods for drainage of pus from a large liver abscess. Percutaneous therapeutic procedures have been increasingly performed compared with open surgical drainage (SD).6 This paradigm shift has been fueled by a drive for a low-risk and less-invasive procedure. SD has increasingly been reserved for those that have failed the percutaneous option.7
However, the true merits of percutaneous drainage (PD) and SD procedures for large liver abscesses are difficult to ascertain from the literature. Successful results have been reported for the percutaneous route, but these reports stem from nonsurgical departments and fail to assess patient comorbidities.8 When comparisons with surgically treated patients are made, historical controls of the preimaging era are used9 or include patients who failed attempts at percutaneous treatment. SD has become associated with higher morbidity and mortality rates, often used as a salvage procedure and seen as inferior.9,10 Large abscesses, especially if multiloculated or containing thick viscid pus, are prone to failure by percutaneous treatment. This can delay definitive treatment, increase complication rates, prolong hospital stay, and increase cost of treatment.11 Open SD allows for breakdown of loculations and complete drainage of viscid pus and necrotic debris that may provide quicker and more effective drainage with faster resolution of sepsis than PD.
To date, no comparative study or randomized trial has addressed the issue of managing large liver abscesses with PD and SD as first-line treatment to compare their relative efficacies. At our institution, all liver abscesses larger than 5 cm are referred to the surgical unit. We hypothesize that SD can produce better clinical outcome than PD for large liver abscesses.
PATIENTS AND METHODS
A search of the departmental database was carried out for patients who underwent treatment of pyogenic liver abscess. The 3-year period of January 2000 till December 2002 inclusive was reviewed. Patients were included in the study when they had an acute pyogenic liver abscess (single or multiple) 5 cm or larger confirmed on US or CT. Patients were excluded if the abscess was not amenable to PD, ruptured at initial presentation, or if there was a concomitant pathology that required urgent surgical intervention. This included patients with cholecystitis, perforated appendicitis, and cholangitis from choledocholithiasis. Amebic and fungal abscesses or those that resulted from a superinfection of an underlying tumor or cyst were also excluded.
In all patients, the clinical parameters, hematologic and biochemical findings, microbiological assays, and radiologic findings were documented and obtained from the case records and a computerized database. Size of abscess was determined by the widest diameter of the largest abscess identified. Multiloculation was defined as an abscess with 2 or more septations within its cavity. After initial workup of blood cultures, broad-spectrum antibiotics in the form of ceftriaxone and metronidazole were given parenterally. This was modified accordingly when bacterial cultures and sensitivities were subsequently available.
The approach to drainage of the abscess was determined and carried out by the individual surgeons. The unique situation in the department was that 1 team of surgeons favored the use of PD and 1 favored SD of liver abscesses larger than 5 cm for first-line treatment. Their choice of treatment was consistent over the period of the study, and this allowed for comparison of treatment between the 2 groups. For PD, both US and CT scan–guided procedures were used. Initial therapeutic aspiration of pus was carried out followed by placement of an 8- to 10-Fr pigtail catheter by the modified Seldinger technique. If the patient's condition failed to improve or worsened clinically, PD was abandoned and SD was carried out.
All patients that underwent SD had their CT scans reviewed prior to the procedure to assess the position and best route of drainage. Intraoperative ultrasonography (IOUS) was used to locate and mark the extent of the abscess. The position of major vascular and biliary pedicles was also noted. Diagnostic aspiration with a 20-Fr needle was used to confirm the location of the abscess and mark the site for drainage. Drainage was carried out by complete removal of all pus. Intra-abscess loculations were broken down by gentle blunt dissection. Following drainage, the abscess cavity was flushed with saline for clearance of residual pus and necrotic debris. IOUS was used to confirm the complete drainage and hemostasis was secured. Large-bore soft tube drains (28 Fr) were placed within the abscess cavity proper. Individual consultant surgeons of the teams supervised all surgical procedures. In both drainage procedures, abscess cavities were followed up by US or CT imaging. Drainage tubes are removed only after drainage is minimal. All patients were subsequently followed up for at least 3 months after discharge from hospital.
The clinical end points used in the study include the following:
Time to defervescence of fever to 37.5°C or less for 2 consecutive days. The time was defined as the number of days from the day of initiation of drainage to the time that the body temperature had declined to 37.5°C or less. The first of the 2 consecutive afebrile days was used.
Failure of treatment was defined as death or deterioration of clinical status requiring additional procedures related to the liver abscess during the same hospital admission.
Secondary procedure was defined as the need for a repeated procedure related to progression or persistence of sepsis, recurrence of liver abscess, or related to the underlying cause of the abscess.
Mortality was defined as death within 30 days or within the same hospital admission.
Length of hospital stay was defined as the number of days of hospital stay from the day PD or SD was carried out.
The data were compiled and analyzed using a commercial statistical software program (SPSS for Windows, version 9.0). All continuous data were expressed as median and range. The treatment groups were compared using the χ2 test with Yates correction or the Fisher exact test where appropriate for categorical variables. For continuous variables, the Mann-Whitney U test was applied. A significant result was taken as P < 0.05.
RESULTS
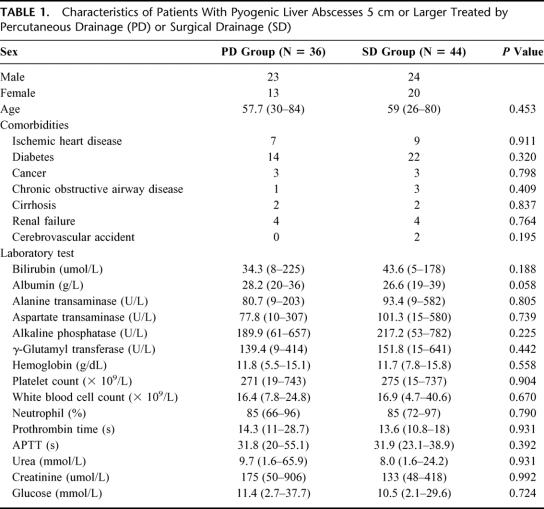
A total of 96 patients were treated with SD and PD over the 3-year period of the study. After applying the inclusion and exclusion criteria of the study, 80 patients were eligible for further analysis. Sixteen patients were excluded; 8 had suspected rupture of abscess, 7 had concomitant intra-abdominal infections requiring urgent surgical intervention, and 1 had a liver carbuncle requiring liver resection. There were a total of 47 men and 33 women, with a median age of 60 years (range: 24–84). The peak incidence of liver abscess in our cohort occurred in the 60 to 69 age group (37.5%). All patients were ethnic Chinese, Malay, and Indian except for 3 (1 Vietnamese and 2 Eurasian patients). Thirty-six patients underwent PD, and the other 44 underwent SD as first-line treatment. The clinical and laboratory parameters of both patient groups are compared in Table 1. There was no significant difference in the patient demographics, comorbidities, and laboratory parameters between the 2 treatment arms.
TABLE 1. Characteristics of Patients With Pyogenic Liver Abscesses 5 cm or Larger Treated by Percutaneous Drainage (PD) or Surgical Drainage (SD)
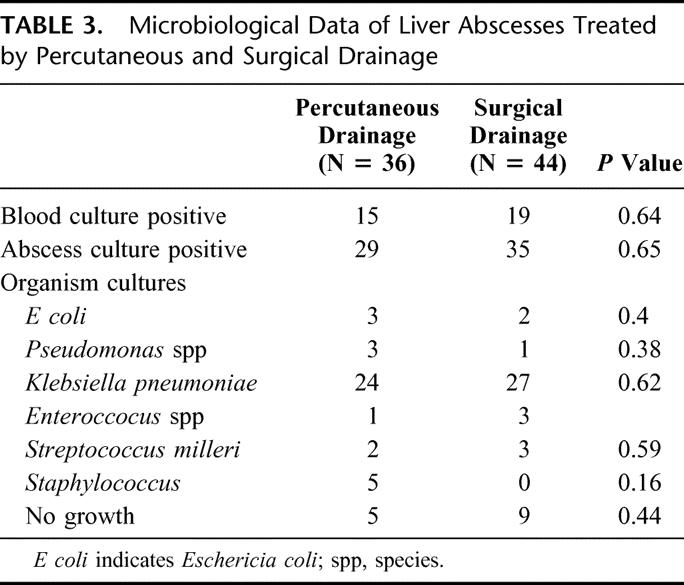
The characteristics of the abscesses treated in both groups are shown in Table 2. All abscesses were larger than 5 cm, and the site, size, distribution, and multiplicity of abscesses in both groups are similar. Eighty percent (64/80) of abscesses treated were multiloculated at presentation and still underwent both SD and PD as first-line treatment. The commonest identifiable underlying etiology is related to biliary stones, although the majority of liver abscesses are cryptogenic. Microbiological assessment of the liver abscesses is displayed in Table 3. Klebsiella pneumoniae is the commonest organism isolated and comprised 63.8% of the cohort.
TABLE 2. Characteristics of Liver Abscesses Treated by Percutaneous and Surgical Drainage
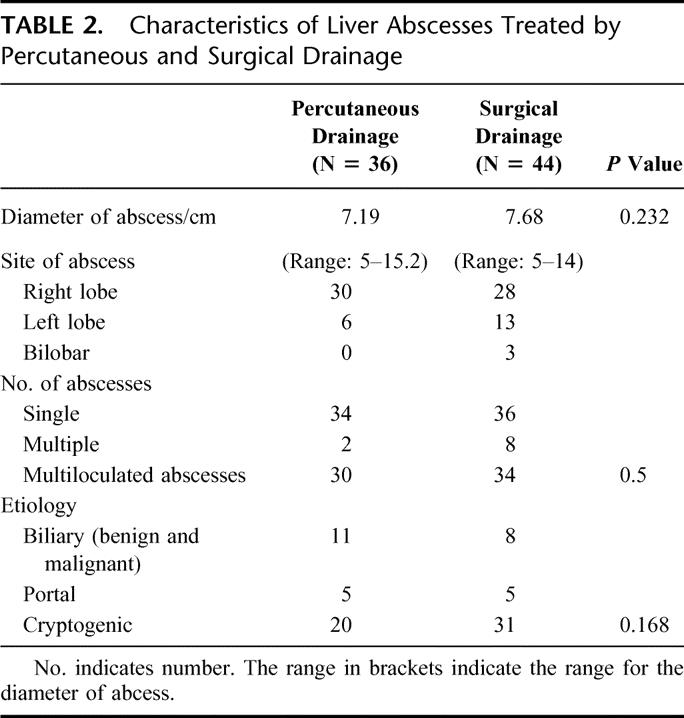
TABLE 3. Microbiological Data of Liver Abscesses Treated by Percutaneous and Surgical Drainage
The mortality rate for the 80 patients that underwent treatment was 3.8% (3/80). The mortality rate for SD and PD was 4.5% and 2.8%, respectively. In the SD arm, the median time of surgery was 94.5 ± 7 (range 25-265) minutes. In addition to drainage, 23 patients also had a concomitant cholecystectomy. Twelve patients were monitored in the intensive care unit (ICU) after surgery (27.3%). The overall morbidity for SD was 27.2%, and the general and specific complications are shown in Table 4. In the PD arm, 4 patients (11.1%) required ICU monitoring after drainage. The overall morbidity was comparable in both PD and SD groups (30.5% versus 27.2%, P = 0.74).
TABLE 4. Morbidity of Patients Treated by Percutaneous and Surgical Drainage
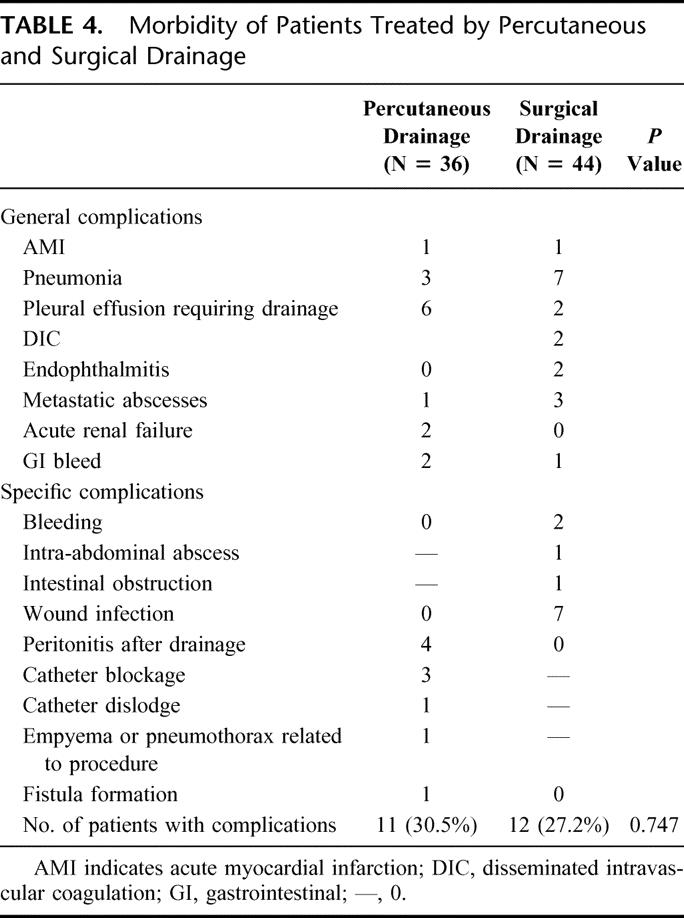
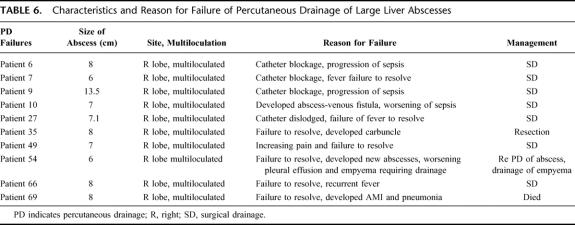
The clinical outcome in both treatment arms is shown in Table 5. There was no significant difference between PD and SD in time to defervescence of fever when treatment was successful. The number of successful treatments for SD was significantly better. Three patients failed SD (6.8%), and of these, 2 patients required a relaparotomy. One patient required 2 laparotomies for hemostasis from hemorrhage from the abscess bed. The other patient had a residual abscess collection, as well as development of multiple subphrenic abscesses that were not amenable to PD. She also had a history of pancreatic carcinoma with local pancreatic bed and nodal recurrence. The third failure occurred in a patient who had a history of diabetes and ischemic heart disease. Although there was resolution of his sepsis from the liver abscess, he developed acute myocardial infarction on the 10th postoperative day and died. In the PD arm, there were 10 failures. The causes of failures are detailed in Table 6.
TABLE 5. Clinical Outcomes of Patients Treated by Percutaneous and Surgical Drainage
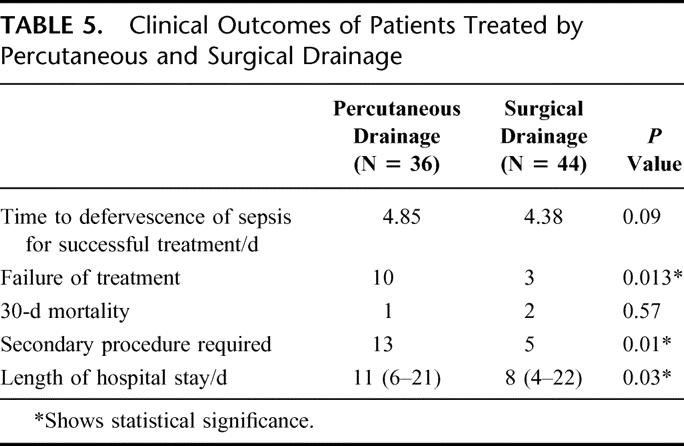
TABLE 6. Characteristics and Reason for Failure of Percutaneous Drainage of Large Liver Abscesses
There were also more secondary procedures carried out in the PD group than in the SD group (13 versus 5; P = 0.01). In the PD group, this was composed of 9 open SD procedures for progression of sepsis- and catheter-related complications, 2 delayed laparoscopic to open cholecystectomy for biliary stones, 1 PD of chest empyema, and 1 PD of a recurrent abscess. In the SD group, 4 relaparotomies were carried out. These were 1 for bleeding from the abscess bed, 1 for drainage of residual abscess, 1 for intestinal obstruction and 1 for delayed drainage of a subphrenic abscess after discharge. The last patient required a surgical debridement of the wound infection and secondary closure of the wound. The length of stay after drainage of the abscess was significantly shorter in the SD group than in the PD group (8 versus 11 days, P = 0.03) (see Table 5).
DISCUSSION
The introduction of the percutaneous approach to liver abscesses was initially described by MaFadzean et al12 from Hong Kong in 1953. The body of literature in recent years suggests that PD of liver abscess is a safe and effective method for drainage of pyogenic liver abscess.9,13,14 As a result, this modality has become viewed as the treatment of choice, being superior to SD.10,15 The attraction of PD is related to its minimally invasive approach and the ability to perform the procedure without general anesthesia. Selected series of PD drainage report success rates of more than 95%.14,16,17 However, PD is not suitable for all patients with liver abscesses. In cases of rupture, difficult access to the abscess due to anatomic location, coexisting pathology requiring open surgery, SD becomes the only treatment option.8 In addition, the abscesses that fail to resolve after PD will also have to rely on an open surgical approach for successful management. Although the trend is toward PD, it is difficult to assess the true merits of each procedure to say which modality produces better clinical outcome.
We hypothesized that SD can provide better clinical outcomes for liver abscesses larger than 5 cm. To show this, SD mostly produced better resolution of fever and better success but with equally good morbidity and mortality rates as PD. There is some evidence from surgical reported series to suggest this. Bertel et al11 reported in a series of 39 patients and showed that the success rates were lower and hospitalization stay was longer than for the PD group. Similarly, Farges et al8 also reported better success with SD (9.5 versus 36%), and more recently Herman et al18 reemphasized their good results of SD when compared with PD for successful treatment (91.5% versus 69.2%). However, such comparison of PD with SD was largely difficult and criticized for selection bias.
Our paper is the first study in the literature that compares the 2 modalities for treatment of large liver abscesses >5 cm. Abscesses that were amenable to only SD, like rupture or concomitant surgical pathology requiring urgent surgical exploration, were excluded from the study according to our criteria listed. In addition, the dichotomous first-line treatment preferences for PD and SD, unique in our department, allowed for a reasonable comparison between the groups. The patient, clinical, and abscess profiles between the 2 groups are similar.
In this study, we have demonstrated that the surgical approach can provide a better clinical outcome than the percutaneous approach for abscesses larger than 5 cm. This was significant in terms of number of treatment failures, number of secondary procedures, and hospitalization stay (see Table 5). The resolution of sepsis as defined by defervescence of fever showed a trend towards faster resolution by SD than by PD, but this did not reach statistical significance. Most important, the use of SD did not come at an increased morbidity or mortality for our patients.
Drainage of large liver abscesses may be more difficult to achieve percutaneously. There are several advantages that SD may have over PD that can account for these results. First, in our series, multiloculation is found in 80% of the abscesses treated. The role of PD in drainage of septated and multiloculated abscesses is not clear. Farges et al8 and Barakate et al19 suggested that multiloculation contributes to poorer drainage by compartmentalization of the abscess, reducing the effectiveness of PD. SD allows for breakdown of loculations and more complete drainage. Second, the use of IOUS provides better resolution of the abscess intraoperatively than during PD procedures. This allows for accurate localization, planning of drainage route, localization, and avoidance of vascular and biliary structures. In addition, complete drainage of the abscess cavity can be checked. This has also contributed to the lower-than-previously reported complications related to SD. We advocate the routine use of IOUS for all SD procedures. Third, SD allows for the accurate drainage and placement of the drainage catheter to all portions of the liver and from all angles, with mobilization of the liver if necessary. This is often aided by palpation of the abscess wall if superficial. Fourth, SD allows for a soft large-bore tube drain to be placed for better drainage of residual viscid pus and necrotic debris. This would not be possible with the pigtail catheter or cope loop used for PD, resulting in a larger number of catheter-related complications. Fifth, intraoperative US also allows for localization and drainage of satellite abscesses that may be missed on CT. Last, the patients compared here underwent SD and PD as first-line treatments rather than SD as a backup modality for failed PD. This represents a more aggressive approach to liver abscess treatment. However, it also reflects the ability of SD to deal summarily with large abscesses, leading to less failures and shorter hospital stay.
SD did not show a statistically significant difference to PD in terms of time to defervescence of fever, although there was a trend towards faster resolution of sepsis. However, this involved comparison of only successful treatments within the PD and SD group. The number of failures in the PD group contributed to the increased number of secondary procedures required and also the increase in length of hospital stay after drainage. This leads indirectly to an increase in cost of treatment of each patient. Failures in PD occurred in 27.8% of cases. The major causes were catheter-related and progression of sepsis. Catheter-related complications include 3 blockages, 1 slippage, and 1 development of a empyema of the chest after the drainage track traversed the pleural space. Some of these could have been circumvented with the use of larger drainage catheters and further improved with better interventional radiology expertise and better case selection for PD.
Previously, authors reported in-hospital mortality rates for operative treatment to be up to 15% to 25%.3,6,20 However, this was not seen in our series. The mortality rate for SD and PD is 4.5% and 2.8%, respectively. This is much lower than previously reported for the SD group. This suggests that SD as first-line treatment produces equally good mortality outcomes as PD. Delay in definitive treatment and progression of sepsis may have contributed to apparent operative failures in previously reported series. A notable difference in our series compared with those published that could contribute to our good results is the low number of associated malignancies and high number of cryptogenic abscesses.
Our study can be criticized for its patient and treatment selection, the lack of assessment of quality-of-life issues when comparing SD and PD, as well as the retrospective nature of assessment of outcome. However, it highlights the possibility that SD does have a role in first-line management for large liver abscesses more than 5 cm and should not be regarded as inferior or as a salvage procedure. It can result in better clinical outcomes than PD with comparable morbidity and mortality. This forms the rationale for a randomized trial comparing SD with PD to circumvent the selection bias and address this controversial issue.
Footnotes
Reprints: Yu-Meng Tan, Associate Consultant, Department of Surgery, Singapore General Hospital, Outram Road, Singapore. E-mail: gsutym@sgh.com.sg.
REFERENCES
- 1.Neoptolemos JP, Macpherson DS, Holm J, et al. Pyogenic liver abscess: a study of forty-four cases in two centres. Acta Chir Scand. 1982;148:415–421. [PubMed] [Google Scholar]
- 2.Northover JM, Jones BJ, Dawson JL, et al. Difficulties in the diagnosis and management of pyogenic liver abscess. Br J Surg. 1982;69:48–51. [DOI] [PubMed] [Google Scholar]
- 3.Miedema BW, Dineen P. The diagnosis and treatment of pyogenic liver abscesses. Ann Surg. 1984;200:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silver S, Weinstein AJ, Cooperman A. Changes in the pathogenesis and detection of intraphepatic abscess. Am J Surg. 1979;137:608–610. [DOI] [PubMed] [Google Scholar]
- 5.Herbert DA, Fogel DA, Rothman J, et al. Pyogenic liver abscesses: successful non-surgical therapy. Lancet. 1982;1:134–136. [DOI] [PubMed] [Google Scholar]
- 6.Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg. 1996;223:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeto RK, Rockey DC. Pyogenic liver abscess: changes in etiology, management, and outcome. Medicine (Baltimore). 1996;75:99–113. [DOI] [PubMed] [Google Scholar]
- 8.Farges O, Leese T, Bismuth H. Pyogenic liver abscess: an improvement in prognosis. Br J Surg. 1988;75:862–865. [DOI] [PubMed] [Google Scholar]
- 9.Gerzof SG, Johnson WC, Robbins AH, et al. Intrahepatic pyogenic abscesses: treatment by percutaneous drainage. Am J Surg. 1985;149:487–494. [DOI] [PubMed] [Google Scholar]
- 10.Attar B, Levendoglu H, Cuasay NS. CT-guided percutaneous aspiration and catheter drainage of pyogenic liver abscesses. Am J Gastroenterol. 1986;81:550–555. [PubMed] [Google Scholar]
- 11.Bertel CK, van Heerden JA, Sheedy PF 2nd. Treatment of pyogenic hepatic abscesses: surgical vs percutaneous drainage. Arch Surg. 1986;121:554–558. [DOI] [PubMed] [Google Scholar]
- 12.McFadzean AJS, Chang KPS, Wong CC. Solitary pyogenic abscess treated by closed aspiration and antibiotics: fourteen consecutive cases with recovery. Br J Surg. 1953;41:141–152. [DOI] [PubMed] [Google Scholar]
- 13.McDonald MI, Corey GR, Gallis HA, et al. Single and multiple pyogenic liver abscesses: natural history, diagnosis and treatment, with emphasis on percutaneous drainage. Medicine (Baltimore). 1984;63:291–302. [DOI] [PubMed] [Google Scholar]
- 14.Miller FJ, Ahola DT, Bretzman PA, et al. Percutaneous management of hepatic abscess: a perspective by interventional radiologists. J Vasc Interv Radiol. 1997;8:241–247. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RD, Mueller PR, Ferrucci JT Jr, et al. Percutaneous drainage of pyogenic liver abscesses. AJR Am J Roentgenol. 1985;144:463–467. [DOI] [PubMed] [Google Scholar]
- 16.Baek SY, Lee MG, Cho KS, et al. Therapeutic percutaneous aspiration of hepatic abscesses: effectiveness in 25 patients. AJR Am J Roentgenol. 1993;160:799–802. [DOI] [PubMed] [Google Scholar]
- 17.Giorgio A, Tarantino L, Mariniello N, et al. Pyogenic liver abscesses: 13 years of experience in percutaneous needle aspiration with US guidance. Radiology. 1995;195:122–124. [DOI] [PubMed] [Google Scholar]
- 18.Herman P, Pugliese V, Montagnini AL, et al. Pyogenic liver abscess: the role of surgical treatment. Int Surg. 1997;82:98–101. [PubMed] [Google Scholar]
- 19.Barakate MS, Stephen MS, Waugh RC, et al. Pyogenic liver abscess: a review of 10 years’ experience in management. Aust N Z J Surg. 1999;69:205–209. [DOI] [PubMed] [Google Scholar]
- 20.Chu KM, Fan ST, Lai EC, et al. Pyogenic liver abscess: an audit of experience over the past decade. Arch Surg. 1996;131:148–152. [DOI] [PubMed] [Google Scholar]