Abstract
Objective:
The purpose of this study was to test the hypothesis that CEACAM6 expression is an indicator of adverse pathologic features and clinical outcome in pancreatic adenocarcinoma.
Summary Background Data:
Previously, we have demonstrated carcinoembryonic antigen–related cell adhesion molecule 6 (CEACAM6) to be an oncoprotein that plays an important role in the biology of pancreatic adenocarcinoma. Suppression of CEACAM6 expression reduces tumorigenesis and metastasis in vivo.
Methods:
A tissue microarray was constructed using tumor specimens obtained from 89 consecutive patients who had undergone pancreatic resection for pancreatic adenocarcinoma with curative intent. A second microarray containing 54 pancreatic intraepithelial neoplasia (PanIN) lesions was constructed using tissues from a separate cohort of 44 patients. Both arrays were immunostained using a specific anti-CEACAM6 monoclonal antibody. Tumoral CEACAM6 expression was dichotomized into negative and positive immunoreactivity groups. The log-rank test was used to evaluate univariate associations of CEACAM6 expression with prognosis. Survival curves were derived using the Kaplan-Meier method.
Results:
Tumoral CEACAM6 expression was detected in 82 (92%) pancreatic adenocarcinoma specimens. CEACAM6 expression was more prevalent in high-grade than in low-grade PanIN lesions (P = 0.0002). Negative tumoral CEACAM6 expression was associated with absence of lymph node metastases (P = 0.012), lower disease stage (P = 0.008), and longer postoperative survival (P = 0.047).
Conclusions:
CEACAM6 is a novel biomarker for pancreatic adenocarcinoma. CEACAM6 warrants further evaluation as both a prognostic factor and a therapeutic target in pancreatic cancer.
Tumoral expression of carcinoembryonic antigen–related cell adhesion molecule 6 (CEACAM6) was evaluated in human pancreatic adenocarcinoma specimens. The presence of CEACAM6 expression was found to be associated with adverse clinicopathological features, including shorter postoperative survival. CEACAM6 is a novel biomarker in pancreatic cancer.
Pancreatic cancer is the 4th leading cause of cancer-related death in the United States.1 Because of its propensity for aggressive invasion and early metastasis and the lack of effective early detection strategies, only around 10% of patients are surgical candidates at the time of diagnosis. Even among patients who are able to undergo surgical resection with curative intent, median postoperative survival times remain in the region of 17 months.2
The strategy most likely to yield significant advances in our ability to care for patients with this disease is to increase our understanding of the biology of pancreatic cancer, particularly of the molecular mechanisms driving the initiation and progression of this cancer. Potential dividends of this approach include identification of novel therapeutic targets and biomarkers that may be used for early detection, prognostication, and monitoring response to therapy.
We have previously identified carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) to be an oncoprotein that plays an important role in the progression of pancreatic cancer.3 CEACAM6 is a glycosylphosphatidylinositol (GPI)-linked member of the immunoglobulin superfamily that has emerged as an important determinant of gastrointestinal adenocarcinoma malignant cellular behavior and progression.3–10 We have shown that suppression of CEACAM6 expression is associated with decreased resistance to anoikis, a subset of apoptosis normally induced by inadequate or inappropriate cell-substrate adhesion.3 Resistance to anoikis is a feature of malignant cells and a determinant of tumorigenesis and metastasis.9–11 CEACAM6 expression is deregulated in colorectal cancer8 and has recently been identified as a factor of prognostic significance in resectable colorectal cancer.5
Until now, expression of CEACAM6 in pancreatic precursor ductal lesions and adenocarcinoma has not been explored or correlated with clinical outcomes. We therefore performed immunohistochemical analysis of CEACAM6 expression in pancreatic epithelial neoplasia (PanIN) lesions and in pancreatic adenocarcinoma specimens derived from patients having undergone resection for pancreatic cancer at our institutions. The purpose of the study was to test the hypothesis that CEACAM6 represents a determinant of pancreatic adenocarcinoma progression and that expression of CEACAM6 is indicative of adverse pathologic features and prognosis. Expression levels of CEACAM6 were correlated with clinicopathological data from these patients. Our results demonstrate that expression of CEACAM6 is an early molecular abnormality in pancreatic carcinogenesis and a factor of prognostic significance in pancreatic cancer.
PATIENTS, MATERIALS AND METHODS
Patients and Clinicopathological Parameters
Under an institutional review board–approved study protocol, pathology reports were searched to identify patients who underwent curative surgical resection for pancreatic adenocarcinoma between the years 1991 and 2002, at the Brigham and Women's Hospital. Eighty-nine patients with both complete clinical data and adequate tissue for inclusion in this study were identified. Clinical information, including age, gender, and use of chemotherapy, was gathered retrospectively from patient records. Pathologic findings, including tumor size, stage, lymphovascular invasion (LVI), perineural invasion (PNI), differentiation, surgical resection margin status, and lymph node status, were obtained from original pathology reports. Pathologic staging was updated according to current American Joint Committee on Cancer guidelines.
Tissue Microarray Construction
Original formalin-fixed, paraffin-embedded specimens were used to construct a pancreatic adenocarcinoma tissue microarray (TMA). Hematoxylin and eosin (H&E)-stained standard slides from each tumor specimen were reviewed by a single pathologist (MR), who was blinded to specimen CEACAM6 status. Representative tumor regions were selected from each tissue block and 2 to 3 tissue cores (0.6 mm in diameter) were taken from each region using an automated tissue arrayer (Beecher Instruments, Sun Prarie, WI). Cores were transferred to individual recipient blocks. In some cases, cores were from taken normal adjacent pancreas for use as internal controls. Five-micron sections were cut from each recipient block. Sections were stained with H&E to confirm the presence of tumor within each core, and immunohistochemical analysis of CEACAM6 expression was performed, as described below. A separate PanIN array was constructed in a similar fashion from 44 patients undergoing Whipple resection for pancreatic adenocarcinoma at the Johns Hopkins Hospital, as previously described.12
Immunohistochemistry and Scoring
TMA slides were deparaffinized, rehydrated through graded alcohol, washed with Tris-buffered saline, and processed using a streptavidin–biotin–peroxidase complex method. Antigen retrieval was performed by microwave-heating sections in 10 mM sodium citrate buffer (pH 6) for 10 minutes. After quenching of endogenous peroxidase activity and blocking of nonspecific binding, monoclonal anti-CEACAM6 antibody (By114, Imgenex, San Ramon, CA)13,14 was added at a 1:200 dilution, after which slides were incubated at 4°C overnight. The secondary biotinylated rabbit antimouse antibody (DAKO, Carpinteria, CA) was used at a dilution of 1:200 for 30 minutes at 37°C. After further washing with Tris-buffered saline, sections were incubated with StrepABComplex/horseradish peroxidase (1:100, DAKO) for 30 minutes at 37°C. Chromogenic immunolocalization was performed by exposure to 0.05% 3,3′-diaminobenzidine tetrahydrochloride. Our prior study has confirmed the specificity of this antibody by demonstrating the presence of only a single band at 90 kDa corresponding to CEACAM6 on Western blot analysis.3 Other cores containing pancreatic adenocarcinoma served as positive controls for CEACAM6 expression. Normal serum was used in the place of primary antibody as a negative control. Slides were counterstained with hematoxylin before dehydration and mounting.
Slides were reviewed by 2 independent observers blinded to clinical and pathologic data. CEACAM6 was scored according to membrane and cytoplasmic staining intensity as follows: 0, no staining or weak intensity staining in less than 5% of cells; 1, weak intensity; 2, moderate intensity; 3, strong intensity. For statistical analyses, expression was dichotomized into a CEACAM6-negative group (score 0) and a CEACAM6-positive group (scores 1, 2, or 3). In cases of disagreement, a consensus was reached by joint review.
Statistical Analysis
The association between CEACAM6 expression and individual clinicopathological variables was assessed using Fisher exact test. Survival time was defined as the time from resection to death or censoring based on the date of last contact. Deaths within 30 days of surgery were excluded from the analysis. Survival curves were derived using the method of Kaplan and Meier and compared using the log-rank test. All statistics were two-tailed with P value ≤ 0.05 considered significant.
RESULTS
Patient Characteristics
As shown in Table 1, the sample consisted of 89 patients with a diagnosis of pancreatic adenocarcinoma (42 men and 47 women). The mean age at diagnosis was 63 years (median 63 years; range 34–84 years). Only 39 patients were alive at the time of censoring. Median survival was 16.6 months (range 91–3462 days). The actuarial 1-year survival rate was 70.3%, with a 5-year survival of 8.1%. The median tumor size was 2.0 cm, with the majority of specimens having at least one lymph node with a metastatic deposit. Most tumors (47/89, 53%) were moderately differentiated, 8 (9%) were well differentiated, and 34 (38%) were poorly differentiated. Thirteen tumors were categorized as AJCC15 stage I, 74 as stage II, none as stage III, and 2 as stage IV (Table 1).
TABLE 1. Clinicopathological Characteristics of the Pancreatic Adenocarcinoma Cohort

CEACAM6 Expression in Normal Tissue and Pancreatic Adenocarcinoma Specimens
When the immunohistochemical staining protocol was applied to the specimens, we found positive expression of CEACAM6 in 92% of tumors. The CEACAM6 staining was localized to the membrane and cytoplasm (Fig. 1). Entire pathology case review of the 7 specimens with absent tumoral CEACAM6 expression demonstrated no atypical features for pancreatic adenocarcinoma. CEACAM6 was very weakly expressed in some normal pancreatic ducts.

FIGURE 1. Immunohistochemical staining for CEACAM6 in normal pancreas and pancreatic adenocarcinoma specimens. (A) Normal pancreas (CEACAM6-negative). (B) Pancreatic adenocarcinoma (CEACAM6-negative). (C) Pancreatic adenocarcinoma (CEACAM6-positive). (Magnification ×100).
CEACAM6 Expression in PanIN Lesions
In a separate tissue array, constructed from 44 patients with pancreatic adenocarcinoma, 54 PanIN lesions of all histologic grades were evaluated for CEACAM6 expression. The majority of PanIN 1A lesions 12/18 (66%) had no CEACAM6 expression; the remainder of PanIN 1A lesions had only weak CEACAM6 expression. In contrast, 13/20 (65%) of PanIN 1B demonstrated some, albeit weak, CEACAM6 staining. The prevalence of CEACAM6 expression was similar among PanIN 2 and PanIN 1B lesions; however, the staining was more intense among PanIN 2 lesions, with some demonstrating moderate to heavy CEACAM6 levels. All 6 of the PanIN 3 lesions expressed CEACAM6 at moderate to heavy levels (Table 2). Relative to low grade PanIN lesions (IA and IB), higher grade PanIN lesions (2 and 3) demonstrated significantly greater CEACAM6 expression (P < 0.0001, Fig. 2).
TABLE 2. CEACAM6 Expression in PanIN Lesions

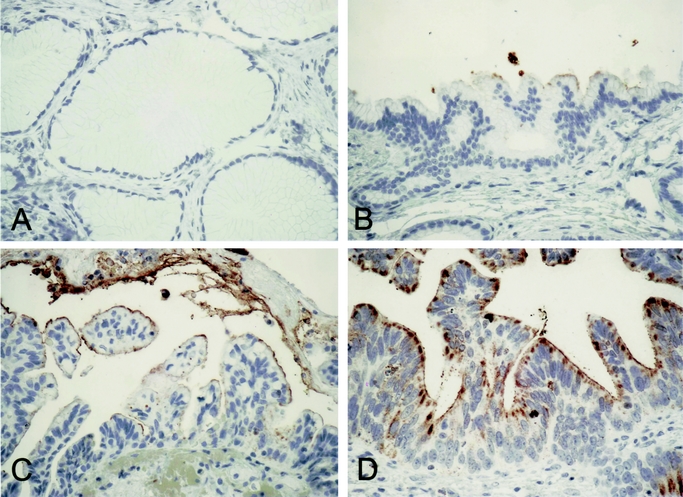
FIGURE 2. CEACAM6 expression in PanIN lesions. There was no detectable CEACAM6 expression in most PanIN 1A lesions (A). Although some PanIN 2 lesions were negative, most weakly expressed CEACAM6 (B). In contrast, PanIN 3 lesions expressed CEACAM6 at moderate to high levels (C and D).
Association of Immunohistochemical CEACAM6 Expression With Clinicopathological Variables
Clinicopathological variables were dichotomized and their association with CEACAM6 expression in pancreas adenocarcinoma was analyzed. The distribution of patients with positive or negative CEACAM6 expression with respect to age, gender, tumor size, differentiation, LVI, PNI, receipt of chemotherapy, and margin status showed no statistically significant differences. However, positive CEACAM6 expression was significantly overrepresented in patients with positive lymph nodes, and those with T3 (tumor extending beyond the pancreas) versus T2 (tumor limited to pancreas) disease (Table 3).
TABLE 3. Association Between CEACAM6 Expression and Clinicopathological Variables

Immunohistochemical Absence of CEACAM6 Expression Predicts Favorable Prognosis
To perform survival analysis, using the Kaplan-Meier method, patients were divided into 2 groups: 1 with positive CEACAM6 expression (CEACAM6+) and another with negative CEACAM6 expression (CEACAM6−). Patients with absent CEACAM6 expression had significantly longer overall postoperative survival (mean 5.42 years; median 8.81 years), compared with those with CEACAM6-positive tumors (mean 1.37 years; median 1.32 years; P = 0.047, log rank; Fig. 3).
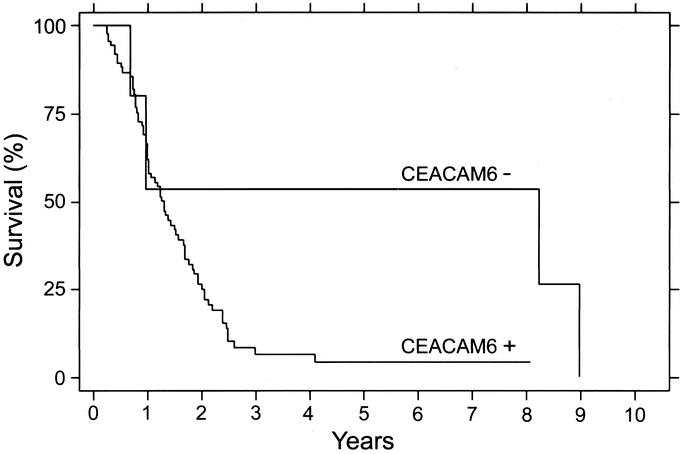
FIGURE 3. Kaplan-Meier analysis for overall survival for pancreatic adenocarcinoma patients according to CEACAM6 expression. CEACAM6-: immunohistochemically CEACAM6-negative; CEACAM6+: immunohistochemically CEACAM6 positive (P = 0.047, log-rank test).
DISCUSSION
The purpose of this study was to examine CEACAM6 expression in pancreatic adenocarcinoma, as well as in PanIN lesions, which represent a potential precursor of this devastating malignancy. CEACAM6 expression was observed in more than 90% of pancreatic adenocarcinoma specimens and was associated with lymph node–positive disease and extrapancreatic spread. The median survival time of patients with CEACAM6-positive tumors was significantly shorter than that of patients with CEACAM6-negative disease. Furthermore, we observed differential expression of CEACAM6 among PanIN lesions, with higher grade lesions exhibiting increased levels of CEACAM6 expression. Taken together, these observations indicate that CEACAM6 is not only a potentially useful biomarker, but may also play a role in the oncogenic changes that occur during progression of premalignant PanIN lesions to invasive pancreatic cancer.
Pancreatic adenocarcinoma is an almost invariably fatal disease and remains among the leading causes of cancer-related death worldwide. Although the majority of patients present with surgically incurable malignancy, even with apparently curative resection, most patients die of their disease. In addition, nonsurgical treatments such as chemotherapy using the principal agent gemcitabine offer minimal survival benefit.16 Earlier and more reliable identification of patients likely to benefit from surgical intervention would therefore represent a significant advance. The identification of precursor lesions has had significant clinical impact in other gastrointestinal malignancies, such as colorectal cancer, by allowing earlier detection and curative treatment of lesions that may progress to invasive cancer. To date, treatment of PanIN lesions has been limited by inadequate detection and, in turn, a paucity of data regarding their natural history. A better understanding of the clinical significance of PanIN lesions may be gained through studying the molecular and genetic similarities and differences between these lesions and invasive pancreatic cancer.
CEACAM6 expression appears to be an early event in the progression to pancreatic cancer. Overall, 50% of the low-grade PanIN lesions demonstrated some staining for CEACAM6 expression, whereas 100% of PanIN3 lesions were CEACAM6-positive. “Early” events in the putative PanIN-cancer progression sequence include abnormal expression of diverse genes including cycle cell regulators such as p16, the apomucin MUC5, and the novel tumor marker prostate stem cell antigen.12 The early detection of PanIN lesions, before they progress to invasive cancer, may be 1 strategy to maximize the therapeutic impact of surgical intervention in this disease.
Based on the frequency of CEACAM6 expression in PanIN tissues, it was not surprising to find that the majority of invasive adenocarcinomas expressed CEACAM6. The demonstration that, in our cohort, 92% of tumors demonstrated CEACAM6 expression suggests that CEACAM6 may have a place among existing tumor markers, such as CEA or CA19-9, as a diagnostic aid or adjunct for monitoring disease status postresection. Although there are currently no data available on the serum expression of CEACAM6 in patients with either pancreas or colorectal cancer, based upon the homology of CEACAM6 to its immunoglobulin superfamily member CEA as well as its location on the cell surface, it is likely shed into the serum as is CEA. Perhaps more interesting, however, was the identification of a subpopulation of patients with immunohistochemically CEACAM6-negative tumors. These patients exhibited significantly longer survival than patients with CEACAM6-positive tumors. Expression of CEACAM6 has also been reported to be a factor of independent prognostic significance in colorectal cancer patients.5 Although ours is among the largest immunohistochemical studies of pancreatic adenocarcinoma, the relatively small size of our cohort limits interpretation beyond the results presented. These findings need to be independently validated in a larger cohort. However, absence of CEACAM6 immunostaining may be useful in identifying a subgroup of patients who survive longer after surgical resection, and by inference, theoretically derive greater benefit from surgical management of their disease. Beyond its prognostic value, CEACAM6 itself shows promise as a therapeutic target in the setting of pancreatic cancer.3,17 Further studies are under way to explore this possibility.
Our data regarding the role of CEACAM6 in pancreatic adenocarcinoma disease progression and patient outcome are consistent with the findings of a variety of preclinical studies. Pancreatic cancers cells are known to differentially express CEACAM6.18 Our group has recently shown that levels of CEACAM6 expression correlate with the ability of cancer cells to survive under anchorage-independent conditions, and to form metastases in the mouse.3 Resistance to apoptosis induced by loss of cell anchorage, termed anoikis,19 is a feature of transformed cells that is associated with tumorigenesis, invasion, and metastasis in a broad range of malignancies.3,4,9–11,20–24 The association of tumor CEACAM6-negativity with lower levels of lymph node metastasis and extrapancreatic spread, as well as improved survival in our study, is consistent with a putative role for CEACAM6 as an inhibitor of anoikis, a hypothesis that is supported by in vitro studies.3 This association does, however, remain speculative and will require further investigation.
CEACAM6 appears to play a role in a variety of malignancies. CEACAM6 expression status has been shown to be related to grade of differentiation in gastrointestinal cancer6 and artificial overexpression of CEACAM6 blocks colonocyte and myogenic differentiation.4,25 The higher levels of CEACAM6 expression exhibited by PanIN lesions of increasing grade is consistent with a model in which CEACAM6 acts as either a determinant, or a marker, of abnormal differentiation. The findings of the present study, taken together with these other data, support a fundamental role for CEACAM6 in both early gastrointestinal carcinogenesis and subsequent tumor progression.
In summary, CEACAM6 expression appears to be an early event in pancreatic carcinogenesis, and its expression is associated with adverse pathologic features and prognosis in patients undergoing resection for pancreatic adenocarcinoma. This study demonstrates, for the first time, that CEACAM6 expression may represent a useful biomarker that may aid in the identification of patients more likely to derive benefit from surgical management of their disease. CEACAM6 warrants further evaluation as a novel biomarker marker in all stages of this malignancy.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Jan Rounds and Martina Storz Schweizer.
Footnotes
Supported by American Cancer Society (RSG-04-221-01-CCE), National Pancreas Foundation, Departmental funding from the Department of Surgery, Brigham and Women's Hospital.
An equal contribution was made by authors M.S.D. and E.M.
Reprints: Edward E. Whang, MD, Department of Surgery, Brigham and Women's Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115. E-mail: ewhang1@partners.org.
REFERENCES
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. [DOI] [PubMed] [Google Scholar]
- 2.Zervos EE, Rosemurgy AS, Al Saif O, et al. Surgical management of early-stage pancreatic cancer. Cancer Control. 2004;11:23–31. [DOI] [PubMed] [Google Scholar]
- 3.Duxbury MS, Ito H, Zinner MJ, et al. CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene. 2004;23:465–473. [DOI] [PubMed] [Google Scholar]
- 4.Ilantzis C, DeMarte L, Screaton RA, et al. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia. 2002;4:151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jantscheff P, Terracciano L, Lowy A, et al. Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J Clin Oncol. 2003;21:3638–3646. [DOI] [PubMed] [Google Scholar]
- 6.Kodera Y, Isobe K, Yamauchi M, et al. Expression of carcinoembryonic antigen (CEA) and nonspecific crossreacting antigen (NCA) in gastrointestinal cancer; the correlation with degree of differentiation. Br J Cancer. 1993;68:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ordonez C, Screaton RA, Ilantzis C, et al. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000;60:3419–3424. [PubMed] [Google Scholar]
- 8.Scholzel S, Zimmermann W, Schwarzkopf G, et al. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol. 2000;156:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streuli CH, Gilmore AP. Adhesion-mediated signaling in the regulation of mammary epithelial cell survival. J Mammary Gland Biol Neoplasia. 1999;4:183–191. [DOI] [PubMed] [Google Scholar]
- 10.Yawata A, Adachi M, Okuda H, et al. Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells. Oncogene. 1998;16:2681–2686. [DOI] [PubMed] [Google Scholar]
- 11.Shanmugathasan M, Jothy S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int. 2000;50:273–279. [DOI] [PubMed] [Google Scholar]
- 12.Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–912. [DOI] [PubMed] [Google Scholar]
- 13.Mayne KM, Pulford K, Jones M, et al. Antibody By114 is selective for the 90 kD PI-linked component of the CD66 antigen: a new reagent for the study of paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1993;83:30–38. [DOI] [PubMed] [Google Scholar]
- 14.Tooze JA, Saso R, Marsh JC, et al. The novel monoclonal antibody By114 helps detect the early emergence of a paroxysmal nocturnal hemoglobinuria clone in aplastic anemia. Exp Hematol. 1995;23:1484–1491. [PubMed] [Google Scholar]
- 15.American Joint Committee on Cancer (AJCC) Cancer Staging Manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 16.Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 17.Duxbury MS, Ito H, Zinner MJ, et al. CEACAM6 gene silencing impairs anoikis resistance and suppresses metastasis of pancreatic adenocarcinoma. J Surg Res. 2003;114:241. [Google Scholar]
- 18.Ryu B, Jones J, Blades NJ, et al. Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res. 2002;62:819–826. [PubMed] [Google Scholar]
- 19.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvelo F, Poupon MF. [Molecular and cell aspects of the cancer metastasis]. Acta Cient Venez. 2001;52:304–312. [PubMed] [Google Scholar]
- 21.Fiucci G, Ravid D, Reich R, et al. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–2375. [DOI] [PubMed] [Google Scholar]
- 22.Graff JR, Deddens JA, Konicek BW, et al. Integrin-linked kinase expression increases with prostate tumor grade. Clin Cancer Res. 2001;7:1987–1991. [PubMed] [Google Scholar]
- 23.Swan EA, Jasser SA, Holsinger FC, et al. Acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer. Oral Oncol. 2003;39:648–655. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Z, Sanchez-Sweatman O, Huang X, et al. Anoikis and metastatic potential of cloudman S91 melanoma cells. Cancer Res. 2001;61:1707–1716. [PubMed] [Google Scholar]
- 25.Rojas M, DeMarte L, Screaton RA, et al. Radical differences in functions of closely related members of the human carcinoembryonic antigen gene family. Cell Growth Differ. 1996;7:655–662. [PubMed] [Google Scholar]


