Abstract
In CD4+ UE160 cells with inducible expression of gp160, mechanisms of apoptosis induced by human immunodeficiency virus (HIV) Env protein were analyzed. Induction of gp160 caused intracellular calcium increase followed by the release of cytochrome c from mitochondria, which was inhibited by calcineurin inhibitors. Association of BAD with Bcl-xL was observed, and a portion of BAD was dephosphorylated after induction of gp160. These data suggested that calcineurin plays a role in the HIV Env-induced apoptosis in a mitochondrion-dependent way.
Human immunodeficiency virus (HIV) infection leads to AIDS, characterized by loss of CD4+ T cells in the immune system (3), and the loss of CD4+ T cell has been postulated as the main reason of the immunodeficiency in AIDS. Viral genes env, nef, tat, and vpr have been shown to have cytopathogenic properties (13). Although it is presumable that the apoptotic cell death in vivo in AIDS patients is a complexity of various mechanisms, in an attempt to clarify the mechanism of Env-induced apoptosis, we established an experimental system in which induced expression of gp160 of HIV type 1 in CD4+ cells led to apoptosis (5, 6, 8). We have reported that this apoptosis was preceded by the elevation of intracellular calcium concentration (4, 14).
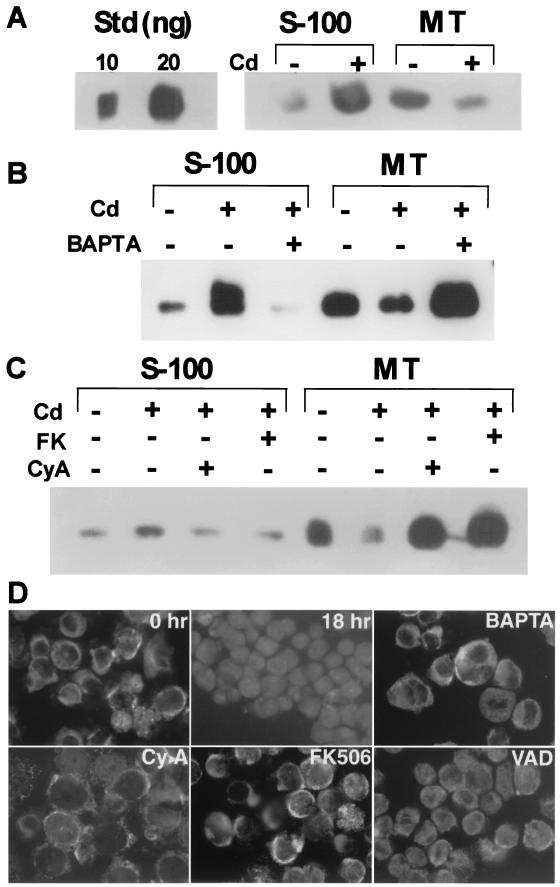
An HIV gp160-expressing transfectant cell line, UE160, derived from CD4+ U937-2, was established previously (5). As we have reported (8), UE160 showed apoptotic cell death after induction of gp160 with 15 μM CdCl2, while treatment of U937-2, a parental cell line, with the same concentration of CdCl2 did not induce cell death. The same treatment also induced a dissipation of the mitochondrial transmembrane potential, ΔΨm (7), in a population of UE160 cells (data not shown), indicating mitochondrial disturbances in the cells. We thus examined the redistribution of cytochrome c after induction of gp160 in UE160 cells by fractionation of intracellular proteins into mitochondrial and S-100 fractions. Before CdCl2 treatment, most of cytochrome c was detected in the mitochondrial fraction of UE160 cells (Fig. 1A). However, translocation of cytochrome c from mitochondria to S-100 was evident in UE160 cells treated with CdCl2 for 24 h. In our previous studies, the increase of intracellular calcium concentration after induction of gp160 protein was responsible for apoptotic cell death (4, 14). Treatment of cells with BAPTA-am, a cell-permeating calcium chelator, inhibited the release of cytochrome c in CdCl2-treated UE160 cells (Fig. 1B), indicating the involvement of calcium in the cytochrome c release.
FIG. 1.
Release of cytochrome c after induction of gp160 in UE160. (A) UE160 cells were treated with CdCl2 for 24 h. The S-100 fraction and mitochondrial fraction (MT) were prepared by centrifugation and examined for cytochrome c by Western blotting. As a control, cytochrome c (10 or 20 ng per lane) was electrophoresed (Std). (B) UE160 cells were cultured in the presence of CdCl2 for 12 h either with or without BAPTA-am at 10 μM. Cytochrome c in S-100 and mitochondrial fractions was examined. (C) UE160 cells were cultured in the presence of CdCl2 for 6 h with FK506 (FK) (0.5 μM) or cyclosporine (CyA) (0.5 μM). S-100 and mitochondrial fractions were examined for cytochrome c. (D) Immunofluorescence staining of cytochrome c in CdCl2-treated UE160 cells. In untreated cells or cells treated with CdCl2 in the presence of calcium signal blockers, cytochrome c was stained in a punctate pattern excluding nuclei, whereas in Cd-treated cells either with or without z-VAD, cytochrome c was stained homogeneously.
We then explored the possibility that calcium-activated calcineurin might be responsible for this redistribution of cytochrome c, since a recent line of evidence shows that calcium-dependent activation of calcineurin, a calcium-dependent serine-threonine phosphatase, induced apoptosis in some types of cells (18). Sustained increases in intracellular calcium lead to activation of calcineurin, which in turn dephosphorylates BAD, a proapoptotic member of the Bcl-2 family, thereby associating with and disturbing Bcl-xL. As shown in Fig. 1C, addition of either cyclosporine or FK506, specific calcineurin inhibitors, clearly inhibited the release of cytochrome c from mitochondria to S-100. Immunofluorescence staining of cytochrome c (Fig. 1D) also revealed the release of cytochrome c in CdCl2-treated cells, which was clearly inhibited by BAPTA-am or calcineurin inhibitors. Addition of z-VAD-fmk, a broad-spectrum caspase inhibitor, showed only a partial inhibitory effect on the release of cytochrome c, indicating the release is primarily caspase independent. Thus, in UE160 cells, activation of calcineurin by the gp160-induced increase of intracellular calcium concentration brought about the release of cytochrome c from mitochondria.
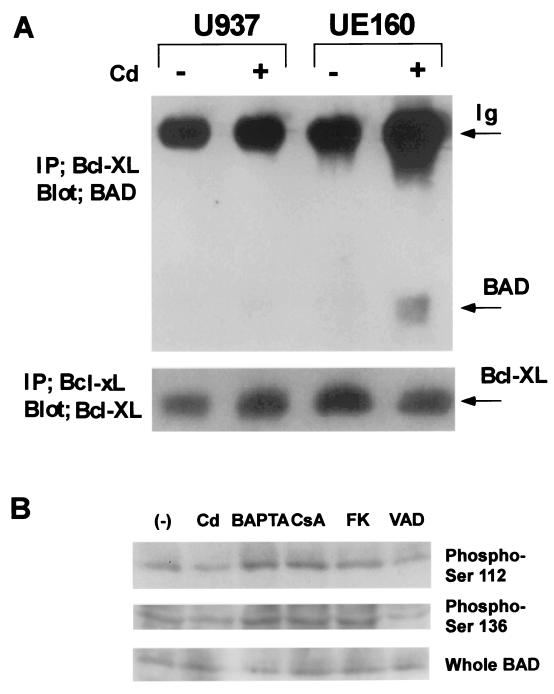
We also examined the association of BAD with Bcl-xL in UE160 cells or in parental U937-2 cells. In CdCl2-treated UE160 cells, association of BAD with Bcl-xL was confirmed by coprecipitation of BAD with Bcl-xL (Fig. 2A). In UE160 cells before CdCl2 treatment, however, no coprecipitation of BAD with Bcl-xL was detected. In the parental U937-2 cells either with or without CdCl2 treatment, no association of BAD with Bcl-xL was observed. We also examined the phosphorylation status of BAD after induction of gp160, and as expected, 18 h after induction of gp160, a portion of BAD was dephosphorylated both at Ser112 and at Ser136 compared with BAD before induction of gp160 (Fig. 2B). Addition of BAPTA-am or calcineurin inhibitors suppressed the dephosphorylation of BAD at the two residues, whereas z-VAD-fmk showed no effect. Taken together, it was indicated that, in response to the induction of gp160, a portion of BAD that was dephosphorylated presumably by activated calcineurin associated with Bcl-xL.
FIG. 2.
Association with Bcl-xL and dephosphorylation of BAD in UE160. (A) U937 (parental cell line) or UE160 cells were treated with CdCl2 for 24 h. Association of BAD with Bcl-XL was examined after immunoprecipitation of Bcl-XL and Western blotting of BAD. The amount of Bcl-xL in the immunoprecipitates (IP) was examined as an internal control by blotting with anti-Bcl-xL antibodies. The amount of BAD in UE160 did not change after CdCl2 treatment (see panel B). (B) UE160 cells were cultured in the presence of CdCl2 for 18 h either in the presence or absence (−) of indicated reagents (CsA, cyclosporine). Cell lysates were examined for total amount of BAD (Whole BAD), phosphorylated forms of BAD at Ser 112 (Phospho-Ser 112), or at Ser 136 (Phospho-Ser 136), with anti-BAD, anti-phospho-Ser112, or anti-phospho-Ser136 antibodies (Upstate Biotechnology, Lake Placid, N.Y.).
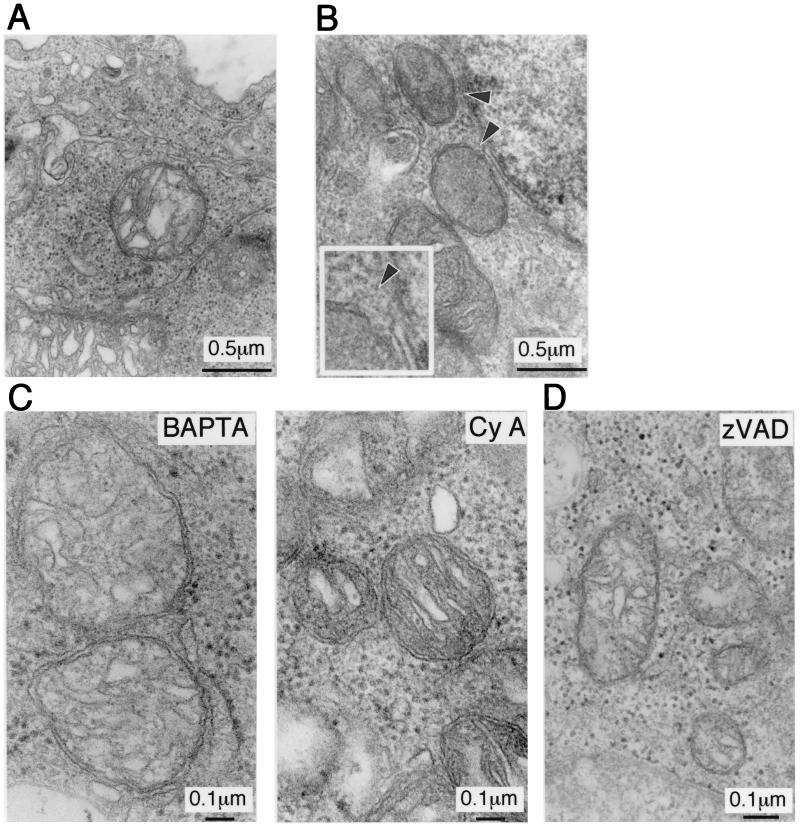
Electron microscopic examination of Cd-treated UE160 cells revealed characteristics typical of apoptosis, both at 24 and 48 h after CdCl2 treatment (8). In these apoptotic cells, we also noticed that approximately 20% of mitochondria appeared swollen and even showed rupture of outer membranes at as early as 6 h after Cd treatment (Fig. 3B). Both in BAPTA-am- and in FK506-treated UE160 cells after induction of gp160, mitochondrial membrane rupture was inhibited (Fig. 3C), demonstrating that the calcium-mediated pathway was responsible for these ultrastructural changes. However, treatment of the cells with z-VAD-fmk also inhibited these mitochondrial changes (Fig. 3D), indicating that the morphological changes in mitochondria was not a primary event but occurred downstream of caspases, unlike the situation in which mitochondrial swelling was the primary event of apoptosis in response to growth factor deprivation (17).
FIG. 3.
Transmission electron microscopic analysis of mitochondria of UE160 cells. (A) Untreated cells. (B) UE160 cells 6 h after CdCl2 treatment. Arrowheads show ruptures of outer membranes. The inset shows the same view at a higher magnification. Transverse mitochondrion sections were chosen for mitochondrial membrane ruptures; the abrupt disappearance of outer membranes accompanied with apparently normal inner membranes was considered to indicate rupture of the outer membranes. (C) UE160 cells treated with CdCl2 in the presence of BAPTA-am (left) or cyclosporine (CyA) (right). (D) UE160 cells treated with CdCl2 in the presence of z-VAD-fmk.
Others and we have reported that binding of gp160 via its C terminal transmembrane domain to calmodulin is pivotal in Env-induced apoptosis (4, 12, 15, 16). Moreover, Pan et al. reported that calmodulin antagonist inhibited apoptosis of CD4+ cells in AIDS patients (11), indicating that calcium- and calmodulin-regulated apoptotic pathways play roles in vivo. Therefore, the mechanism for HIV-induced cytotoxicity may involve, in part, disturbances of calmodulin-regulated cellular functions and the resultant failure of the maintenance of calcium homeostasis. Mitochondrial perturbations are also observed in HIV type 1-infected patients (1, 2, 9, 10), suggesting that mitochondrial pathways of apoptosis may be activated in vivo. Although our system is meant to experimentally illustrate the mechanisms of Env-induced apoptosis and its relevance in vivo has yet to be proved, our observations could provide a possible link between Env-induced calcium alterations and mitochondrion-dependent apoptotic pathways for the apoptosis of HIV-infected cells. Thus, we submit that disturbances of calcium metabolism induced by the expression of gp160 in CD4+ cells activates mitochondrial pathways of apoptosis and may be critically involved in the pathogenesis of AIDS.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (H.Y.).
We thank J. C. Reed and D. R. Green for comments and K. Imaizumi and T. Lin for helpful discussions.
REFERENCES
- 1.Castedo, M., A. Macho, N. Zamzami, T. Hirsch, P. Marchetti, J. Uriel, and G. Kroemer. 1995. Mitochondrial perturbations define lymphocytes undergoing apoptotic depletion in vivo. Eur. J. Immunol. 25:3277–3284. [DOI] [PubMed] [Google Scholar]
- 2.Cossarizza, A., C. Mussini, N. Mongiardo, V. Borghi, A. Sabbatini, B. De Rienzo, and C. Franceschi. 1997. Mitochondria alterations and dramatic tendency to undergo apoptosis in peripheral blood lymphocytes during acute HIV syndrome. AIDS 11:19–26. [DOI] [PubMed] [Google Scholar]
- 3.Fauci, A. S. 1993. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science 262:1011–1018. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa, H., M. Sasaki, S. Noda, and Y. Koga. 1998. Apoptosis induction by the binding of the carboxyl terminus of human immunodeficiency virus type 1 gp160 to calmodulin. J. Virol. 72:6574–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koga, Y., K. Nakamura, M. Sasaki, G. Kimura, and K. Nomoto. 1994. The difference in gp160 and gp120 of HIV type 1 in the induction of CD4 downregulation preceding single-cell killing. Virology 201:137–141. [DOI] [PubMed] [Google Scholar]
- 6.Koga, Y., M. Sasaki, H. Yoshida, H. Wigzell, G. Kimura, and K. Nomoto. 1990. Cytopathic effect determined by the amount of CD4 molecules in human cell lines expressing envelope glycoprotein of HIV. J. Immunol. 144:94–102. [PubMed] [Google Scholar]
- 7.Kroemer, G., N. Zamzami, and S. A. Susin. 1997. Mitochondrial control of apoptosis. Immunol. Today 18:44–51. [DOI] [PubMed] [Google Scholar]
- 8.Lu, Y. Y., Y. Koga, K. Tanaka, M. Sasaki, G. Kimura, and K. Nomoto. 1994. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J. Virol. 68:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macho, A., M. Castedo, P. Marchetti, J. J. Aguilar, D. Decaudin, N. Zamzami, P. M. Girard, J. Uriel, and G. Kroemer. 1995. Mitochondrial dysfunctions in circulating T lymphocytes from human immunodeficiency virus-1 carriers. Blood 86:2481–2487. [PubMed] [Google Scholar]
- 10.Moretti, S., S. Marcellini, A. Boschini, G. Famularo, G. Santini, E. Alesse, S. M. Steinberg, M. G. Cifone, G. Kroemer, and C. De Simone. 2000. Apoptosis and apoptosis-associated perturbations of peripheral blood lymphocytes during HIV infection: comparison between AIDS patients and asymptomatic long-term non-progressors. Clin. Exp. Immunol. 122:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan, G., T. Zhou, W. Radding, M. S. Saag, J. D. Mountz, and J. M. McDonald. 1998. Calmodulin antagonists inhibit apoptosis of CD4+ T-cells from patients with AIDS. Immunopharmacology 40:91–103. [DOI] [PubMed] [Google Scholar]
- 12.Pan, Z., W. Radding, T. Zhou, E. Hunter, J. Mountz, and J. M. McDonald. 1996. Role of calmodulin in HIV-potentiated Fas-mediated apoptosis. Am. J. Pathol. 149:903–910. [PMC free article] [PubMed] [Google Scholar]
- 13.Roshal, M., Y. Zhu, and V. Planelles. 2001. Apoptosis in AIDS. Apoptosis 6:103–116. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki, M., J. Uchiyama, H. Ishikawa, S. Matsushita, G. Kimura, K. Nomoto, and Y. Koga. 1996. Induction of apoptosis by calmodulin-dependent intracellular Ca2+ elevation in CD4+ cells expressing gp 160 of HIV. Virology 224:18–24. [DOI] [PubMed] [Google Scholar]
- 15.Srinivas, S. K., R. V. Srinivas, G. M. Anantharamaiah, R. W. Compans, and J. P. Segrest. 1993. Cytosolic domain of the human immunodeficiency virus envelope glycoproteins binds to calmodulin and inhibits calmodulin-regulated proteins. J. Biol. Chem. 268:22895–22899. [PubMed] [Google Scholar]
- 16.Tencza, S. B., M. A. Miller, K. Islam, T. A. Mietzner, and R. C. Montelaro. 1995. Effect of amino acid substitutions on calmodulin binding and cytolytic properties of the LLP-1 peptide segment of human immunodeficiency virus type 1 transmembrane protein. J. Virol. 69:5199–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Heiden, M. G., N. S. Chandel, P. T. Schumacker, and C. B. Thompson. 1999. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 3:159–167. [DOI] [PubMed] [Google Scholar]
- 18.Wang, H. G., N. Pathan, I. M. Ethell, S. Krajewski, Y. Yamaguchi, F. Shibasaki, F. McKeon, T. Bobo, T. F. Franke, and J. C. Reed. 1999. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284:339–343. [DOI] [PubMed] [Google Scholar]