Abstract
The replicase protein nsP2 of Semliki Forest virus (SFV) has a 648RRR nuclear localization signal and is transported to the nucleus. SFV-RDR has a single amino acid change which disrupts this sequence and nsP2 nuclear transport. In BHK cells, SFV4 and SFV-RDR replicate to high titers, but SFV-RDR is less virulent in mice. We compared the replication of SFV4 and SFV-RDR in adult mouse brain. Both SFV4 and SFV-RDR were neuroinvasive following intraperitoneal inoculation. SFV4 spread rapidly throughout the brain, whereas SFV-RDR infection was confined to small foci of cells. Both viruses infected neurons and oligodendrocytes. Both viruses induced apoptosis in cultured BHK cells but not in the cells of the adult mouse brain. SFV-RDR infection of mice lacking alpha/beta interferon receptors resulted in widespread virus distribution in the brain. Thus, a component of the viral replicase plays an important role in the neuropathogenesis of SFV.
The molecular and cell biology of the alphavirus Semliki Forest virus (SFV) have been extensively studied, as has the pathogenesis of SFV infection in laboratory mice (6, 10). Generally, the virus is neuroinvasive, and extraneural inoculation results in a high-titer plasma viremia with infection of central nervous system (CNS) cells by virus passage across the blood-brain barrier (24, 25, 11, 36). Strains of SFV vary in their neurovirulence in adult mice. Irrespective of the route of inoculation, adult mice infected with the L10, V13, or prototype strains or virus derived from the prototype molecular clone SFV4 rapidly succumb to a fulminant encephalitis, whereas adult mice infected with the A7 or A7(74) strain develop a subclinical encephalitis (8, 27). The nominally avirulent strains spread more slowly in the CNS, and host responses prevent widespread fatal infection and eventually eliminate infectious virus (7, 11), though they generate lesions of immune-mediated demyelination (12, 37). In SJL mice these lesions are active for many months (9, 35). In contrast to their differences in adult mice, all strains of SFV are virulent in embryonic, neonatal, and suckling mice (5, 11, 13, 15). For the adult-avirulent strains A7 and A7(74) there is a sharp age-related transition (22, 23). In suckling mouse brain and in culture, many infected brain cells rapidly undergo apoptosis (3, 17), whereas in immunocompromised adult mice, virus can persist in CNS cells without any apparent cell death for many months (4, 12). Age-related neurovirulence may be related to changes in the propensity of CNS cells to undergo apoptosis on infection (2, 3).
The SFV4, A7, and A7(74) strains have been cloned and sequenced (14, 16, 31, 32, 39, 40, 41). Between the strains there are multiple changes scattered throughout the genome (41). The difference in virulence between SFV4 and A7 or A7(74) appears to be polygenic, and to date, changes in the E2 gene, the 5′ untranslated region, and the nsP3 gene have been shown to be determinants of virulence between these particular strains (32, 33, 40, 41). As with all viruses, there will also be many other sites where changes in the genome sequence will affect viral virulence.
Interestingly for a positive-strand RNA virus which replicates in the cytoplasm, the SFV nsP2 nonstructural protein has a nuclear targeting signal. This sequence, 648RRRV (the numbering refers to the SFV4 amino acid sequence), is present in all three cloned and sequenced strains of the virus. In SFV4-infected cells about half of the nsP2 synthesized is translocated to the nucleus (19, 26, 29). nsP2 is involved at a number of stages of viral RNA replication; it has single-stranded-RNA-stimulated ATPase and GTPase activities, RNA triphosphatase activity, and RNA helicase activity; it regulates synthesis of the 26S subgenomic RNA; it is involved in the cessation of negative-strand synthesis; and it contains a papain-like proteinase domain responsible for processing the nonstructural polyprotein (18, 20, 30, 38, 42). The mechanism by which RNA viruses such as SFV trigger the apoptotic response of the infected cell remain unclear; events in the nucleus would be one possibility.
SFV-RDR virus has a single amino acid change, from SFV4 649R to D, which disrupts the nsP2 nuclear localization signal. The engineering of this virus from the SFV4 cDNA infectious clone has been described previously (28). In BHK-21 cells transfected with infectious RNA transcribed from the SFV-RDR plasmid, nsP2 expression is confined to the cytoplasm, infected cells have only a partial shutoff of host cell DNA synthesis, and there are only marginal differences in the one-step growth curves of the two viruses (28). In order to determine the importance of the nsP2 649R-to-D mutation and to establish whether this has any role in neuroinvasion, neurovirulence, tropism, or ability of the virus to trigger apoptosis, we compared the course of CNS infection in mice infected with SFV-RDR and SFV4.
Four- to six-week-old female BALB/c mice, 129 mice, or 129 mice with a genetic disruption of the alpha/beta interferon receptor gene (21) were kept under specific-pathogen-free conditions in the animal unit at the University of Edinburgh Laboratory for Clinical and Molecular Virology. Two groups of 10 female BALB/c mice were inoculated intraperitoneally with 200 PFU of SFV4 or SFV-RDR in 0.1 ml of phosphate-buffered saline (PBS) containing 0.75% bovine serum albumin. A further two groups of 10 female BALB/c mice were inoculated intracerebrally with 50 PFU of SFV4 or SFV-RDR in 0.025 ml of PBS containing 0.75% bovine serum albumin. Following intraperitoneal inoculation, mice were sampled at days 4 and 7. Following intracerebral inoculation, mice were sampled at days 2, 3, 4, and 7. In all cases, brains were removed and bisected sagitally down the midline. One half of each brain was snap frozen and stored (−70°C) for the viral infectivity assay, and the other half was immersion fixed in 4% phosphate-buffered formalin before being processed for paraffin-based histology. Plaque assays were performed on subconfluent monolayers of BHK-21 cells as described previously (11). Paraffin-embedded sections at 5 μm were cut onto adhesive-coated slides and immunostained for viral antigens. Prior to immunostaining, to increase sensitivity and resolution, an antigen retrieval step was carried out by heating in a microwave oven in 0.01 M citrate buffer. Sections were then rinsed in PBS, fixed by immersion in paraformaldehyde lysine periodate for 20 min, and digested with proteinase K before immunostaining using a rabbit polyclonal anti-SFV antiserum or staining with the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) technique prior to immunostaining as described previously (11, 34).
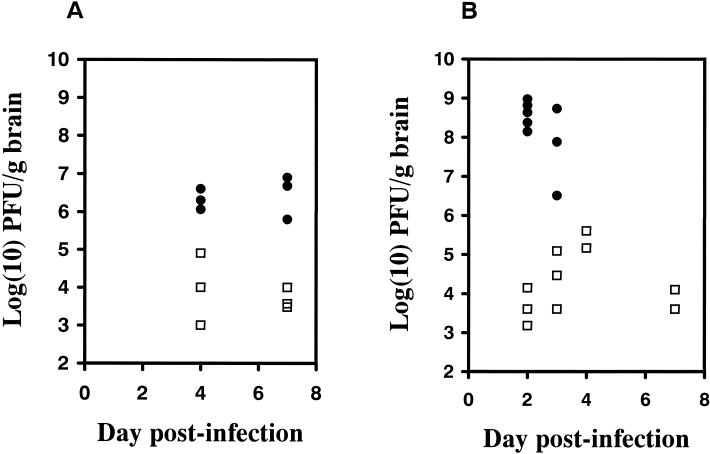
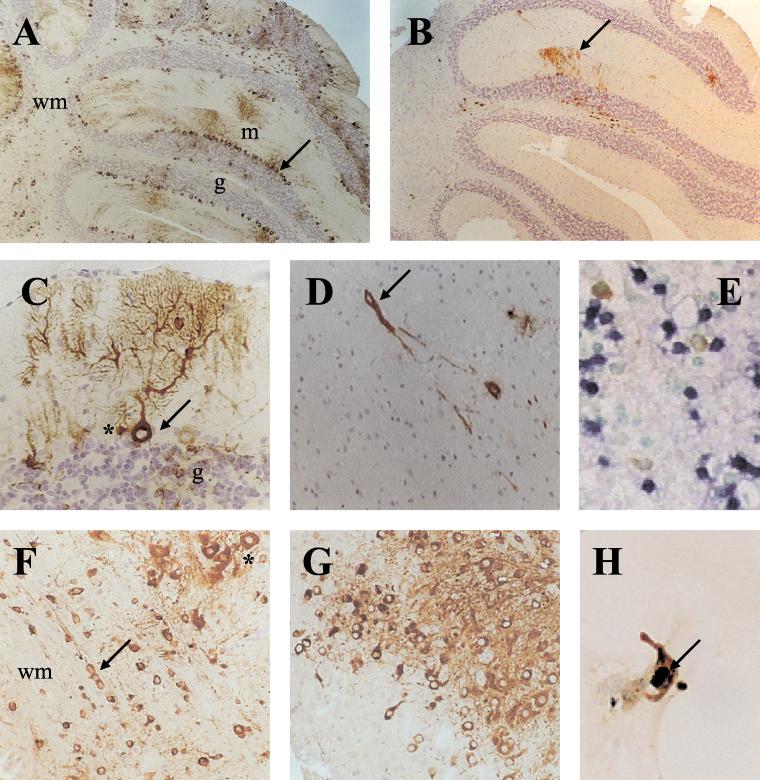
Following intraperitoneal inoculation with 200 PFU of SFV4 or SFV-RDR, BALB/c mice survived at least 42 days, at which time the study was terminated; these mice did not develop clinical signs during this time. In contrast, BALB/c mice inoculated intracerebrally with SFV4 developed clinical signs within 2 days and were all dead by 4 days (Table 1). Intracerebral inoculation of SFV-RDR did not result in any mortality or clinical signs over the 42-day study period. The brain virus titers of the sampled mice are shown in Fig. 1. Both viruses were neuroinvasive and entered the brain in all animals inoculated intraperitoneally. SFV4 brain virus titers were significantly higher than SFV-RDR titers for both routes of infection (Fig. 1). Immunostaining studies on the distribution and extent of infection in the brain confirmed this finding, with many more cells being infected by SFV4 than by SFV-RDR (Fig. 2A and B). SFV-RDR virus was restricted to replication in individual cells or small groups of cells scattered throughout the brain (Fig. 2D). This was particularly apparent following intracerebral inoculation, where foci of infection were most frequent around the needle tract and in the corpus callosum and the deep layers of the cortex close to the site of inoculation. No major differences in virus cell-type tropism were observed. Both viruses infected cells which based on their morphology were clearly neurons, for example, cerebellar Purkinje cells, and cells distributed in chains in the white matter, presumably oligodendrocytes (Fig. 2C and F). Neither strain of virus resulted in positive staining of ependymal, meningeal, or choroid plexus cells.
TABLE 1.
Percent mortality and average survival time in mice infected with SFV4 or SFV-RDR
| Mouse straina | % Mortality (avg survival time [days])
|
|
|---|---|---|
| SFV4 | SFV-RDR | |
| BALB/c mice (i.p.) | 0 | 0 |
| BALB/c mice (i.c.) | 100 (3.3) | 0 |
| 129 IFN-α/β-R+/+ mice (i.p.) | 0 | 0 |
| 129 IFN-α/β-R0/0 mice (i.p.) | 100 (2.2) | 100 (5.2) |
Mice inoculated intraperitoneally (i.p.) received 200 PFU; mice inoculated intracerebrally (i.c.) received 50 PFU. Survival was observed for 42 days.
FIG. 1.
Brain virus titers determined by plaque assay on BHK-21 cells. Mice were inoculated intraperitoneally (A) or intracerebrally (B) with 200 PFU of SFV4 (•) or SFV-RDR (□). SFV4 titers were significantly (P < 0.05) higher than SFV-RDR titers by Student’s t test at several time points following inoculation (intraperitoneal inoculation, P = 0.065 and 0.007 on days 4 and 7, respectively; intracerebral inoculation, P = 0.008 and 0.072 on days 2 and 3, respectively). Mice inoculated intracerebrally with SFV4 did not survive after day 3. The limit of detection of the assay was 2.7 log (10) PFU/g of brain.
FIG. 2.
(A) Immunostaining for viral proteins showing widespread distribution of SFV4 in the cerebellum of an adult mouse, 3 days postinfection. Cells in the all layers of the cerebellum are infected, including cells in the white matter tracts (wm), granule cells (g), and Purkinje cells (arrow). Purkinje cell processes extending into the molecular layer (m) are clearly infected. (B) SFV-RDR in the cerebellum, 3 days postinfection. As with SFV4, cells in all layers of the cerebellum are infected and the infection includes the dendritic tree of Purkinje cells, but the number of infected cells is far lower (cf. panel A). (C) Higher-power view showing infection of a single cerebellar Purkinje cell. Both the cell body (arrow) and dendritic tree contain viral proteins, SFV4, 3 days postinfection. Granule cells (g) and cells adjacent to the Purkinje cells which are probably Bergmann glia cells (∗) are also infected. (D) An SFV-RDR-positive cell (arrow) and its processes in the frontal cortex, 3 days postinfection. Single positive cells or small foci of positive cells are characteristic of this infection. (E) Control immunostaining (brown) and TUNEL staining (blue) in the olfactory bulb of a 4-day-old mouse infected intranasally (48 h earlier) with SFV A7(74). This section was stained in the same assay as sections F and G. (F) Widespread infection of SFV4 includes white matter (wm) tracts and cells distributed in chains (arrow), a characteristic of oligodendrocytes. Infected neurons often had large pale nuclei (∗). This section was also stained for TUNEL, and no TUNEL-positive cells were observed (cf. panel E). (G) Widespread distribution of SFV-RDR in the cortex of an IFN-α/β-R0/0 mouse, 5 days postinfection. This section was also stained for TUNEL, and no positive cells were observed (cf. panel E). (H) SFV-RDR infection of a BHK cell in culture (48 h postinfection) immunostained for viral proteins (brown) in the cytoplasm and TUNEL (blue-black) in the nucleus (arrow).
The difference in brain virus titers and the extent of spread of these two viruses was marked and could have resulted from a number of factors. One possibility is that SFV-RDR is incapable of widespread brain infection; alternatively, there could have been insufficient time for this virus to become widespread before its control by host responses. To distinguish between these possibilities, the course of SFV4 and SFV-RDR infection was investigated in groups of 10 alpha/beta interferon receptor knockout (IFN-α/β-R0/0) and parental (wild-type) 129 mice (21). Disruption of the single alpha/beta interferon receptor results in an absence of alpha/beta interferon responses. Intraperitoneal inoculation of 200 PFU of SFV4 into IFN-α/β-R0/0 mice resulted in death of all mice within 3 days with minimal brain infection, whereas intraperitoneal inoculation of 200 PFU of SFV-RDR into IFN-α/β-R0/0 mice resulted in death between 4 and 6 days. As with BALB/c mice, wild-type 129 mice, which have an intact interferon system, survived intraperitoneal infection with both strains of virus (Table 1). Examination of the brains of moribund SFV-RDR-infected IFN-α/β-R0/0 mice at 5 days revealed widespread infection in the frontal cortex (Fig. 2G), brain stem, pons, and thalamus with infection of ependymal cells, meningeal cells, cerebellar Purkinje cells, cerebellar granule cells, and oligodendrocytes.
In adult mouse brain, in the absence of appropriate immune responses the avirulent SFV A7(74) strain can persist for many months (4, 12), and in contrast to the situation in the developing brain, infected cells do not undergo apoptosis (2). To ascertain whether SFV4 infection of adult brain cells results in apoptosis and whether the nsP2 649R-to-D mutation changes this situation, brain sections were stained by the TUNEL technique to detect apoptotic cells and immunostained to detect virally infected cells. Sections of SFV A7(74)-infected neonatal mouse brain were used as positive controls and showed clear TUNEL staining (Fig. 2E), as shown previously (3). Fixed cultures of SFV4- and SFV-RDR-infected BHK-21 cells were also double labeled. In both the SFV4- and SFV-RDR-infected adult BALB/c mouse brains, the majority of SFV protein-positive cells had the morphologic appearance of neurons and showed enlarged palely staining nuclei (Fig. 2F). None of these infected cells were TUNEL positive. Double labeling was also carried out on sections from the brains of SFV-RDR-infected IFN-α/β-R0/0 mice, and again, neurons with enlarged palely staining nuclei but no TUNEL-positive infected cells were observed (Fig. 2G). In contrast to mature neurons, both SFV4- and SFV-RDR-infected BHK-21 cells exhibited TUNEL-positive nuclei (Fig. 2H). These results demonstrate that an intact RRR nuclear localization sequence is not required for triggering of apoptosis in SFV-infected BHK-21 cells or for the reduced apoptosis observed in SFV-infected adult brain neurons.
In conclusion, an intact nsP2 649RRR sequence is not required in cultured BHK-21 cells for apoptosis or in adult mice for neuroinvasion, determination of CNS cell tropism, or inhibition of apoptosis. In contrast, an intact nsP2 RRR sequence enhances spread of virus in the mature mouse brain, exacerbating neuropathology and increasing virulence. Thus, a single mutation in the multifunctional RNA replicase protein can limit virus spread in the brain, indicating the importance for pathogenesis of events associated with RNA replication or nsP2 nuclear transport. The nsP3 replicase protein has been shown to be important in the attenuated neurovirulence of the A7(74) strain of SFV (41). We recently constructed an SFV mutant with an internal deletion in nsP3 which also has attenuated neurovirulence in mice (43). Further support for the importance of RNA replicase components in neuropathogenesis comes from the observation that inhibition of palmitoylation of nsP1 also attenuates SFV neurovirulence (1). Our present results obtained with mice with no functional alpha/beta interferon system show that mutation of the nsP2 RRR sequence does not prevent virus spread but results in a lower rate of spread. Thus, RNA replication as such is not inhibited, but it may be slowed sufficiently in neuronal cells for the interferon system to limit the infection. This effect could also underlie the attenuating changes in nsP1 and nsP3. Further characterization of these mutant viruses in neuronal cultures as well as in immunodeficient mice is needed to identify the role of the RNA replication apparatus in the neuropathogenicity of this virus.
REFERENCES
- 1.Ahola, T., P. Kujala, M. Tuittila, T. Blom, P. Laakkonen, A. Hinkkanen, and P. Auvinen. 2000. Effects of palmitoylation of replicase protein nsP1 on alphavirus infection. J. Virol. 74:6725–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allsopp, T. E., and J. K. Fazakerley. 2000. Altruistic cell suicide and the specialized case of the virus-infected central nervous system. Trends Neurosci. 23:284–290. [DOI] [PubMed] [Google Scholar]
- 3.Allsopp, T. E., M. F. Scallan, A. Williams, and J. K. Fazakerley. 1998. Virus infection induces neuronal apoptosis: a comparison with trophic factor withdrawal. Cell Death Differ. 5:50–59. [DOI] [PubMed] [Google Scholar]
- 4.Amor, S., M. F. Scallan, M. M. Morris, H. Dyson, and J. K. Fazakerley. 1996. Role of immune responses in protection and pathogenesis during Semliki Forest virus encephalitis. J. Gen. Virol. 77:281–291. [DOI] [PubMed] [Google Scholar]
- 5.Atkins, G. J., J. Carter, and B. J. Sheahan. 1982. Effect of alphavirus infection on mouse embryos. Infect. Immun. 38:1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkins, G. J., B. J. Sheahan, and P. Liljeström. 1999. The molecular pathogenesis of Semliki Forest virus: a model virus made useful. J. Gen. Virol. 80:2287–2297. [DOI] [PubMed] [Google Scholar]
- 7.Balluz, I. M., G. M. Glasgow, H. M. Killen, M. F. Mabruk, B. J. Sheahan, and G. J. Atkins. 1993. Virulent and avirulent strains of Semliki Forest virus show similar cell tropism for the murine central nervous system but differ in the severity and rate of induction of cytolytic damage. Neuropathol. Appl. Neurobiol. 19:233–239. [DOI] [PubMed] [Google Scholar]
- 8.Bradish, C. J., K. Allner, and H. B. Maber. 1971. The virulence of original and derived strains of Semliki Forest virus for mice, guinea pigs and rabbits. J. Gen. Virol. 12:141–160. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly, S. M., B. J. Sheahan, and G. J. Atkins. 1997. Long-term effects of Semliki Forest virus infection in the mouse central nervous system. Neuropathol. Appl. Neurobiol. 23:235–241. [PubMed] [Google Scholar]
- 10.Fazakerley, J. K., S. Amor, and A. A. Nash. 1997. Animal model systems of MS, p. 255–273. In W. C. Russell (ed.), Molecular biology of multiple sclerosis. John Wiley & Sons, New York, N.Y.
- 11.Fazakerley, J. K., S. Pathak, M. Scallan, S. Amor, and H. Dyson. 1993. Replication of the A7(74) strain of Semliki Forest virus is restricted in neurons. Virology 195:627–637. [DOI] [PubMed] [Google Scholar]
- 12.Fazakerley, J. K., and H. E. Webb. 1987. Semliki Forest virus-induced, immune-mediated demyelination-adoptive transfer studies and viral persistence in nude mice. J. Gen. Virol. 68:377–385. [DOI] [PubMed] [Google Scholar]
- 13.Fleming, P. 1977. Age-dependent and strain-related differences of virulence of Semliki Forest virus in mice. J. Gen. Virol. 37:93–105. [DOI] [PubMed] [Google Scholar]
- 14.Garoff, H., A. Frischauf, K. Simons, H. Lehrach, and H. Delius. 1980. Nucleotide sequence of cDNA coding for Semliki Forest virus membrane glycoproteins. Nature 288:236–241. [DOI] [PubMed] [Google Scholar]
- 15.Gates, M. C., B. J. Sheahan, M. A. O’Sullivan, and G. J. Atkins. 1985. The pathogenicity of the A7, M9 and L10 strains of Semliki Forest virus for weanling mice and primary mouse brain cell cultures. J. Gen. Virol. 66:2365–2373. [DOI] [PubMed] [Google Scholar]
- 16.Glasgow, G. M., H. M. Killen, P. Liljestrom, B. J. Sheahan, and G. J. Atkins. 1994. A single amino-acid change in the E2 spike protein of a virulent strain of Semliki Forest virus attenuates pathogenicity. J. Gen. Virol. 75:663–668. [DOI] [PubMed] [Google Scholar]
- 17.Glasgow, G. M., M. M. McGee, B. J. Sheahan, and G. J. Atkins. 1997. Death mechanisms in cultured cells infected by Semliki Forest virus. J. Gen. Virol. 78:1559–1563. [DOI] [PubMed] [Google Scholar]
- 18.Gomez de Cedron, M., N. Ehsani, M. Mikkola, J. A. Garcia, and L. Kääriäinen. 1999. RNA helicase activity of Semliki Forest virus replicase protein NSP2. FEBS Lett. 448:19–22. [DOI] [PubMed] [Google Scholar]
- 19.Kujala, P., M. Rikkonen, T. Ahola, M. Kelve, M. Saarma, and L. Kääriäinen. 1997. Monoclonal antibodies specific for Semliki Forest virus replicase protein nsP2. J. Gen. Virol. 78:343–351. [DOI] [PubMed] [Google Scholar]
- 20.Merits, A., L. Vasiljeva, T. Ahola, L. Kääriäinen, and P. Auvinen. 2001. Proteolytic processing of Semliki Forest virus-specific nonstructural polyprotein by nsP2 protease. J. Gen. Virol. 82:765–773. [DOI] [PubMed] [Google Scholar]
- 21.Muller, U., U. Steinhoff, L. F. L. Reis, S. Hemmi, J. Pavlovic, R. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1924. [DOI] [PubMed] [Google Scholar]
- 22.Oliver, K. R., and J. K. Fazakerley. 1998. Transneuronal spread of Semliki Forest virus in the developing mouse olfactory system is determined by neuronal maturity. Neuroscience 82:867–877. [DOI] [PubMed] [Google Scholar]
- 23.Oliver, K. R., M. F. Scallan, H. Dyson, and J. K. Fazakerley. 1997. Susceptibility to a neurotropic virus and its changing distribution in the developing brain is a function of CNS maturity. J. Neurovirol. 3:38–48. [DOI] [PubMed] [Google Scholar]
- 24.Pathak, S., and H. E. Webb. 1974. Possible mechanisms for the transport of Semliki Forest virus into and within mouse brain: an electron microscopic study. J. Neurol. Sci. 23:175–184. [DOI] [PubMed] [Google Scholar]
- 25.Pathak, S., and H. E. Webb. 1980. The entry and the transport of arboviruses into and throughout mouse brain: an electron-microscopic study. Electron Microsc. 2:492–493. [Google Scholar]
- 26.Peränen, J., M. Rikkonen, P. Liljeström, and L. Kääriäinen. 1990. Nuclear localization of Semliki Forest virus nonstructural protein nsP2. J. Virol. 64:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pusztai, R., E. Gould, and H. Smith. 1971. Infection pattern in mice of an avirulent and virulent strain of Semliki Forest virus. Br. J. Exp. Pathol. 52:669–677. [PMC free article] [PubMed] [Google Scholar]
- 28.Rikkonen, M. 1996. Functional significance of the nuclear-targeting and NTP-binding motifs of Semliki Forest virus nonstructural protein nsP2. Virology 218:352–361. [DOI] [PubMed] [Google Scholar]
- 29.Rikkonen, M., J. Peranen, and L. Kääriäinen. 1992. Nuclear and nucleolar targeting signals of Semliki Forest virus nonstructural protein nsP2. Virology 189:462–473. [DOI] [PubMed] [Google Scholar]
- 30.Rikkonen, M., J. Peranen, and L. Kääriäinen. 1994. ATPase and GTPase activities associated with Semliki Forest virus nonstructural protein nsP2. J. Virol. 68:5804–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santagati, M. G., P. V. Itaranta, P. R. Koskimies, J. A. Määttä, A. Salmi, and A. E. Hinkkanen. 1994. Multiple repeating motifs are found in the 3′-terminal non-translated region of Semliki Forest virus A7 variant genome. J. Gen. Virol. 75:1499–1504. [DOI] [PubMed] [Google Scholar]
- 32.Santagati, M. G., J. A. Maatta, P. V. Itaranta, A. A. Salmi, and A. E. Hinkkanen. 1995. The Semliki Forest virus E2 gene as a virulence determinant. J. Gen. Virol. 75:47–52. [DOI] [PubMed] [Google Scholar]
- 33.Santagati, M. G., J. A. Määttä, M. Roytta, A. A. Salmi, and A. E. Hinkkanen. 1998. The significance of the 3′-nontranslated region and E2 amino acid mutations in the virulence of Semliki Forest virus in mice. Virology 243:66–77. [DOI] [PubMed] [Google Scholar]
- 34.Scallan, M. F., T. E. Allsopp, and J. K. Fazakerley. 1997. Bcl-2 acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J. Virol. 71:1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth, J. M., B. J. Sheahan, and G. J. Atkins. 1990. Multiplication of virulent and demyelinating Semliki Forest virus in the mouse central nervous system: consequences in BALB/c and SJL mice. J. Gen. Virol. 71:2575–2583. [DOI] [PubMed] [Google Scholar]
- 36.Soilu-Hanninen, M., J. P. Eralinna, V. Hukkanen, M. Roytta, A. A. Salmi, and R. Salonen. 1994. Semliki Forest virus infects mouse brain endothelial cells and causes blood-brain barrier damage. J. Virol. 68:6291–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subak-Sharpe, I., H. Dyson, and J. K. Fazakerley. 1993. In vivo depletion of CD8+ T cells prevents lesions of demyelination in Semliki Forest virus infection. J. Virol. 67:7629–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suopanki, J., D. L. Sawicki, S. G. Sawicki, and L. Kääriäinen. 1998. Regulation of alphavirus 26S mRNA transcription by replicase component nsP2. J. Gen. Virol. 79:309–319. [DOI] [PubMed] [Google Scholar]
- 39.Takkinen, K. 1986. Complete nucleotide-sequence of the nonstructural protein genes of Semliki Forest virus. Nucleic Acids Res. 14:5667–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarbatt, C. J., G. M. Glasgow, D. A. Mooney, B. J. Sheahan, and G. J. Atkins. 1997. Sequence analysis of the avirulent, demyelinating A7 strain of Semliki Forest virus. J. Gen. Virol. 78:1551–1557. [DOI] [PubMed] [Google Scholar]
- 41.Tuittila, M. T., M. G. Santagati, M. Roytta, J. A. Maatta, and A. E. Hinkkanen. 2000. Replicase complex genes of Semliki Forest virus confer lethal neurovirulence. J. Virol. 74:4579–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasiljeva, L., A. Merits, P. Auvinen, and L. Kääriäinen. 2000. Identification of a novel function of the alphavirus capping apparatus. RNA 5′-triphosphatase activity of nsP2. J. Biol. Chem. 275:17281–17287. [DOI] [PubMed] [Google Scholar]
- 43.Vihinen, H., T. Ahola, M. Tuittila, A. Merits, and L. Kääriäinen. 2001. Elimination of phosphorylation sites of Semliki Forest virus replicase protein nsP3. J. Biol. Chem. 276:5745–5752. [DOI] [PubMed] [Google Scholar]