Abstract
Heat shock proteins loaded with viral peptides were shown to induce a CD8+ T cell response and confer protective immunity against challenge with herpes simplex virus (HSV). The delivery system consisted of recombinant human hsp70 coupled to the peptide SSIEFARL, which is the immunodominant peptide epitope, recognized by HSV specific T cells in C57BL/6 mice. Immunization resulted in CD8+ T-cell responses, measured by peptide-specific tetramers and peptide-induced intracellular gamma interferon expression and cytotoxicity, similar to responses resulting from immunization with a recombinant vaccinia virus that expressed SSIEFARL as a minigene (VvgB) and UV-inactivated HSV. However, the durability of the hsp70-SSIEFARL response was less than that resulting from VvgB and HSV immunization and in addition the CD8+ T-cell responses in the memory phase were functionally less effective. Mice challenged soon after immunization showed excellent immunity, but by 90 days postimmunization this had waned to be significantly less than the level of immunity in both VvgB- and HSV-immunized mice.
Peptides are poor immunogens unless given with potent adjuvants that are largely unacceptable or impractical for use in humans (21). However, as observed originally by Srivastava and coworkers, peptides bound to one or more classes of molecular chaperones are often converted into potent immunogens (23, 26). Initially, those chaperone/peptide complexes were isolated from tumor tissue and shown to induce tumor-specific immunity (11). Subsequently, it became apparent that the immunoprotective chaperone complexes contained peptides, which represented the tumor-specific antigens (15, 27). Recent work has demonstrated that the gp96 and hsp70 class of chaperones can be loaded with peptides in vitro and shown to induce immunity in both tumor and virus systems (3, 5, 6, 17). This approach appears to be promising for immunity to several viruses since it elicits primarily a cytotoxic T lymphocyte (CTL) response. For example, recombinant human hsp70 (rhsp70) loaded with a peptide from the lymphocytic choriomeningitis virus (LCMV) induced both CTL and protective antiviral immunity (5). It was not clear, however, how this hsp70-peptide immunization approach compared with other traditional means of inducing antiviral immunity. In the present report, we evaluated the vaccine potential of an rhsp70 bound to SSIEFARL derived from glycoprotein B of herpes simplex virus (HSV). This peptide represents the immunodominant epitope recognized by anti-HSV CTLs in C57BL/6 mice, and accounts for >90% of the CTL response in this mouse strain (28). The magnitude of CTL induction by rhsp70-SSIEFARL was compared with other means of vaccination, including immunization with a recombinant vaccinia vector that also expressed the SSIEFARL peptide. We evaluated the response of these treatments both in the acute phase and into the memory phase. Our results indicate that hsp70-peptide induced comparable CTL responses in the acute phase and that mice were protected from systemic HSV challenge. However, memory CTL responses induced by rhsp70-peptide were 50% lower than those induced by either the virus or recombinant vaccinia virus immunization. The possible explanation for the poor memory response and the implication of such findings to future vaccine development are briefly discussed.
MATERIALS AND METHODS
Mice.
Four- to five-week-old C57BL/6 (H-2b) mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). In conducting the research described in this work, we adhered to the Guide for the Care and Use of Laboratory Animals, as proposed by the committee on care of Laboratory Animal Resources Commission on Life Sciences of the National Research Council. The facilities are fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Peptides.
HSVgB (amino acids [aa] 498 to 505) peptide SSIEFARL and chicken ovalbumin (aa 257 to 264) peptide SIINFEKL and ova265-280 peptide (TEWTSSNVMEERKIKV) were synthesized and supplied by Research Genetics, Huntsville, Ala.
Virus.
HSV-1 KOS and HSV-1 17 strains were grown on Vero cell monolayers (ATCC catalog no. CCL81), titrated, and stored in aliquots at −80°C until used. The recombinant vacccinia virus encoding the minigene SSIEFARL (VvgB) was provided by S. S. Tevethia (4).
Proteins.
rhsp70 was purchased from Stressgen (catalog no. SPP-755). It tested positive for ATPase activity and does not contain DnaK, as demonstrated by Western blot analysis.
Cell lines.
MC38, Vero (African green monkey kidney cell line), EL4 (C57BL/6, H-2b lymphoma), EMT6 (BALB/c mammary adenocarcinoma cells, H-2d) were used. All cell lines were cultured in Dulbecco modified Eagle medium (Life Technologies, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum, 100 U of penicillin G/ml, 100 γg of streptomycin sulfate/ml, and 2 mM l-glutamine.
Antibodies.
Fluorescence-tagged antibodies for fluorescence-activated cell sorting (FACS) staining (also purchased from Becton Dickinson, San Diego, Calif.) included fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labeled immunoglobulin G1 (IgG1) isotype control (catalog no. 20604A and 20605A), FITC- and PE-labeled IgG2a isotype control (20047A and 20075A), FITC- and PE-labeled IgG2b (23244A and 20075A), FITC-labeled anti-IFN-γg (18114A), CD3e (01084A), CD4 (09425A), CD8a (01045A), CD11b (01714A), CD11c (09705A), CD16/CD32 (01241A), CD25 (09985B), CD44 (01225A/01224D), CD62L (01265B), CD69 (01504A/01505B), CD95L (09071A), and CD154 (CD40L) (09025B/09022D).
hsp70 and peptide binding.
The SSIEFARL was loaded onto the hsp70 by the procedure described by Ciupiti et al. (5). In brief, the peptides were incubated with rhsp70 in binding buffer (phosphate-buffered saline with 2 mM MgCl2) at 37°C for 60 min. Then, 0.5 mM ADP (Sigma Chemical Co.) was added, and the incubation was continued for another 60 min at the same temperature. The peptide was complexed to bovine serum albumin (BSA) by glutaraldehyde conjugation.
Immunizations.
C57BL/6 mice were immunized with (i) 2.5 γg of SSIEFARL and 2.5 γg of rhsp70 or BSA, (ii) 2.5 γg of peptide mixed with 2.5 γg of rhsp70 or BSA, or (iii) with binding buffer only. The immunizations were done intraperitoneally (i.p.) on day 0 and day 21. The i.p. route was chosen after experimenting with intramuscular and footpad injections.
Peptide-specific proliferation.
The procedure is exactly as described above, except for the stimulators. The antigen-presenting cells (APCs) from naive C57BL/6 mice were pulsed with either SSIEFARL peptide or irrelevant peptide as control for CD8+ T-cell-specific stimulation. Next, 50 U of interleukin-2 (IL-2)/ml was added to the cultures. The incubation continued for 5 days, with the last 18 h carried out in the presence of [3H]thymidine.
HSV-specific lymphoproliferation.
Splenocytes from experimental mice were restimulated in vitro with X-ray-irradiated APCs that were infected with UV-inactivated HSV (multiplicity of infection before irradiation of 1.5) or uninfected and/or unpulsed APCs and then incubated for 5 days at 37°C. The responders (2 × 106) were serially double diluted, and stimulators (105) were mixed at a responder/stimulator ratio beginning at 20:1, with the addition of 50 U of recombinant IL-2 (rIL-2)/ml, and incubated for 5 days (the last 18 h with [3H]thymidine). As a positive control, concanavalin A (5 γg/ml) and anti-CD3 were added to some samples as a polyclonal stimulator and incubated for 3 days. At 18 h before harvesting the cells, [3H]thymidine was added to the cultures. The cells were harvested and read with an Inotech automatic cell harvester and reader. Proliferative responses tested in quadruplicate wells were expressed as mean counts per minute + the standard deviation.
CTL assays.
The CTL assay was performed as described earlier (13). Briefly, effector cells generated after in vitro expansion (with peptide or HSV) were analyzed for their ability to kill major histocompatibility complex (MHC)-matched antigen-presenting targets. The cells were mixed with the target at various ratios and incubated for 4 h. The targets included 51Cr-pulsed MHC-matched HSV-infected (MC38-HSV), MHC-matched SSIEFARL-pulsed (MC38-SSIEFARL), MHC-mismatched HSV-infected SSIEFARL-pulsed (EMT6-HSV and EMT6-SSIEFARL), and MHC-matched uninfected (MC38) targets. The chromium release results were computed and are expressed as lytic units as described elsewhere (13).
FACS analysis.
Cell suspensions containing 106 cells were incubated with 1 γg each of a FITC- or PE-labeled antibody. The cells were then incubated on ice for 45 min to 1 h. Cells were washed again with FACS buffer (1× phosphate-buffered saline containing 3% fetal calf serum and 0.1% sodium azide) and fixed with 2% paraformaldehyde. Cells were analyzed on Becton Dickinson FACScan by using CellQuest software.
Intracellular IFN-γg staining (ICG assay).
To enumerate the number of IFN-γg-producing cells, intracellular cytokine staining was performed as previously described (14). In brief, 106 freshly explanted splenocytes were cultured in flat-bottom 96-well plates. Cells were either left untreated, stimulated with SSIEFARL peptide (1 γg/ml), or treated with phorbol myristate acetate (10 ng/ml) and ionomycin (500 ng/ml) and then incubated for 6 h at 37°C in 5% CO2. Brefeldin A was added for the duration of the culture period to facilitate intracellular cytokine accumulation. After this period, cell surface staining was performed, followed by intracellular cytokine staining by using the Cytofix/Cytoperm Kit (PharMingen, San Diego, Calif.) in accordance with the manufacturer’s recommendations. For intracellular cytokine staining, the antibodies used were anti-IFN-γg (clone XMG1.2). All antibodies were purchased from PharMingen and analyzed with CellQuest software.
Tetramer staining and flow cytometry.
MHC class I (H-2b) tetramers to measure SSIEFARL-specific T cells were provided by S. S. Tevethia (18). A total of 106 cells obtained from these mice were stained with a mixture of FITC-labeled anti-CD8 (Caltag) and PE-labeled H-2Kb-SIINFEKL tetramers for 45 min at 4 C. The controls included isotype control, stained cells, and unstained cells. They were then analyzed by using a FACSCAN machine and CellQuest software. The percentage values seen are the double-positive cells (CD8+ and SSIEFARL-specific T-cell receptor).
Virus challenge.
A zosteriform challenge experiment was performed as described by Manickan et al. (16). In brief, the left flank area was depilated prior to challenge by a combination of hair clipping and the use of the depilatory chemical (Nair; Carter-Wallace, Inc., New York, N.Y.). The animals were anesthetized with Metofane (Pitman-Moore, Inc., Mundelein, Ill.), and 20 scarifications were made in an ∼4-mm2 area. To such scarifications, 10 γl containing 106 PFU of HSV-1 (strain 17) were added and the area was gently massaged. Animals were inspected daily for the development of zosteriform ipsilateral lesions, general behavior changes, encephalitis, and mortality. The severity of the lesions were scored as follows: 1+, vesicle formation; 2+, local erosion and ulceration of the local lesion; 3+, mild to moderate ulceration; 4+, severe ulceration, hind limb paralysis, and encephalitis; and 5+, ultimate death (mice that were moribund and hence euthanized).
Statistical analysis.
The data were analyzed by dependent-sample t test by using SPSS for Windows, release 10.1.3 (SPSS, Inc., Chicago, Ill.), and the Student t test.
RESULTS
Pattern of in vitro response to hsp70-peptide immunization.
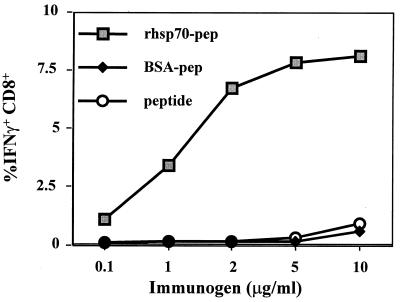
In initial experiments, groups of C57BL/6 mice were immunized i.p. on days 0 and 21 with (i) various doses of hsp conjugated to SSIEFARL peptide (molar ratio ∼75:1 [peptide/rhsp70]), (ii) peptide conjugated to BSA, or (iii) 2.5 γg of rhsp70 mixed but not conjugated to SSIEFARL peptide (75:1 molar ratio of peptide). Animals were killed 7 days after immunization, and their splenocytes were stimulated for 6 h with free peptide. Responsiveness (Fig. 1), measured by detection of intracellular IFN-γg production (ICG), revealed that only mice immunized with SSIEFARL conjugated to rhsp70 responded; optimal results were obtained with 2.5 γg of rhsp70-peptide conjugate. This dose was used for subsequent experiments.
FIG. 1.
Dose-response studies for optimization. C57BL/6 mice were immunized i.p. (after experimentation with inoculation by the intramuscular and subcutaneous routes) with various doses of hsp70-peptide preparation ranging from 0.1 to 10 γg/ml to arrive at an optimum dose to be used in the subsequent analysis of its efficacy. BSA-SSIEFARL and SSIEFARL alone were included as controls. The splenocytes were harvested from these mice and analyzed for the frequency of SSIEFARL-specific CD8+ T cells by peptide-stimulated intracellular IFN-γ assay. Based on the results shown, we chose 2.5 γg/ml as the optimum dose for administration.
In subsequent experiments, the immunogenicity of rhsp70-peptide conjugate, was compared to both mice immunized with recombinant vaccinia virus that encoded SSIEFARL as a minigene and to mice immunized with UV-inactivated HSV-1. Additional control groups received rhsp70 alone, rhsp70 plus SSIEFARL, or SSIEFARL coupled to BSA. Animals were sacrificed either 7 days after the second immunization (i.e., acute phase) or 90 days after the second immunization (i.e., memory phase), and the isolated splenocytes were evaluated for SSIEFARL and HSV specific reactivity. Four assays were used: (i) enumeration of CD8+ T-cell numbers by a SSIEFARL-specific tetramer; (ii) peptide-induced intracellular IFN-γ expression (ICG); (iii) cytotoxicity to both syngeneic peptide and HSV targets; and (iv) proliferative responses to peptide or HSV antigens. The results are shown in Table 1 and Fig. 2. It is clear that both a positive acute and a memory response to hsp70-peptide, VvgB, and HSV was detectable by all four assays. The control groups rhsp70, BSA-peptide, and peptide alone were all negative. Both the quantitative tetramer staining and ICG responses revealed that the rhsp70-peptide responses exceeded those elicited by UV-inactivated HSV during the acute phase. In fact, these assays indicated that rhsp70-peptide responses were comparable in magnitude to those induced by VvgB. Similarly, as reflected by peptide-specific proliferation and CTL responses to peptide targets, maximal responses were observed in mice immunized with rhsp70-peptide. In addition, when splenocytes were stimulated with HSV, and HSV-infected targets were used in the cytotoxicity assays, comparable responses occurred in mice immunized with either rhsp70-peptide, HSV, or VvgB.
TABLE 1.
Comparison of immunity after immunization with different preparationsa
| Immunogen | Phaseb | Frequency (%)
|
CTL (LU)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tetramer | ICG | ICG/Tet%c | Peptide | HSV | HSV/Pep%c | ||||
| rhsp70-SSIEFARL | Acute | 7.8 | 6.9 | 88 | (1) | 72 | 64 | 89 | (4) |
| Memory | 2.1 | 1.1 | 52 | 15 | 4 | 27 | |||
| UV-inactivated HSV | Acute | 6.0 | 5.8 | 97 | (2) | 53 | 50 | 94 | (5) |
| Memory | 3.2 | 2.9 | 91 | 36 | 32 | 89 | |||
| VvgB | Acute | 8.1 | 7.3 | 90 | (3) | 82 | 72 | 87 | (6) |
| Memory | 2.6 | 1.8 | 69 | 32 | 22 | 68 | |||
The mice were sacrificed on day 7 (acute) and day 90 (memory) after the second immunization and analyzed for tetramer-positive CD8 T-cells, peptide-induced IFN-γ production, and CTL activity by 51Cr release assay as described in Materials and Methods. The tetramer and ICG values are the percentage of double-positive cells (CD8+/Tet+ and CD8+/IFN-γ+, respectively). The CTL data are expressed as lytic units (LU) and show results accrued from only the UV-treated HSV, VvgB-, and rhsp70-peptide-immunized groups for SSIEFARL-pulsed and HSV-infected targets. The experiments were done with 10 individual mice in each group at a given time point. The data represent the average result. Individual values were used to compute the statistics by using SPSS software to analyze the significance by the dependent sample t test.
Acute phase, 7 days after the second immunization; memory phase, 90 days after the second immunization.
ICG/Tet%, (intracellular IFN-γ-positive cells/tetramer-positive cells) × 100; HSV/Pep%, (HSV targets/peptide targets) × 100. P values are indicated by numbers in parentheses as follows: 1, P < 0.0001 (t9 = 13.93); 2, P < 0.001 (t9 = 4.87); 3, P < 0.0001 (t9 = 10.74); 4, P < 0.001 (t9 = 17.92); 5, P < 0.242 (t9 = 1.37); and 6, P < 0.008 (t9 = 4.99).
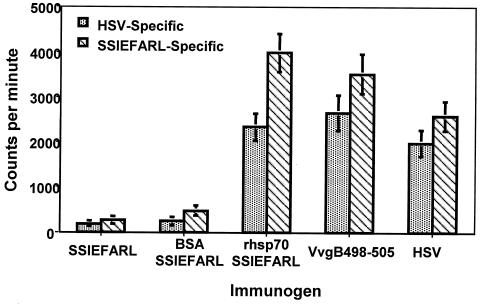
FIG. 2.
hsp70-SSIEFARL immunization induces HSVgB 498-505 (SSIEFARL)-specific CD8+ T-cell proliferative response in B6 mice. C57BL/6 mice were immunized with rhsp70-SSIEFARL, SSIEFARL, VvgB (106 PFU), HSV-1 KOS (106 PFU), or buffer alone i.p. on days 0 and 21. One week later the splenocytes were harvested, and nylon wool-nonadherent T cells were assessed for in vitro proliferative response to SSIEFARL peptide-pulsed or HSV-infected syngeneic APCs. The controls included and not shown are anti-CD3- and concanavalin A-stimulated responders, stimulators alone, and responders with irrelevant peptide-pulsed APCs.
In memory populations (Table 1), the pattern of T-cell responses to the three immunogens (rhsp70-peptide, VvgB, and HSV) showed some differences compared to responses observed in the acute phase. Accordingly, responses as determined by all assays were highest from mice immunized with UV-inactivated HSV. For example, by the tetramer assay, the memory response in HSV immunized mice was determined to be ∼53% compared to that observed in the acute phase. In contrast, with rhsp70-peptide-immunized mice, the tetramer response was only ∼25% of the acute-phase response. In the memory VvgB-immunized mice, the tetramer response was ∼33% that of the acute phase. Differences were also evident in the various groups in CTL responses to peptide- or HSV-specific targets. Animals immunized with HSV showed CTL responses (recorded as lytic units) to peptide and HSV targets that were roughly equal. In contrast, HSV-specific responses of rhsp70-peptide-immunized animals were only 33% of the peptide-specific responses. This demonstrates that the response to HSV targets was less effective in the hsp70-peptide-immunized group than that measured in HSV-immunized animals.
The pattern of responsiveness to the VvgB immunogen was more akin to the pattern in HSV-immunized animals, with the lytic unit response to HSV being 68% of the peptide response. Finally, a comparison of the response to peptide, measured by both tetramer staining and the ICG assay, revealed that the magnitude of the CD8+ T-cell reaction was approximately equal in the HSV-immunized group (ICG assay result = 94% of the tetramer response). However, in the rhsp70-peptide-immunized group, memory responses measured by the ICG assay were only 52% of those measured by the tetramers. Collectively, these results serve to indicate that the memory responses to the rhsp70-peptide complexes was both quantitatively and functionally weaker than the responses to either HSV or VvgB immunization.
Immunity to challenge.
Mice immunized with the various preparations described previously were challenged with five 50% lethal doses (LD50s) of HSV-1 strain-17 both at the acute phase (7 days postimmunization) and during the memory phase (90 days postimmunization). At both stages the mice were scored for zosteriform lesions (ZL) and for signs of encephalitis. Table 2 presents the cumulative results of two separate challenge experiments. Clearly, mice immunized with hsp70-SSIEFARL and challenged in the acute phase all survived infection. Many animals, in contrast to those immunized with HSV, developed skin lesions (ZL average of 2.6 at day 10), but none succumbed to encephalitis. Similar results were observed with the mice immunized with VvgB (ZL average of 2.5 at day 10), with one animal dying of herpes encephalitis. The control mice groups given peptide-coupled BSA, peptide alone, or hsp alone all succumbed to challenge.
TABLE 2.
Zosteriform challenge of mice immunized with SSIEFARL loaded chaperones
| Immunogen | Challenge (HSV-1 = 5 LD50)a at:
|
|||||
|---|---|---|---|---|---|---|
| Acute phase
|
Memory phase
|
|||||
| Mortalityb | Severityc | DDd | Mortalityd | Severityd | DD | |
| SSIEFARL | 100 | 5 | 8 | ND | ||
| rhsp70-peptide | 0 | 2.6 | 80 | 4.5f | 12 | |
| VvgB | 2.5 | 2.5 | 15 | 53 | 3.1 | 14 |
| UV-inactivated HSV | 0 | <1 | 0 | 1.5 | ||
| rshp70 | 100 | 5 | 9 | ND | ||
| BSA-peptide | 100 | 5 | ND | |||
Intracutaneous challenge with 5 LD50s of HSV-1 strain-17. Acute phase, 7 days postimmunization; memory phase, 90 days postimmunization. DD, average day of death.
Average of two separate experiments involving 12 mice per group.
ZL severity as measured at day 10 postchallenge.
Average of two experiments involving 20 mice per group. ND, not done.
ZL severity as measured at day 15 postchallenge.
P < 0.001.
A different pattern of results was evident when mice were challenged at 90 days after the second immunization. Thus, mice immunized with the rhsp70-peptide were more susceptible to challenge (80% of animals dying of encephalitis) than at the acute phase. In contrast, HSV-immunized mice resisted challenge, with only a few animals showing minor lesions. In the VvgB-immunized mice, 55% of the animals survived the challenge, but all animals showed lesions. These lesions were significantly (P < 0.001) less severe than those observed in the rhsp70-peptide-immunized animals.
DISCUSSION
The present results demonstrate that immunization with a molecular chaperone loaded with an immunodominant viral peptide induces CD8+ T-cell immunity and protection from systemic viral challenge. Specifically, we confirm the ability of a recombinant human hsp70 loaded in vitro with SSIEFARL (aa 498 to 505) from the glycoprotein B of HSV to produce a potent acute effect, followed by a weak memory response.
In the acute phase, CD8+ T-cell responses were evaluated by four separate assays and found to be either comparable or elevated beyond those induced by immunization with either HSV alone or with a recombinant vaccinia virus that expressed SSIEFARL as a minigene. However, 3 months after immunization the CD8+ T-cell immunity was still detectable in the rhsp70-peptide-immunized mice, whereas activity levels had diminished to ∼50% of those present in the HSV-immunized animals. Moreover, rhsp70-peptide immune animals remained more susceptible to systemic challenge than the HSV- or VvgB-immunized mice and yet still displayed significant protection compared to control animals. The reduction in immunity in the memory phase, compared to animals immunized with virus or VvgB, appeared to be associated both with a diminished quantitative and functional CD8+ T-cell response. Thus, CD8+ T cells measured by tetramers fell fourfold in rhsp70-peptide immune animals between the acute and memory phase. In addition, CD8+ T cells were less functional, as measured both by the percentage of tetramer positive cells that scored positive by the ICG assay and by the ability of CTLs to kill both HSV- and peptide-sensitized target cells. Our results indicate that rhsp70-peptide represents a useful vaccination strategy, but optimal efficacy may require the optimization of dosage, timing, and choice of epitopes to increase the magnitude and function of the memory CD8+ T-cell response.
Several observations have documented that the feeble immunogenicity of CD8 peptide epitopes can be overcome by the use of appropriate carrier systems and adjuvants (9, 12, 19). The ability of chaperones to possibly function in both capacities is highly attractive, since these conserved proteins have minimal immunogenicity and no apparent toxicity (1, 2, 25). Unexpectedly however, chaperones may act as potent adjuvants when linked either covalently or noncovalently to peptides (10, 20, 24). Initial observations of this effect came from the tumor field, where complexes of stress proteins and tumor peptide antigens were isolated from tumors and shown to be excellent immunogens (11). Subsequently, complexes between various chaperone proteins and peptide prepared in vitro were shown to induce CD8+ T-cell immunity (3). One report documents that the normally intracellular stress protein hsp70 bound to an epitope peptide of LCMV induced CD8+ T-cell immunity and immunoprotection (5). However, this report failed to compare the immunogenicity of the hsp70-peptide with other forms of vaccination, nor did it study the duration of immunity. The findings in this study corroborates many of the results above and also demonstrates for the first time their efficacy in comparison to other forms of vaccination and evaluation of the duration. Our results confirm that immunization with rhsp70 linked noncovalently to an immunodominant CD8 peptide epitope provides an excellent immunogen and confers immunity to viral challenge. Indeed, the rhsp70-peptide approach induced a potent CD8+ T-cell response equivalent to that engendered by exposure to live or UV-inactivated virus. Such observations applied, however, only for the acute phase soon after immunization. According, the durability of immunity induced by rhsp70-peptide was less than that observed with either HSV or VvgB immunization.
This diminished resistance of rhsp70-peptide-immunized mice appeared to have both a quantitative and a functional explanation in terms of the CD8+ T-cell response. Accordingly, when analyzed 3 months postimmunization, the numbers of CD8+ T cells measured by peptide specific tetramers had fallen more notably in rhsp70-peptide-immunized animals than had occurred in animals immunized with HSV. Perhaps of greater consequence, however, the CD8+ T-cell reactivity that did remain showed functional shortcomings. Thus, when we compared the ability of peptide binding cell to produce IFN-γ upon stimulation, a much lower percentage of cells from rhsp70-peptide-immunized animals scored positive than was evident in HSV-immunized mice.
Indeed, the rhsp70-peptide memory population shows functional defects similar to those of the so-called “Sisyphean cell” described by the Ahmed group that occurred in instances in which T-cell help was lacking in mice exposed to LCMV (29). Our group has also observed functionally defective HSV peptide-specific cells in LTαa mice, which lack normal lymphoid function (13). The appearance of such functionally defective cells may reflect the absence of adequate helper cell stimulation by rhsp70-peptide complexes during the immunization process. Conceivably, such helper cell deficiencies could be overcome by coimmunization with helper cell-inducing epitopes by providing help by other means of costimulation or facilitating interaction between responder T cells and cells which present the rhsp70-peptide antigen. Such experiments are currently under way in our laboratory.
An additional explanation for the observation that rhsp70-peptide-immunized mice were less protected in the memory phase than occurred with the other immunogens tested could be that memory CTLs from rhsp70-peptide-immune mice show less avidity. Accordingly, in contrast to CD8+ T cells from HSV-immunized mice, cells from rhsp70-peptide-immunized animals were less able to lyse HSV-infected targets than they were on peptide-sensitized targets. Such a situation has been described as low avidity (8, 22). It has also been noted to occur when CTL precursors were stimulated in vitro with high doses of peptide. Such low-avidity cells were less immunoprotective in adoptive transfer experiments against virus infection (7). Since a major requirement for any vaccine is that it should induce potent long-term memory responses, it will be important to find methods to reinforce the memory responses to rhsp70-peptide immunization. Several approaches are currently being tested in an attempt to improve the memory CTL responses induced by rhsp70-peptide immunization.
Acknowledgments
we thank Satvir S. Tevethia, Pennsylvania State University College of Medicine, Hershey, for providing the recombinant vaccinia virus strain encoding the SSIEFARL and SSIEFARL tetramers. We also thank Mike Newman, DII’s Statistical Consulting Center, University of Tennessee, Knoxville, for help with the SPSS analysis.
This work was supported by National Institutes of Health grants AI 14981 and AI46462.
REFERENCES
- 1.Asea, A., E. Kabingu, M. A. Stevenson, and S. K. Calderwood. 2000. Hsp70 peptide-bearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones 5:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asea, A., S. K. Kraeft, E. A. Kurt-Jones, M. A. Stevenson, L. B. Chen, R. W. Finberg, G. C. Koo, and S. K. Calderwood. 2000. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 6:435–442. [DOI] [PubMed] [Google Scholar]
- 3.Blachere, Z. N. E. Li, R. Y. Chandawarkar, R. Suto, N. S. Jaikaria, S. Basu, H. Udono, and P. K. Srivastava. 1997. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J. Exp. Med. 186:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaney, J. E., Jr., E. Nobusawa, M. A. Brehm, R. H. Bonneau, L. M. Mylin, T. M. Fu, Y. Kawaoka, and S. S. Tevethia. 1998. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72:9567–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciupitu, A. M., M. Petersson, C. L. O’Donnell, K. Williams, S. Jindal, R. Kiessling, and R. M. Welsh. 1998. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J. Exp. Med. 87:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellino, F., P. E. Boucher, K. Eichelberg, M. Mayhew, J. E. Rothman, A. N. Houghton, and R. N. Germain. 2000. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J. Exp. Med. 91:1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derby, M., M. Alexander-Miller, R. Tse, and J. Berzofsky. 2001. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J. Immunol. 166:1690–1697. [DOI] [PubMed] [Google Scholar]
- 8.Derby, M. A., J. Wang, D. H. Margulies, and J. A. Berzofsky. 2001. Two intermediate-avidity cytotoxic T lymphocyte clones with a disparity between functional avidity and MHC tetramer staining. Int. Immunol. 13:817–824. [DOI] [PubMed] [Google Scholar]
- 9.Dyall, R., L. V. Vasovic, A. Molano, and J. Nikolic-Zugic. 1995. CD4-independent in vivo priming of murine CTL by optimal MHC class I-restricted peptides derived from intracellular pathogens. Int. Immunol. 7:1205–1212. [DOI] [PubMed] [Google Scholar]
- 10.Huang, Q., J. F. Richmond, K. Suzue, H. N. Eisen, and R. A. Young. 2000. In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat shock protein 70 fusion proteins maps to a discrete domain and is CD4+ T cell independent. J. Exp. Med. 191:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janetzki, S., N. E. Blachere, and P. K. Srivastava. 1998. Generation of tumor-specific cytotoxic T lymphocytes and memory T cells by immunization with tumor-derived heat shock protein gp96. J. Immunother. 21:269–276. [DOI] [PubMed] [Google Scholar]
- 12.Jennings, R., J. R. Simms, and A. W. Heath. 1998. Adjuvants and delivery systems for viral vaccines—mechanisms and potential. Dev. Biol. Stand. 2:19–28. [PubMed] [Google Scholar]
- 13.Kumaraguru, U., I. A. Davis, S. Deshpande, S. S. Tevethia, and B. T. Rouse. 2001. Lymphotoxin alpha−/− mice develop functionally impaired CD8+ T cell responses and fail to contain virus infection of the central nervous system. J. Immunol. 66:1066–1074. [DOI] [PubMed] [Google Scholar]
- 14.Kumaraguru, U., and B. T. Rouse. 2000. Application of the intracellular gamma interferon assay to recalculate the potency of CD8+ T-cell responses to herpes simplex virus. J. Virol. 74:5709–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Z., and P. K. Srivastava. 1993. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 12:3143–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manickan, E., and B. T. Rouse. 995. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse-models. J. Virol. 9:8178–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moroi, Y., M. Mayhew, J. Trcka, M. H. Hoe, Y. Takechi, F. U. Hartl, J. E. Rothman, and A. N. Houghton. 2000. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc. Natl. Acad. Sci. USA 97:3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mylin, L. M., T. D. Schell, D. Roberts, M. Epler, A. Boesteanu, E. J. Collins, J. A. Frelinger, S. Joyce, and S. S. Tevethia. 2000. Quantitation of CD8+ T-lymphocyte responses to multiple epitopes from simian virus 40 (SV40) large T antigen in C57BL/6 mice immunized with SV40, SV40 T-antigen-transformed cells, or vaccinia virus recombinants expressing full-length T antigen or epitope minigenes. J. Virol. 74:6922–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovich, N. R., P. McInnes, D. L. Klein, and B. F. Hall. 1994. Vaccine technologies: view to the future. Science 265:1401–1404. [DOI] [PubMed] [Google Scholar]
- 20.Roman, E., and C. Moreno. 1996. Synthetic peptides non-covalently bound to bacterial hsp70 elicit peptide-specific T-cell responses in vivo. Immunology 88:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh, M., and D. O’Hagan. 1999. Advances in vaccine adjuvants. Nat. Biotechnol. 17:1075–1081. [DOI] [PubMed] [Google Scholar]
- 22.Speiser, D. E., D. Kyburz, U. Stubi, H. Hengartner, and R. M. Zinkernagel. 1992. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J. Immunol. 49:972–980. [PubMed] [Google Scholar]
- 23.Suto, R., and P. K. Srivastava. 1995. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science 269:1585–1588. [DOI] [PubMed] [Google Scholar]
- 24.Suzue, K., and R. A. Young. 1996. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J. Immunol. 56:873–879. [PubMed] [Google Scholar]
- 25.Suzue, K., X. Zhou, H. N. Eisen, and R. A. Young. 1997. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc. Natl. Acad. Sci. USA 94:13146–13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udono, H., and P. K. Srivastava. 1993. Heat shock protein 70-associated peptides elicit specific cancer immunity. J. Exp. Med. 178:1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Udono, H., D. L. Levey, and P. K. Srivastava. 1994. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc. Natl. Acad. Sci. USA 91:3077–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace, M. E., R. Keating, W. R. Heath, and F. R. Carbone. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 3:7619–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]