Abstract
Introduction:
Radiologic imaging is routinely used to evaluate women with spontaneous nipple discharge (SND), but definitive diagnosis is usually only achieved by surgical terminal duct excision (TDE). Ductoscopy has been reported to result in improved localization of intraductal lesions and may avoid surgery in women with endoscopically normal ducts.
Materials and Methods:
We conducted a retrospective review of the records of 117 consecutive women who underwent ductoscopy to guide ductal excision (scope-DE) or received conventional TDE without ductoscopy. Two women had atypical ductal lavage cytology and the remainder presented with SND from 1 or more duct. Preoperative evaluation included radiologic imaging as clinically indicated.
Results:
Fifty-nine women underwent scope-DE, and 58 underwent conventional TDE. There were no significant differences in age, race, discharge characteristics, or radiologic findings. The proportion of women with intraductal neoplasia was slightly greater in the group undergoing scope-DE (88% vs. 81%, P = 0.2). In the conventional TDE group, 8.5% were found to have atypical hyperplasia or duct carcinoma in situ compared with 18.6% in the scope-DE group. In the ductoscopy group, 22 of 59 (37.3%) had lesions >5 cm from the nipple, compared with 1 of 17 women for whom distance of the lesion from the nipple was known in the conventional group (P = 0.02). Of the ductoscopy-detected cancers, 5 of 6 had no symptoms other than SND, whereas 1 of the 4 malignancies in the conventional group presented as SND alone.
Discussions:
Ductoscopy identifies intraductal lesions in a high proportion of women with SND, and it may contribute to more accurate resection of these. A prospective study is required to obtain an unbiased assessment of these possible advantages.
Ductoscopy (mammary endoscopy) is a technique of direct examination of the ductal lumen, which can be used to localize intraductal neoplasia in women with spontaneous nipple discharge. The yield of pathologic proliferative lesions in surgical specimens obtained by this technique is slightly, but not significantly, higher (88% versus 81%) than in specimens obtained using the conventional surgical approach.
Nipple discharge is responsible for approximately 5% of surgical referrals annually.1 Not all forms of spontaneous nipple discharge (SND) are associated with significant pathologic findings. The clinical features of SND that are associated with a high likelihood of intraductal neoplasia include unilaterality, persistence, emanation from a single duct, and watery, serous, or bloody appearance.2,3 Discharges with these characteristics are classified as pathologic and have traditionally been considered an indication for surgical excision of the involved duct. However, among women with SND who are evaluated surgically, 2% to 25% are diagnosed with either invasive or noninvasive cancer.2–5
When mammography shows significant findings in women with SND, the likelihood of malignancy increases,2 but mammographic imaging is often unhelpful. Galactography has been used to evaluate women with SND with variable success.6,7 When SND is caused by peripheral intraductal lesions, galactography provides localizing information and can also assess the likelihood of malignancy,4 although definitive diagnosis requires central or terminal duct excision (TDE). Duct excision is also therapeutic unless malignancy is discovered. Conventionally, this procedure has been performed blindly, guided by a lacrimal probe or blue dye instillation into the duct or by preoperative ductography and wire localization if the lesion is located more than 2 to 3 cm from the ductal orifice.2,8
Mammary endoscopy (ductoscopy) is a recently introduced technique, which may allow more precise identification and delineation of intraductal disease but is not currently a standard practice among most surgeons. Ductoscopy has been reported to result in improved localization of intraductal lesions9–11 and may avoid surgery in women with endoscopically normal ducts. However, ductoscopy adds to time and expense in the operating room, and the yield of significant pathologic lesions reported in separate series of women who are managed with and without ductoscopy at different institutions is reported to be in the range of 90%.2,9 There has not been a direct comparison of the results achieved with these 2 different approaches in terms of lesion yield or amount of tissue excised. We undertook a comparison of findings in women with SND who were managed with and without ductoscopy at the Lynn Sage Breast Program to assess the value of adding ductoscopy to the management of these patients.
METHODS
We conducted a retrospective review of the records of 117 consecutive patients who presented to the Lynn Sage Comprehensive Breast Center between January 1996 and December 2003 and underwent evaluation and treatment of SND, judged to be suggestive of intraductal neoplasia by clinical characteristics. These included dominance of a single duct; spontaneity; and clear, serous, or bloody fluid. Mammographic findings considered abnormal were clustered calcifications, densities that persisted with spot compressions, and dilated ducts. Ultrasound findings relevant to evaluation of nipple discharge were intraductal nodules and dilated ducts. For those patients who had successfully completed ductograms, significant findings included filling defects and tapered or narrowed ducts. Radiologists at the Lynn Sage Comprehensive Breast Center reviewed studies performed at outside institutions. In this review, we also included 2 women who underwent ductal lavage for risk evaluation and were found to have mildly or markedly atypical cytologic findings, because their ductal excisions were performed under ductoscopy guidance.
Both conventional TDE and ductoscopy-guided excision was performed in the operating room, under local anesthesia, with sedation. For 15 women in the conventional surgery group, the TDE was preceded by a ductogram on the morning of surgery, and a combination of blue dye and radiographic contrast was instilled into the duct. A periareolar incision was centered on the projection of the discharging duct, or the lacrimal probe when one was used, and an areolar skin flap was raised. The discharging duct was identified because it was dilated, or by the palpation of a lacrimal probe or visualization of blue dye. Retroareolar ducts were excised with some underlying breast tissue. In the remaining 43 women, TDE was performed using a periareolar incision centered on the discharging duct. The pathologic duct was identified because the duct was dilated. The ducts were transected at the epidermal surface of the nipple and followed distally in the breast for a distance of 2 to 3 cm and the absence of discharge from the residual duct confirmed at the time of distal transection. If a single discharging duct was not identified, the entire central cone of ductal tissue was excised. For the ductoscopy procedure, the discharging duct was identified and cannulated with a series of graded lacrimal probes, the 0.9-mm Acueity endoscope (Larkspur, CA) was introduced and advanced under direct vision, and all tiers of branches examined until the endoscope could not be advanced further or an obstructing lesion was identified. We attempted to negotiate the endoscope around intraductal lesions whenever possible. If no lesion was identified, the scope was withdrawn and the procedure terminated. However, if a lesion was found, the extent of disease was marked out on the skin by transillumination at the most proximal and most distal lesions, and the axial extent was marked (eg, from 10 o'clock to 12 o'clock) when disease was present in multiple peripheral branches of the same ductal tree. The outer cannula of the ductoscope was left in place, and resection of the diseased duct was performed. In some instances, the incision used was peripheral and radial, and in some, it was circumareolar, depending on the location and extent of the disease.
Until April 2001, all patients underwent conventional excision without ductoscopy, with preoperative evaluation and the precise surgical approach depending on surgeon preference. After ductoscopy became available at Northwestern Memorial Hospital, the majority treated by 1 surgeon (SAK) was offered ductoscopy-guided excision, whereas others were offered ductoscopy as thought to be appropriate by the treating surgeon. Two women did not have SND, but had undergone ductal lavage in association with their high-risk status and had marked cytologic atypia. The records of all women were reviewed for age, race, fluid characteristics, imaging studies, ductoscopy findings, histologic findings, presence or absence of cytologic atypia, and complications.
Statistical comparison between groups was performed using contingency tables and the Fisher exact test, and logistic regression to derive odds ratios.
RESULTS
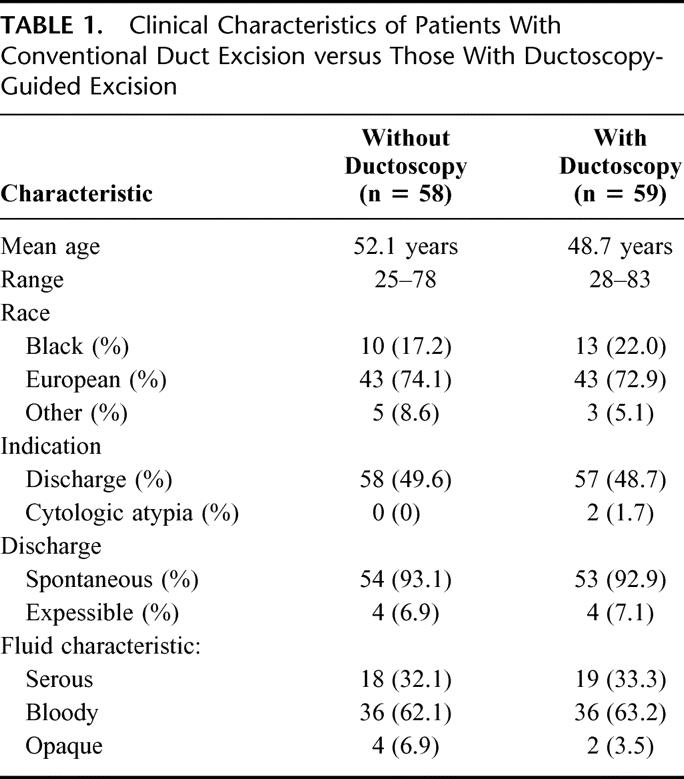
One hundred seventeen breasts from 114 women were evaluated for nipple discharge or abnormal ductal lavage cytology over the 8-year period between January 1996 and December 2003. Because the number of bilateral procedures was small, the data are not presented separately by woman. The patients’ ages ranged from 25 to 83 with a mean of 50.5 years. The study population was divided almost evenly between those who had surgery alone (58) and those with ductoscopic guidance (59). Demographic and clinical characteristics of the 2 groups are presented in Table 1. Ductoscopy was attempted in 68 breasts and was unsuccessful in 9 cases, either related to duct perforation (8 breasts, 11.8%) or duct stricture (1 breast, 1.4%). These women proceeded to duct excision without endoscopic guidance at the same procedure and have been included in the conventional surgery group for purposes of analysis, although their results are also presented separately. Of the 59 breasts that were endoscoped, 49 (83%) had 1 duct examined and 10 (17%) had more than 1 duct examined, usually 2.
TABLE 1. Clinical Characteristics of Patients With Conventional Duct Excision versus Those With Ductoscopy-Guided Excision
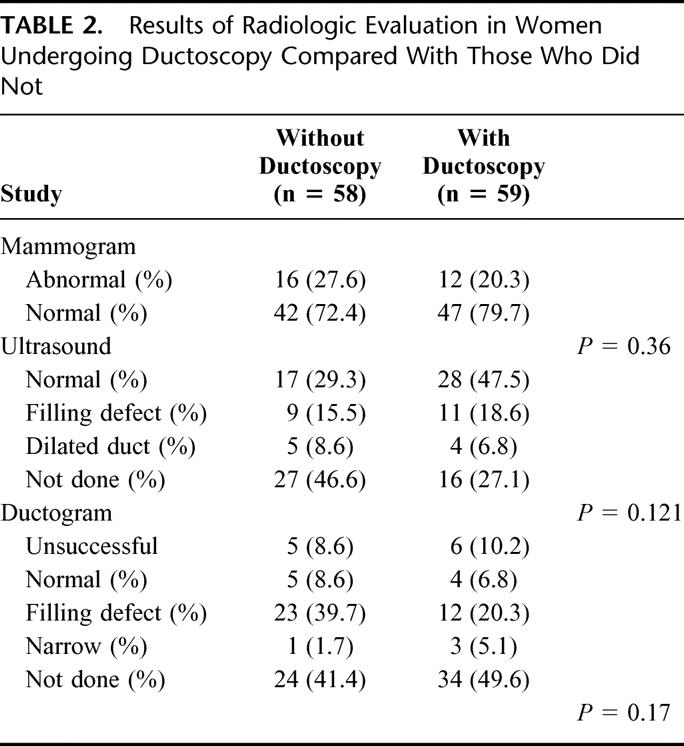
Results of radiographic evaluation are shown in Table 2 and demonstrate no major differences between the groups. Ultrasound was performed somewhat more frequently in the ductoscopy group, but the distribution of abnormal ultrasound findings is not different between the groups. Ductograms were successfully completed in 47 of 58 attempts (81%). Results were normal in 9 (19.1%), filling defects were found in 35 (74.5%), and dilated ducts in 4 (8.5%).
TABLE 2. Results of Radiologic Evaluation in Women Undergoing Ductoscopy Compared With Those Who Did Not
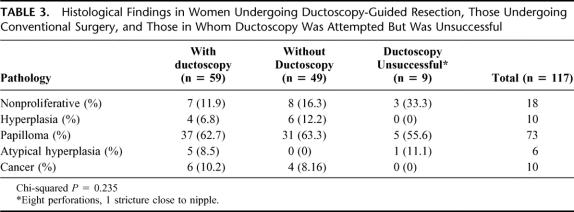
Histologic findings are summarized in Table 3. Overall, nonproliferative benign change was found in 18 women (15.4%) and hyperplasia of the usual type (HUT) in 10 (8.6%). The most common intraductal neoplastic finding was papilloma in 73 patients (62.4%). Atypical hyperplasia was present in 6 specimens (5.1%), and duct carcinoma in situ (DCIS) was found in 10 specimens (8.6%). The yield of pathologic findings explaining the discharge was not significantly different between patients undergoing conventional or endoscopically guided resection, although overall yield was slightly higher in the ductoscopy group. This was true if hyperplasia without atypia was included as an explanation for the discharge (88.1% in the ductoscopy group vs. 81% in the conventional surgery group, 2-sided Fisher exact test, P = 0.2) and also if only findings of papilloma, atypia hyperplasia, or ductal carcinoma in situ were considered (81.3% in the ductoscopy group versus 70.7% in the conventional surgery group, 2-sided Fisher exact test, P = 0.3).
TABLE 3. Histological Findings in Women Undergoing Ductoscopy-Guided Resection, Those Undergoing Conventional Surgery, and Those in Whom Ductoscopy Was Attempted But Was Unsuccessful
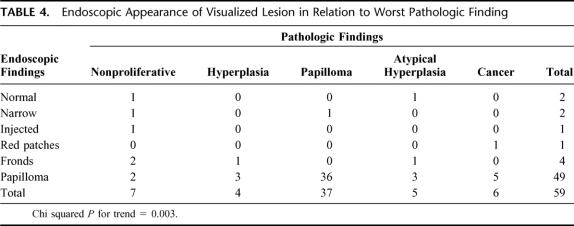
The endoscopic findings correlated well with the pathologic diagnoses (see Table 4); a papilloma or more severe lesion was found in 44 of 49 (90%) ducts where an intraductal neoplasm was seen on ductoscopy. Examples of endoscopic findings are shown in Figure 1 and of cytology–histology correlation in Figure 2. In 1 patient, a papillomatous lesion was visualized on endoscopy, but was not recovered from the pathology specimen. This lesion was within 1 cm of the nipple duct orifice, and the duct was transected on the undersurface of the nipple skin, so it is possible that this lesion prolapsed from the duct during pathologic processing and was lost. A second papilloma identified during ductoscopy was not recovered in the pathologic examination, although the resection extended beyond the point where it was visualized. Nevertheless, there was a significant correlation between endoscopic and pathologic findings (2-tailed Fisher exact test, P = 0.015). Of the 49 papillomatous lesions visualized, 36 (73.5%) were indeed papilloma, 5 (10.2%) were cancer, 3 (6.1%) were atypical hyperplasia, and 3 were HUT. Pathologically there were 37 papillomata diagnosed, and 36 (97.3%) of these were correctly identified at the time of ductoscopic evaluation. In the single papilloma that was not identified a duct stricture was present, which could not be passed with the endoscope. The resection was carried beyond the stricture, and the papilloma was recovered in the specimen on pathologic examination. Of the 5 atypical hyperplasias, 3 were thought to be papilloma at the time of ductoscopy, whereas 1 appeared as frond-like material. The final one, which consisted of atypical lobular hyperplasia, showed no intraductal abnormality and was probably an incidental finding. Five of the 6 DCIS lesions that were identified endoscopically were visualized as papillomatous lesions. The remaining cancer was a DCIS, which appeared as red patches and an irregular wall endoscopically, and yielded malignant cytology. We observed a significant correlation between endoscopically visualized disease and the presence of intraductal neoplasia. The total number of lesions listed in Table 4 is the same as the number scoped, because patients having more than 1 type of finding on endoscopy had only the most suspicious lesion listed. For instance, if a woman had red patches and intraductal growth consistent with papilloma, papilloma was listed as the finding.
TABLE 4. Endoscopic Appearance of Visualized Lesion in Relation to Worst Pathologic Finding
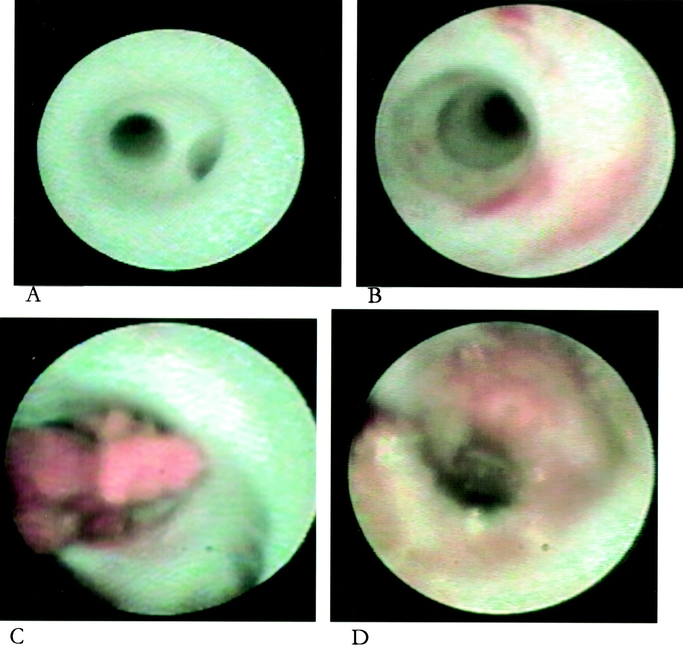
FIGURE 1. Endoscopic appearance of A, normal duct; B, flat red patches of duct hyperplasia; C, intraductal papilloma; and D, duct carcinoma in situ with microscopic invasion.
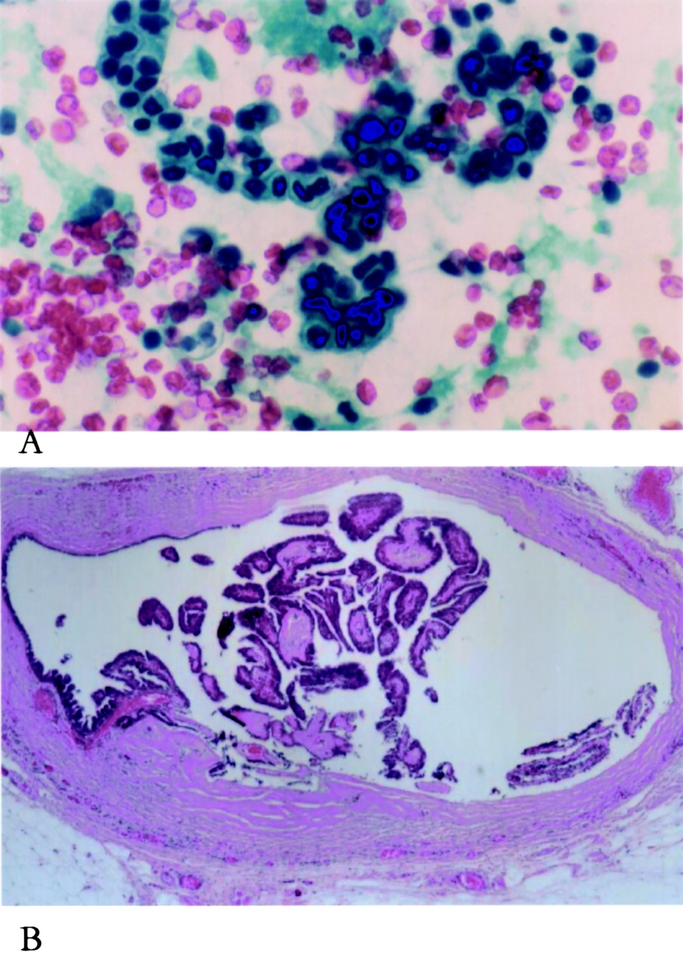
FIGURE 2. Cytologic appearance of ductoscopy washing material with papillary structure (A) and matching histologic appearance of surgically excised lesion (B).
The visualization of neoplastic lesions (papilloma or more severe) was not as good with imaging studies as with ductoscopy; of 9 women with a normal ductogram, 7 (78%) were found to have a papilloma or more severe lesion pathologically, whereas of 35 women with a filling defect on ductography, 28 (80%) had a corresponding lesion seen pathologically (either papilloma or more severe) (P = 0.93). There were 11 women with papilloma or more severe lesion who did not undergo either ductography or ultrasound. There were 26 women who underwent both ductography and ductoscopy. Of these, 20 had papillomata visualized on ductoscopy, but only 10 (50%) were seen on ductogram. In the 10 cases in which papillomata were seen endoscopically but not on ductography (7 normal and 3 unsuccessful ductograms), pathologic findings of papilloma in 8 and DCIS in 2 were demonstrated.
The odds of finding a proliferative lesion were significantly higher if a lesion was seen on ductoscopy (odds ratio [OR], 1.8; 95% confidence interval [CI], 1.2–2.8; P = 0.009). The maximal distance of visualized lesions from the nipple on ductoscopy was 10 cm (mean distance 4 cm), whereas on ductography, it was 5 cm (mean distance 2 cm, 2-tailed P = 0.0001). Among the ductoscopy group, in 22 (37%) women, the most centrally located abnormality was 5 cm or more from the nipple, and 6 of the 11 severe lesions (AH or DCIS) were included in this group. The number of lesions visualized was greater on ductoscopy than ductography. The mean number of lesions seen on ductography was 1, where as on ductoscopy, it was 1.7 (2-tailed P = 0.002). The odds of finding a pathologic diagnosis of hyperplasia, or a more severe lesion, were significantly increased with increasing number of lesions visualized (OR, 8.1; 95% CI, 2–33; P = 0.003).
Ten women in this study had cancer; all were DCIS lesions, although 1 woman had a 2-mm focus of invasion. Four cancers were found in the conventional surgery group and 6 in the ductoscopy group. Three of the cancers diagnosed in women undergoing conventional excision presented with diffuse mammographic calcifications (2) or a large palpable mass (1) in addition to SND. Of the 6 women who had intraoperative ductoscopy, 5 (83.3%) had SND as the only presenting symptom and did not have corresponding radiologic or physical findings.
DISCUSSION
Nipple discharge is the presenting breast complaint for less than 10% of women at any age presenting to breast practices.8,12 Of those who have discharge, 35% to 65% are considered clinically benign or physiological and do not require surgical intervention.2,3,8 Surgical evaluation of patients with discharge suggestive of intraductal neoplasia renders a diagnosis of cancer in only 2% to 25% of cases. Ductoscopy has emerged recently as a method of identifying intraductal neoplasia, and intraductal diagnostic biopsy techniques are evolving. There have been several published reports regarding the ability to visualize papillomata and other changes, but there has been no attempt to compare the yield of ductoscopy-guided resection with that of conventional approaches using ductography, blue dye, or probe-guided resection for the diagnosis of intraductal lesions that cause SND. We have undertaken such an analysis, because adding ductoscopy to the evaluation of these patients results in added cost in terms of equipment, supplies, and operating room time. We report here the first analysis comparing results based on treatment with and without the use of ductoscopy. We find that the range of pathologic diagnoses for both groups is similar, although there is a nonsignificant trend toward a higher yield of ductal neoplasia with the use of ductoscopy compared with women who had conventional surgery (overall yield 88% vs. 81%).
The spectrum of lesions in our population and their frequency is within that reported in the literature and in particular is very similar to that reported in a series of women with SND who were evaluated with ductoscopy, where the reported yield was also 88%.13 Our ductoscopy success rate is also similar; although we encountered more perforations that strictures as a cause of unsuccessful ductoscopy than Dietz et al,9 the overall ductoscopy failure rate was similar. These findings are in accordance with pathologic findings associated with SND that have been reported in the literature. However, we believe ductoscopy is a useful adjunct to the evaluation of SND because we were able to identify multiple papillomata significantly more frequently (mean 1.7 with ductoscopy vs. 1.0 with conventional surgery); we identified peripheral disease in a higher proportion of women, and we found a higher proportion of atypical ductal hyperplasia with ductoscopy. We see a trend toward the identification of more severe intraductal disease, which may merit further therapy (atypical hyperplasia or DCIS) with ductoscopy than with conventional surgery. In the ductoscopy group, 11 of 59 (18.6%) women were found to have these more severe lesions. In the conventional surgery group, there were 5 of 58 such women (8.6%). Thus, lesions that have a larger impact on breast cancer risk (peripheral papillomata, multiple papillomata, and atypical hyperplasia) were found more often with ductoscopy-guided excision than with conventional surgery.
Also of note, the cancers detected in the ductoscopy group presented as SND alone in 5 of the 6 women, whereas 3 of the 4 cancers in the conventional surgery group also had other symptoms. Although this is a retrospective analysis of a single institution experience, the 2 groups were well balanced in terms of race, age, indication for investigation, and fluid characteristics, and results of radiographic evaluation. All patients in this study had mammograms, and most were normal, in keeping with the finding that mammography is insensitive in the detection of lesions associated with nipple discharge.4 Ductography was performed in 59 women, 26 of whom also had ductoscopy. Among these 26 women, 20 were found to have papillomata on ductoscopy, but filling defects were seen on ductography in half that number. The relative insensitivity of ductography for the detection of intraductal neoplasia has been reported previously,6,8 although in studies in which a low threshold has been used for defining abnormal ductography, the sensitivity has been high but the specificity low.2 Cabioglu et al found that 80 of 84 patients with SND had abnormal galactograms, but benign and malignant abnormalities were indistinguishable on galactography. They also concluded that mammographic and sonographic abnormalities were more prevalent in patients with cancerous lesions than in those with benign histology. Our findings are similar in the conventional surgery group, where 3 of 4 cancers detected had other abnormalities such as masses or suspicious calcifications, but not in the ductoscopy-guided group, in which 5 of 6 cancers detected had no associated imaging or palpable abnormalities.
The correlation between endoscopic and pathologic findings is statistically significant (P = 0.003), showing that a luminal growth identified at the time of ductoscopy correlated well with having a proliferative pathologic diagnosis. However, it is not clear from this experience that reliable distinction between benign and malignant lesions is possible based on the endoscopic appearance. Of lesions labeled papilloma at the time of ductoscopy, 8 cases were found to have more serious disease pathologically (cancer = 5, AH = 3). The gross appearance of intraductal lesions is therefore relatively ambiguous, and tissue diagnosis is certainly required. In the future, evaluation with methods such as optical spectroscopy may improve the ability to diagnose these lesions in situ.
We identified more peripheral lesions and more multiple papillomata using ductoscopy than was possible in the conventional surgery group. Because both peripheral location and multiplicity of papillomata has been associated with increased risk of breast cancer,8 the ability to identify these features of papillary neoplasia may be significant in terms of risk assessment. The association of atypical hyperplasia with papillomata increases breast cancer risk to the same extent as atypical hyperplasia alone, and the subsequent carcinomas have been reported to arise in the same location as the original papilloma.14 Thus, complete resection of disease around papillomata may have significance in terms of breast cancer risk assessment and use of preventive interventions. Furthermore, in a 3-dimensional reconstruction study of 26 women, peripheral papillomata were shown to arise from the terminal duct–lobular unit and to be associated with coexisting DCIS in one third of women.15 In another study of multiple papillomata, the same authors used immunohistochemistry to examine carcinoembryonic antigen (CEA) and actin expression in these lesions, and found CEA-positive, myoepithelial cell-free carcinomatous areas that were anatomically associated with benign-appearing papillary lesions, suggesting malignant transformation of intraductal papillomata.16
Other advantages of using the ductoscope to localize intraductal neoplasms include conservation of breast tissue and function. The ability to visualize and spare the central, normal-appearing portion of duct in women who have disease beginning at 3 or 4 cm from the ductal orifice may minimize the disruption of adjacent normal ductal structures, potentially benefiting women in their childbearing years who plan to breast feed. When peripheral papillomata are visualized with ductography, wire localization and excision without approaching the central ducts is also possible, but it appears form the data presented here that visualization of peripheral disease does not occur as often with ductography as with ductoscopy.
Ductoscopy is an emerging technology, not yet widely adopted in the United States. Other applications of ductoscopy that have been proposed include the delineation of intraductal disease in women undergoing breast-conserving surgery for cancer17 and the investigation of women with atypical cytologic findings on ductal lavage.18,19 The use of these has yet to be established, and as greater experience accumulates with this tool, issues that need to be addressed include the cost of the procedure as well as the biologic significance of the additional disease that is identified by using it. The development of ablative methods may, and of methods to distinguish benign from malignant ductal lesions may, in the future, afford the possibility of safely ruling out the need for surgical intervention in select cases. A large-scale prospective study with expanded indications is needed to demonstrate the potential for quick, facile but effective intraductal evaluation.
Footnotes
Supported by grants P50 CA89018, from the National Cancer Institute, National Institutes of Health, and the Bluhm Family Program for Breast Cancer Early Detection and Prevention.
Reprints: Seema A. Khan, MD, Department of Surgery, 675 N. St. Clair Street, Galter 13-174, Chicago, IL 60611. E-mail: SKhan@nmh.org.
REFERENCES
- 1.Dixon JM, Mansel RE. ABC of breast diseases. Symptoms assessment and guidelines for referral. BMJ. 1994;309:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354–364. [DOI] [PubMed] [Google Scholar]
- 3.King TA, Carter KM, Bolton JS, et al. A simple approach to nipple discharge. Am Surg. 2000;66:960–965. [PubMed] [Google Scholar]
- 4.Hou MF, Huang TJ, Liu GC. The diagnostic value of galactography in patients with nipple discharge. Clin Imaging. 2001;25:75–81. [DOI] [PubMed] [Google Scholar]
- 5.Wong L, Chung YF, Wong CY. Microdochectomy for single-duct nipple discharge. Ann Acad Med Singapore. 2000;29:198–200. [PubMed] [Google Scholar]
- 6.Dawes LG, Bowen C, Venta LA, et al. Ductography for nipple discharge: no replacement for ductal excision. Surgery. 1998;124:685–691. [DOI] [PubMed] [Google Scholar]
- 7.Van Zee KJ, Ortega PG, Minnard E, et al. Preoperative galactography increases the diagnostic yield of major duct excision for nipple discharge. Cancer. 1998;82:1874–1880. [DOI] [PubMed] [Google Scholar]
- 8.Gioffre’ Florio MA, Manganero T, Pollicino A, et al. Surgical approach to nipple discharge: a ten year experience. J Surg Oncol. 1999;71:235–238. [DOI] [PubMed] [Google Scholar]
- 9.Dietz JR, Crowe JP, Grundfest S, et al. Directed duct excision by using mammary ductoscopy in patients with pathologic nipple discharge. Surgery. 2002;132:582–587. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga T, Ohta D, Misaka T, et al. Mammary ductoscopy for diagnosis and treatment of intraductal lesions of the breast. Breast Cancer. 2001;8:213–221. [DOI] [PubMed] [Google Scholar]
- 11.Shen KW, Wu J, Lu JS, et al. Fiberoptic ductoscopy for patients with nipple discharge. Cancer. 2000;89:1512–1519. [PubMed] [Google Scholar]
- 12.Hughes LE, Mansel RE, Webster DJ. Benign Disorders and Diseases of the Breast: Concepts and Clinical Management, 2nd ed. 2000.
- 13.Dietz JR, Kim JA, Dawson A, et al. Mammary ductoscopy and ductal washings for the evaluation of patients with pathologic nipple discharge [Abstract]. Ann Surg Oncol. Proceedings of the 55th Annual Cancer Symposium, S15. 2002. [DOI] [PubMed]
- 14.Page DL, Salhany KE, Jensen RA, et al. Subsequent breast carcinoma risk after biopsy with atypia in a breast papilloma. Cancer. 1996;78:258–266. [DOI] [PubMed] [Google Scholar]
- 15.Ohuchi N, Abe R, Kasai M. Possible cancerous change of intraductal papillomas of the breast. A 3-D reconstruction study of 25 cases. Cancer. 1984;54:605–611. [DOI] [PubMed] [Google Scholar]
- 16.Papotti M, Gugliotta P, Ghiringhello B, et al. Association of breast carcinoma and multiple intraductal papillomas: an histological and immunohistochemical investigation. Histopathology. 1984;8:963–975. [DOI] [PubMed] [Google Scholar]
- 17.Dooley WC. Routine operative breast endoscopy during lumpectomy. Ann Surg Oncol. 2003;10:38–42. [DOI] [PubMed] [Google Scholar]
- 18.Khan SA, Baird C, Staradub VL, et al. Ductal lavage and ductoscopy: the opportunities and the limitations. Clin Breast Cancer. 2002;3:185–191. [DOI] [PubMed] [Google Scholar]
- 19.Dietz JR, Rastelli A, Bokern J, et al. Mammary ductoscopy to further characterize ductal lavage-diagnosed atypia: correlation between cytology, endoscopy, and surgical pathology [Abstract]. Ann Surg Oncol. 2004;11(suppl):S101–S102. [Google Scholar]