Abstract
Objective:
To evaluate the feasibility, safety, efficacy, amount of hemorrhage, postoperative complications, and ischemic injury of selective clamping in patients undergoing minor liver resections.
Summary Background Data:
Inflow occlusion can reduce blood loss during hepatectomy. However, Pringle maneuver produces ischemic injury to the remaining liver. Selective hemihepatic vascular occlusion technique can reduce the severity of visceral congestion and total liver ischemia.
Patients and Methods:
Eighty patients undergoing minor hepatic resection were randomly assigned to complete clamping (CC) or selective clamping (SC). Hemodynamic parameters, including portal pressure and the hepatic venous pressure gradient (HVPG), were evaluated. The amount of blood loss, measurements of liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), and postoperative evolution were also recorded.
Results:
No differences were observed in the amount of hemorrhage (671 ± 533 mL versus 735 ± 397 mL; P = 0.54) or the patients that required transfusion (10% versus 15%; P = 0.55). There were no differences on postoperative morbidity between groups (38% versus 29%; P = 0.38). Cirrhotic patients with CC had significantly higher ALT (7.7 ± 4.6 versus 4.5 ± 2.7 μkat/L, P = 0.01) and AST (10.2 ± 8.7 versus 4.9 ± 2.1μkat/L; P = 0.03) values on the first postoperative day than SC. The multivariate analysis demonstrated that high central venous pressure, HVPG >10 mm Hg, and intraoperative blood loss were independent factors related to morbidity.
Conclusions:
Both techniques of clamping are equally effective and feasible for patients with normal liver and undergoing minor hepatectomies. However, in cirrhotic patients selective clamping induces less ischemic injury and should be recommended. Finally, even for minor hepatic resections, central venous pressure, HVPG, and intraoperative blood loss are factors related to morbidity and should be considered.
In cirrhotic patients, selective clamping induces less cytolysis, with similar operative and postoperative complications, and may be recommended during liver resections. Even for minor hepatic resections, high central venous pressure, portal hypertension, and intraoperative blood loss are factors related to morbidity and should be considered.
Intraoperative bleeding is a main concern during liver resections, and mortality and morbidity are clearly correlated with the amount of blood loss.1 Inflow occlusion through clamping of the portal triad (Pringle maneuver) or total vascular exclusion can reduce blood loss during transection of the hepatic parenchyma.2,3 However, Pringle maneuver produces ischemic injury to the remaining liver and intestinal congestion.4 The degree of ischemic injury to the hepatocytes may be accentuated in the presence of underlying liver disease. Several strategies have been used to minimize ischemic injury during liver surgery, especially in patients with abnormal liver parenchyma. Intermittent total pedicular clamping has been shown to improve parenchymal tolerance.5 Ischemic preconditioning has also been proposed as an alternative to intermittent clamping.6
In 1987, Bismuth7 and Makuuchi et al8 proposed a hemihepatic vascular occlusion technique to reduce the severity of visceral congestion and total liver ischemia. However, portal vein and artery dissection to perform selective clamping is time consuming, and previous comparisons by means of retrospective9,10 and randomized11 studies did not show any difference in the postoperative outcome. In the “en bloc” technique for hemihepatic clamping of the portal triad is occluded without dissection of the 3 vessels. This technique may reduce hilar dissection time, and the possibility to damage the bile ducts can also be minimized.9,12,13
To address these issues, we designed a prospective, randomized, controlled trial comparing the intermittent complete pedicular clamping (CC) and intermittent selective clamping (SC) using the Glisson approach in patients undergoing minor hepatectomies.
The main objective was to compare the ischemic damage of the 2 procedures, which was evaluated with alanine aminotransferase (ALT) levels on postoperative day 1. The secondary objective was to evaluate the feasibility, safety, efficacy, amount of hemorrhage, and postoperative complications of the 2 procedures. Finally, we wanted to determine whether cirrhotic patients take more benefit from SC.
PATIENTS AND METHODS
From September 1999 to December 2003, 80 patients undergoing minor hepatic resection were randomly assigned to undergo hepatectomy with intermittent pedicular CC (Pringle maneuver, group CC) or intermittent pedicular SC (group SC). Patients who required concomitant bowel resection or contralateral hepatic resection were not included in the study. Minor hepatectomy was defined as the resection of 2 or less liver segments.
This study was conducted in accordance with the Declaration of Helsinki; the research review board of our hospital approved the protocol, and informed written consent was obtained from each patient before surgery. Because intraoperative ultrasonography might reveal new tumoral nodules and, therefore, lead to changes in the technique of surgical resection, potential study patients were not randomized until full hepatic exploration had been completed. Randomization was performed using sealed envelopes and stratified to include similar number of cirrhotic patients in each study group.
Surgical Technique
The description of the technique has been extensively described previously.14 Briefly, after ultrasonographic study, the liver was mobilized and the gallbladder was removed. A vascular catheter (Venocath 14 G.; Abott Ireland, Sligo, Republic of Ireland) was placed at a branch of the superior mesenteric vein to measure portal pressure.
In the CC group, the entire hilar pedicle was encircled with a rubber tape to perform a Pringle maneuver with a tourniquet (Fig. 1). In the SC group, the control of the intrahepatic portal triad was achieved by a hepatotomy near the corresponding portal pedicle by the “Glissonean” approach technique, as previously described.14 During the hepatotomy, a short period of Pringle maneuver is usually needed to minimize blood loss. Either the “en bloc” right or left portal pedicle was isolated and encircled with a rubber tape (Fig. 2). In both groups, transection of the liver was performed under intermittent clamping by means of occlusion of blood inflow, both pedicular or selective, 15 minutes and then release for 5 minutes. Separate clamping of accessory left hepatic artery was performed when present. During liver transection, the vessels on the resected side were clamped with metallic clips, and vessels on the preserved side were ligated with silk. Potential sites of biliary leakages were identified after resection by injection of methylene blue–dyed saline solution through the cystic duct. The liver cut surface at the end of the procedure was sealed with 5 mL of fibrin glue (Tissucol; Baxter-Immuno, Vienna Austria). Suction abdominal drainage was placed routinely.
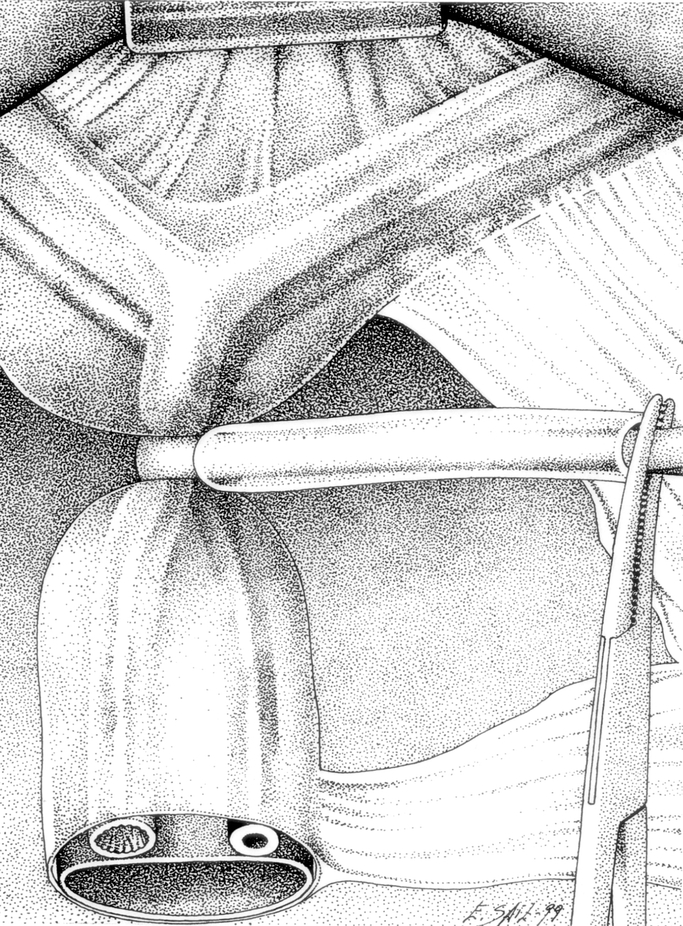
FIGURE 1. The entire hilar pedicle is encircled with a rubber tape to perform a Pringle maneuver with a tourniquet.
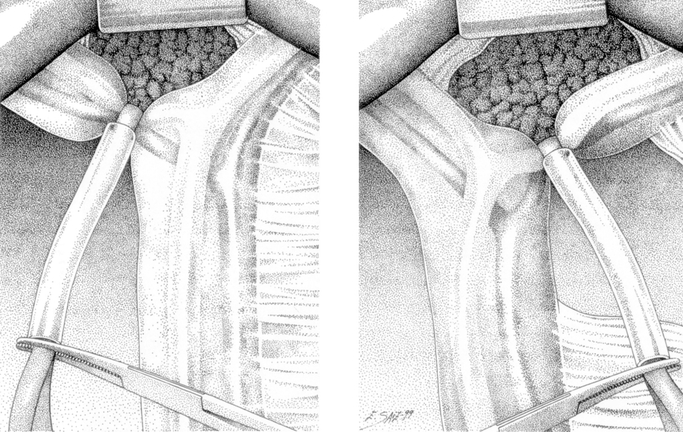
FIGURE 2. Either the “en bloc” right or left portal pedicle is isolated and encircled with a rubber tape.
Protocol Design
For the purposes of this study the operation was divided into 4 phases: (1) the hepatic mobilization phase, including laparotomy, ultrasonographic exploration, catheter vein placement, and liver mobilization; (2) the hilar dissection phase, including cholecystectomy and encircling of the portal triad structures of the pedicle, either “completely” or “selectively” using the “Glissonean” approach; (3) the parenchyma transection phase with intermittent pedicular clamping either “complete” or “selective;” and (4) The hemostasis phase: including hemostasis of the liver cut surface, biliary dye injection, and abdominal closure.
Intra- and postoperative hemodynamic monitoring included a venous central jugular catheter and venous mesenteric catheter. The hemodynamic parameters monitored throughout the operation were mean arterial pressure, heart rate (HR), portal pressure (PPr), central venous pressure (CVP), and end tidal CO2 concentration (EtCO2). The hepatic venous pressure gradient (HVPG) was calculated by the following equation HVPG = PPr − CVP. All these parameters were recorded at the following times (T): T1: at the beginning of the phase 1; T2: 10 minutes after clamping; T3: 10 minutes after release.
The amount of blood loss was measured from the volume of blood collected in the container of the aspirator and the ultrasonic dissector and from the weight of the soaked gauzes.
Lactate levels were measured at T1 and T3. On postoperative days 1, 2, 3, 5, and 7, measurements of liver enzyme ALT, aspartate aminotransferase (AST), prothrombin time, bilirubin, platelets count, hemoglobin, urea, and creatinine and lactate levels were recorded. All patients underwent ultrasonographic abdominal study and chest x-ray before leaving the hospital. Patient demographic data, complications, postoperative evolution, hospital stay, and results of histopathological study were prospectively introduced in a computer data base. All patients were followed in the outpatient clinic at 1 month, 3 months, and every 6 months thereafter with blood biochemistry and spiral CT-scan of the abdomen.
Biliary leak was defined as any drainage through the catheter with a bilirubin content higher that the plasma levels. Hepatic insufficiency was defined by a prothrombin time of less than 50% of normal and/or by serum bilirubin more than 50 μmol/L on postoperative day 5 or thereafter and/or encephalopathy. Postoperative ascites was defined by an abdominal output greater than 500 mL/d or ascites that required medical treatment to be controlled.
Statistical Analysis
Continuous data were analyzed using the Student t test. The Fisher exact test and the Pearson χ2 test were used to analyze categorical data. P < 0.05 was considered statistically significant.
Data were initially analyzed on the intention-to-treat principle. The hemodynamic study showed that some patients included in the SC group had a hemodynamic profile during the clamping period similar to the CC group. To analyze the influence of the type of clamping in cirrhotic patients, a “per-protocol” analysis was performed in this group of patients.
To assess whether other parameters apart from the main variable of the study (type of clamping) may influence on the development of complications, a univariate analysis of all baseline studied parameters was performed (“post hoc” analysis). Variables that were significant (P < 0.05) in this univariate analysis were entered into a logistic regression analysis to define which parameters were independently associated with the development of complications.
All data analysis was performed on an IBM-compatible PC using SPSS 10.0 for windows (SPSS Inc., Chicago, IL).
Sample size was calculated with the PS (Power and Sample Size) program by Dupont and Plummer.15 Sample size needed to detect a true difference of 30% in population postoperative ALT mean, with the specified power and type I error probability of 0.05, given a standard deviation of 4 and a control patient ratio mean of ALT of 6.9 μkat/L (mean of first postoperative day ALT after minor hepatectomy in previous patients at our institution), is 36 patients per group. Assuming the possibility of some missing from protocol after randomization, we decided to include 80 patients.
The trial was powered to detect differences in ALT levels because we thought that it was the most accurate and discriminant of the evaluated parameters. It has to be noted that this trial might be underpowered to detect differences in the other outcome parameters.
RESULTS
Baseline Characteristics and Surgical Feasibility
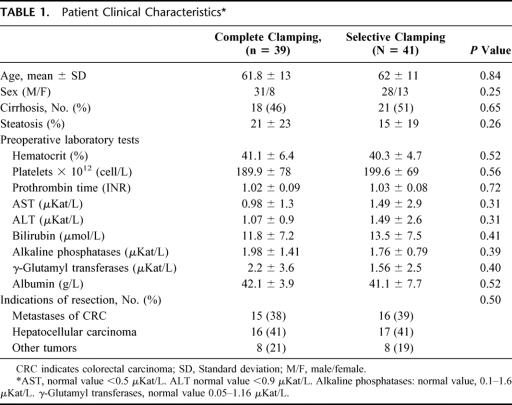
Thirty-nine patients were included in the CC group and 41 in the SC group. The groups were equally matched according to age, sex, preoperative laboratory tests, diagnosis, and degree of steatosis of the nontumorous liver (Table 1). Patients with liver disease were distributed homogeneously between groups.
TABLE 1. Patient Clinical Characteristics
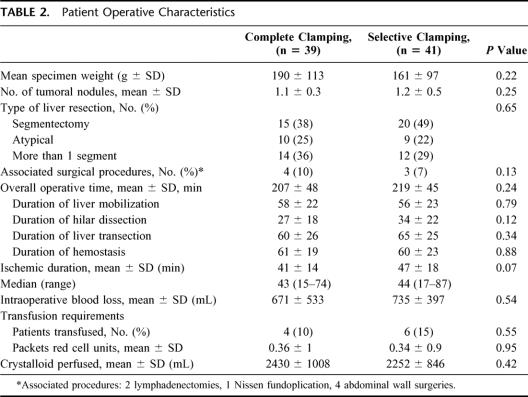
SC was possible in all patients whenever indicated, and no patients required switching to the CC group due to technical reasons. There were no intraoperative complications related with the surgical technique. There were no significant differences between the 2 groups regarding the mean resected specimen weight, number of tumoral lesions, type of liver resection performed or number of associate surgical procedures (Table 2). The duration of the whole procedure was approximately 3.5 hours, and there were no differences between groups neither on the whole procedure or on the duration of each phase of the procedure (Table 2). The overall ischemia time was slightly longer in the SC group, but there were no statistically significant differences. However, in the SC group the ischemia time included the minutes of pedicle clamping required to perform the pedicle dissection (5.9 ± 5.3 minutes). The “real” selective ischemia period during liver transection in the SC group was similar to the total ischemia period in the CC group (41 ± 14 versus 42 ± 19). No differences were observed in the amount of hemorrhage or the volume of crystalloid administered. Only 10 patients required transfusion intraoperatively (12.5%), without any difference being recorded between groups (Table 2). The amount of packed red blood cell units in transfused patients was low (median, 2 units).
TABLE 2. Patient Operative Characteristics
Influence of Type of Clamping on Intraoperative Hemodynamic Evolution
There were no differences on basal parameters between groups, but it has to be noted that there were 11 (27%) patients in the SC group with T1 HVPG greater than 10 mm Hg and only 2 (7%) in the CC group (P = 0.02). CVP during the clamping period was slightly lower in the CC group; in the CC group, the CVP had a median decrease of 25% compared with a median decrease of 11% in the SC group. HR was stable in the SC group, while in the CC group, the HR increased significantly during the clamping period and after unclamping. After unclamping, the EtCO2 was significantly higher in the CC group (Table 3).
TABLE 3. Intraoperative Hemodynamic Evolution
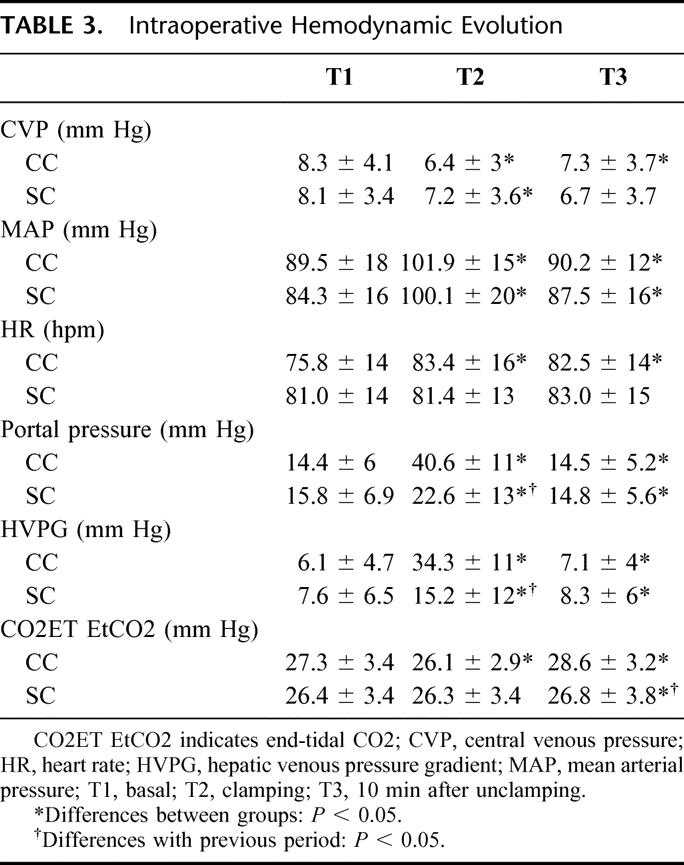
As expected, PPr and HVPG during the clamping period were significantly higher in the CC group; the HVPG increase (HVPG during clamping period minus HVPG at the beginning) was significantly different between groups (28.1 ± 11 versus 7.5 ± 11.7 mm Hg; P < 0.0001) (Fig. 3). However, 8 patients (20%) in the SC group showed a hemodynamic profile during the clamping phase similar to that of the CC group. This profile was characterized by a rise in the PPr, an HVPG increase after clamping higher than 10 mm Hg to the basal levels and increase of EtCO2 after unclamping. These changes were supposed to be due to a functionally complete occlusion of the pedicle, although the tourniquet was applied only to the right of left pedicle.
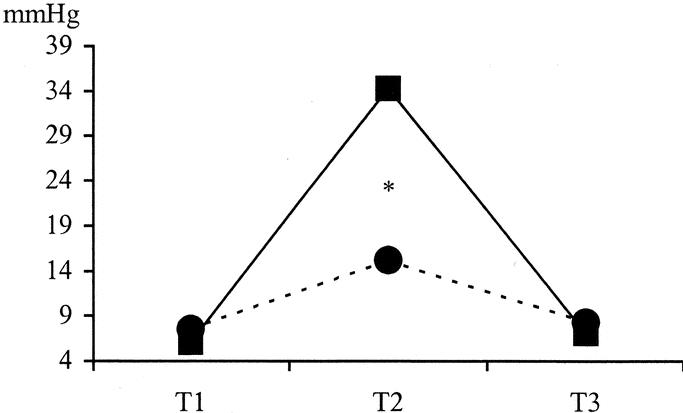
FIGURE 3. Intraoperative evolution of hepatic venous pressure gradient. T1: basal; T2: clamping; T3: 10 minutes after unclamping. * P < 005; comparison between groups. ▪, CC group; •, SC group.
Influence of Type of Clamping on Postoperative Evolution (Intention to Treat Analysis)
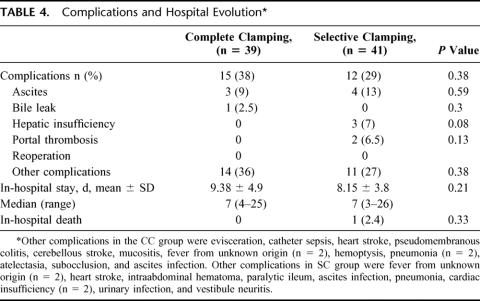
Twenty-seven patients experienced some complication. There were no differences on postoperative morbidity between groups, but 3 patients of the SC group had transitory or temporary hepatic insufficiency. The median in-hospital stay was 7 days in both groups (range 3–26 days). There were no intraoperative deaths. The overall mortality rate within 30 days was 1.2% (1 out of 80 patients). There was 1 death in the SC group as a result of hepatic insufficiency in a patient with hepatitis C virus (HCV) cirrhosis who had a basal HVPG of 30 mm Hg; his blood loss during the operation was 2120 mL, and he required 5 units of RBC transfusion (Table 4).
TABLE 4. Complications and Hospital Evolution
The evolution of postoperative AST, ALT, bilirubin, prothrombin time, and lactate was similar between both groups. ALT levels on postoperative day 1 were similar between groups (CC 6.7 ± 4.3 versus SC 6.2 ± 3.9; P = 0.57; all data not shown; further analysis was performed on cirrhotic patients).
Influence of Type of Clamping on Postoperative Evolution in Cirrhotic Patients (per-Protocol Analysis)
The data were further analyzed to determine whether patients with underlying liver disease had a different tolerance to SC. The hemodynamic analysis has already shown that some patients with SC had functionally a CC; these patients were analyzed in the CC group (per-protocol analysis). So there were 21 cirrhotic patients in the CC group and 18 in the SC group when analyzed “per protocol.”
There were no intraoperative deaths. The overall mortality rate within 30 days was 2.5% (1 out of 39 patients). There were no differences on overall postoperative morbidity between groups (CC 8/21, 38%, versus SC 9 /18, 50%; P = 0.45). Seven patients (18%) had postoperative ascites without differences between groups (CC 3/21, 20%, versus SC 4 /18, 27%; P = 0.67). In-hospital stay was also similar (CC 9.5 ± 4.9 days versus SC 9.6 ± 4.9 days; P = 0.98).
The degree of ischemic injury of the liver was studied by postoperative peak serum transaminase levels. Patients with CC had significantly higher ALT levels on first postoperative day (7.7 ± 4.6 versus 4.5 ± 2.7 μkat/L, P = 0.01) when compared with the SC group (Fig. 4a). Similarly, AST values on the first postoperative day (10.2 ± 8.7 versus 4.9 ± 2.1 μkat/L; P = 0.03) were significantly higher in the CC group when compared with patient receiving “selective” clamping (Fig. 4b). Lactate levels after unclamping were slightly higher in the CC group (4.09 ± 1.5 versus 3.45 ± 1.4 μmol/L, P = 0.2), although the difference was not statistically significant (Fig. 4c).
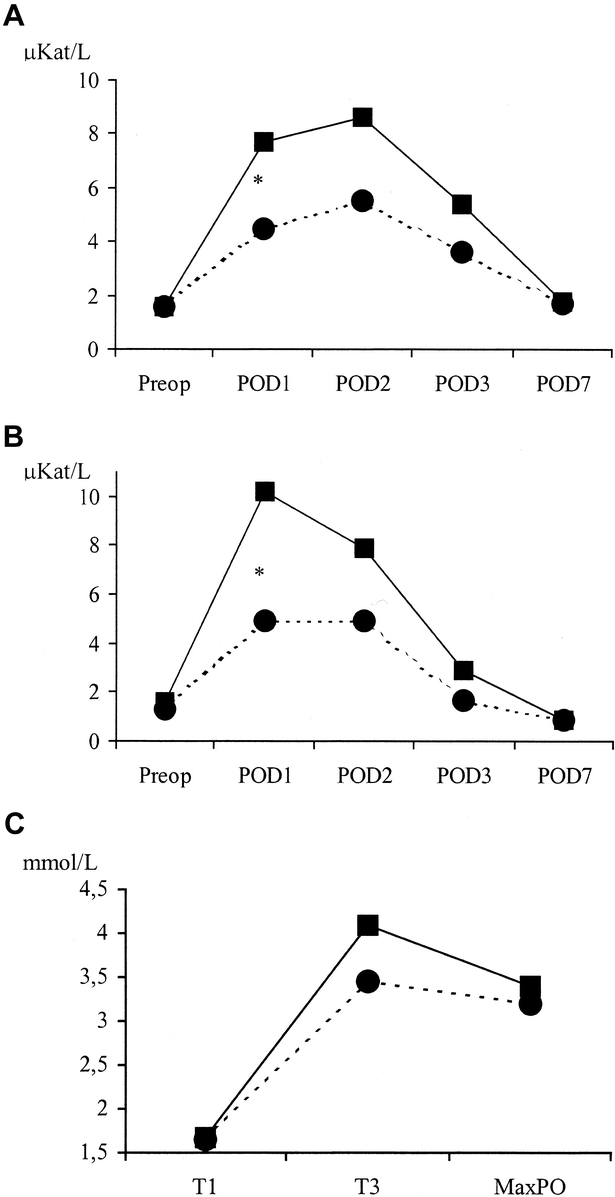
FIGURE 4. Evolution of ischemic injury parameters. A, Postoperative evolution of ALT. (μKat/L). POD, postoperative day.* The levels on postoperative day 1 were significantly higher in the CC group (P = 0.01). ALT normal value <0.9 μkat/L. B, Postoperative evolution of AST (μkat/L). POD, postoperative day.* The levels on postoperative day 1 were significantly higher in the CC group (P = 0.03); normal value <0.6 μkat/L. C, Evolution of lactate levels (mmol/L). T1: basal; T3: 10 minutes after unclamping. MaxPO, maximum value on postoperative period. Normal value <2 mmol/L. ▪, CC group; •, SC group.
Factors Related to Complications (Post Hoc Analysis)
Because clamping seemed to have no influence on postoperative complications, we next reanalyzed the data to elucidate which factors may be related to postoperative complications.
The univariate analysis confirmed that the type of clamping had no influence on morbidity. Moreover, no differences were found between patients with complications and those without them in preoperative data, such as age, sex, indication of resection, preoperative laboratory data, operative time, ischemic duration, and patients requiring transfusion. However, the univariate analysis showed that the following parameters were significantly associated with the development of complications: complicated (n = 27) versus not complicated (n = 53); HCV cirrhosis (11 [41%] versus 10 [19%] P = 0.04), high HVPG at the beginning of procedure (10 [37%] versus 4 [7.5%], P = 0.001), initial high CVP (9.56 ± 3.6 versus 41.6 ± 4.4, P = 0.02), intraoperative blood loss (882 ± 523 versus 613 ± 411, P = 0.01), and high lactate levels after unclamping (3.9 ± 1.3 versus 3.2 ± 1.3, P = 0.03). As expected, in-hospital stay was significantly longer in patients with complications (12.7 ± 5.5 versus 6.7 ± 1.5; P < 0.01).
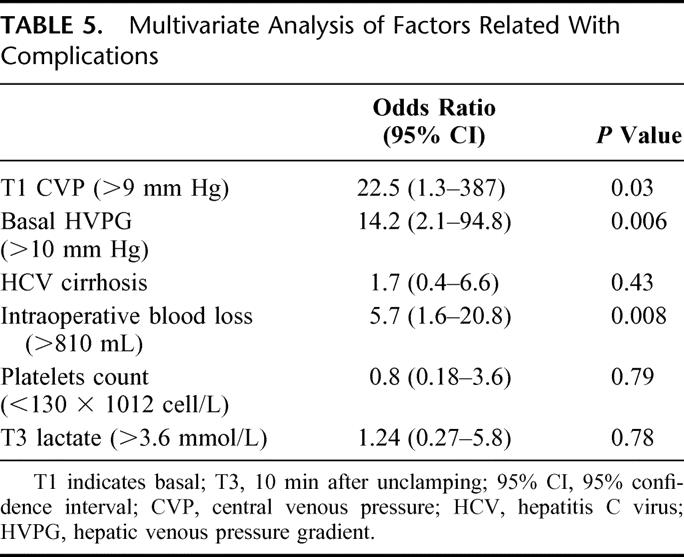
The logistic regression analysis confirmed that the initial CVP > 9 mm Hg, the initial HVPG >10 mm Hg and intraoperative blood loss were independent predictive factors for postoperative complications (Table 5).
TABLE 5. Multivariate Analysis of Factors Related With Complications
DISCUSSION
Although some minor liver resections may be performed without vascular clamping, these hepatectomies are also performed safer and with lower blood requirements using some type of clamping.16 However, experimental and clinical studies have shown than even short periods of clamping produce some degree of ischemia-reperfusion injury that can result in hepatocellular damage, this damage being especially important in patients with abnormal liver parenchyma such as steatosis and cirrhosis.17,18 The intermittent hemihepatic inflow occlusion, or SC, has been shown to be useful avoiding warm ischemia and circulatory disturbances, particularly in cirrhotic patients.10,11 However, this method is time consuming and, more important, can destroy the pericholedochal collateral vessels, which can lead to biliary complications.9 Furthermore, in case of major hepatectomy SC is not always applicable, and bleeding from the contralateral liver may make necessary the conversion to portal triad clamping in as much as 20% of cases.19
The aim of this study was to evaluate whether the use of SC should be generalized to all patients undergoing minor hepatectomies. The first evidence of our study is that SC was feasible in all patients whenever indicated. There were no complications related to the technique. Moreover, operative time, blood loss, and perioperative evolution were similar to the CC; thus, the SC is a safe and effective vascular occlusion procedure. It has to be noted that we perform the SC by means of the control of the intrahepatic portal triad by the “Glissonean” approach technique.9,12,14 In all previous reported series of SC, it was performed by hilar dissection, and the hepatic artery and portal vein to each lobe of the liver were individually taped.8–11,19 However, in our study 8 patients (20%) in the group of SC, using the technique of enbloc control of the hemihepatic vascular inflow, had a hemodynamic profile during the clamping period similar to the CC group. This profile was characterized by a rise in the PPr greater than 10 mm of Hg to the basal levels and an increase of EtCO2 after unclamping. These changes were supposed to be due to a functionally partial occlusion of the contralateral pedicle. This fact may be related to technical factors. It has to be noted that all 8 patients of the selective group with this hemodynamically complete occlusion had a right SC. The right pedicle is short and wide, and probably this may predispose to an accidental contralateral occlusion. Thus, it is very important to place accurately the rubber tape, to avoid the contralateral accidental occlusion.
This study demonstrates that SC with the “Glisson” approach allows safe minor hepatectomies with functionally preserved portal flow to the remnant liver, no splanchnic congestion, and without hemodynamic consequences in 80% of cases.
When we compared all the patients included in the 2 groups of the study, patients in the SC group demonstrated a better hemodynamic stability during hepatectomy. However, there were no differences on postoperative morbidity, mortality, or liver cell injury. Consequently, it is probable that the SC is unnecessary for minor liver resections in noncirrhotic patients.
One of the concerns about SC is the risk of bleeding from the contralateral liver.19 We have compared the bleeding rates depending on the performed segmentectomy, and we have found no differences (SC group: segments with section line perfused [IV, V, or VIII]: 830 ± 366 versus other segments: 674 ± 409; P = 0.22).
As expected, the type of clamping had more influence in cirrhotic patients. Even with the short ischemic time required for these minor hepatectomies, ischemic cellular injury, as reflected by ALT, AST, and lactate levels, were higher in the CC group. Postoperative evolution was similar in both groups.
The group of cirrhotic patients with HVPG higher than 10 mm Hg included 13 patients; a per-protocol analysis showed that 4 cases were in the CC group, while the other 9 patients were in the SC group. In this special group of patients, postoperative complications were higher than in noncirrhotic patients, or cirrhotic with HVPG <10 mm Hg (69% versus 27%; P = 0.001). But there were no differences between groups; CC group 3/4 (75%) versus SC group 6/9 (67%), P = 0.76.
This study also confirms previous results20 suggesting that HCV cirrhosis, high CVP, intraoperative blood loss, HVPG higher than 10 mm Hg, and low lactate clearance are the main prognostic factor of hepatic decompensation after liver resection, particularly in cirrhotic patients. The multivariate analysis demonstrated that initial CVP >9 mm Hg, initial HVPG >10 mm Hg, and intraoperative blood loss are independent factors related to morbidity. These factors have been previously shown by other authors.1,20,21 However, there is a lot of controversy about the clinical importance of these factors. For instance, selection of candidates for resection in oriental countries rely on Child-Pugh classification and ICG retention,22 and they do not use the HVPG to decide. Other authors23 also rely on the preoperative levels of ALT. We think that the conclusions about risk factors for postoperative complications of this prospective randomized study may help to draw conclusions in future analysis.
In view of our results, we can conclude that both techniques of clamping are safe, equally effective, and feasible for patients with normal liver and undergoing minor hepatectomy; thus, it seems unnecessary in this setting to perform an SC. However, in cirrhotic patients SC induces less cytolysis, with similar operative and postoperative complications. In these patients, especially if other factors such as high CVP or HVPG are present, SC may be recommended. Finally, even for minor hepatic resections, high basal CVP, HVPG higher than 10 mm Hg, and intraoperative blood loss are factors related to morbidity and should be considered whenever performing this type of surgery, particularly in patients with cirrhosis.
ACKNOWLEDGMENTS
This is study was partially supported by a grant from “August Pi i Sunyer Foundation,” Ciutat Sanitaria i Universitaria de Bellvitge, Barcelona, Spain; and by a grant from “Fundació August Pi i Sunyer,” Hospital Universitario de Bellvitge, Barcelona, Spain. The authors thank all anesthesiologist of the liver team for their help in data collection and all surgery nurses for their help and patience to perform this study. We also thank Eduardo Saiz for the drawings and Maria Ortiguela, MD, from Boston for reviewing the English language.
Footnotes
Reprints: Joan Figueras, MD, Department of Surgery, (IdIBGi), Hospital Universitari de Girona, “Josep Trueta,” 17007 Girona, Spain. E-mail: cgd.jfigueras@htrueta.scs.es.
REFERENCES
- 1.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belghiti J, Noun R, Zante E, et al. Portal triad clamping or hepatic vascular exclusion for major liver resection: a controlled study. Ann Surg. 1996;224:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Man K, Fan ST, Ng I, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C-C, Huang C-R, Lin T-J, et al. Effects and limitations of prolonged intermittent ischemia for hepatic resection of the cirrhotic liver. Br J Surg. 1996;83:121–124. [DOI] [PubMed] [Google Scholar]
- 5.Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clavien PA, Selzner M, Rüdiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:6–9. [DOI] [PubMed] [Google Scholar]
- 8.Makuuchi M, Mori T, Gunven P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987;164:155–158. [PubMed] [Google Scholar]
- 9.Gotoh M, Monden M, Sakon M, et al. Hilar lobar vascular occlusion for hepatic resection. J Am Coll Surg. 1994;178:6–10. [PubMed] [Google Scholar]
- 10.Takayama T, Makuuchi M, Inoue K, et al. Selective and unselective clamping in cirrhotic liver. Hepato Gastroenterol. 1998;45:376–380. [PubMed] [Google Scholar]
- 11.Wu C-C, Yeh D-C, Ho W-H, et al. Occlusion of hepatic blood inflow for complex central liver resections in cirrhotic patients. Arch Surg. 2002;137:1369–1376. [DOI] [PubMed] [Google Scholar]
- 12.Launois B, Jamiesson GG. The posterior intrahepatic approach for hepatectomy or removal of segments of the liver. Surg Gynecol Obstet. 1992;174:155–158. [PubMed] [Google Scholar]
- 13.Nakai T, Koh K, Funai S, et al. Comparison of controlled and Glisson's pedicle transections of hepatic hilum occlusion for hepatic resection. J Am Coll Surg. 1999;189:300–304. [DOI] [PubMed] [Google Scholar]
- 14.Figueras J, Lopez-Ben S, Lladó L, et al. Hilar dissection versus the “Glissonean” approach and stapling of the pedicle for major hepatectomies: a prospective, randomized trial. Ann Surg. 2003;238:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont WD, Plummer WD. Vanderbilt Medical Center. Available at: http://www.mc.vanderbilt.edu/prevmed/ps/index.htm.
- 16.Ezaki T, Seo Y, Tomoda H, et al. Partial hepatic resection under intermittent hepatic inflow occlusion in patients with chronic liver disease. Br J Surg. 1992;79:224–226. [DOI] [PubMed] [Google Scholar]
- 17.Grace PA. Ischaemia-reperfusion injury. Br J Surg. 1994;81:637–647. [DOI] [PubMed] [Google Scholar]
- 18.Franco D, Smadja C, Meakins JL, et al. Improved early results of elective hepatic resection for liver tumors: one hundred consecutive hepatectomies in cirrhotic and non-cirrhotic patients. Arch Surg. 1989;124:1003–1037. [DOI] [PubMed] [Google Scholar]
- 19.Malassagne B, Cherqui D, Alon R, et al. Safety of selective vascular clamping for major hepatectomies. J Am Coll Surg. 1998;187:482–486. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. [DOI] [PubMed] [Google Scholar]
- 21.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;620–625. [DOI] [PubMed] [Google Scholar]
- 22.Fan ST, Lo CM, Lam CM, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noun R, Jagot P, Farges O, et al. High preoperative serum alanine transferase levels: effect on the risk of liver resection in Child grade A cirrhotic patients. World J Surg. 1997;21:390–394. [DOI] [PubMed] [Google Scholar]