Abstract
Objective:
To assess the effect of isocaloric isonitrogenous parenteral glutamine supplementation on intestinal permeability and nitrogen loss in newborns and infants after major digestive-tract surgery.
Summary Background Data:
Glutamine supplementation in critically ill and surgical adults may normalize intestinal permeability, attenuate nitrogen loss, improve survival, and lower the incidence of nosocomial infections. Previous studies in critically ill children were limited to very-low-birthweight infants and had equivocal results.
Methods:
Eighty newborns and infants were included in a double-blind, randomized trial comparing standard parenteral nutrition (sPN; n = 39) to glutamine-supplemented parenteral nutrition (GlnPN; glutamine target intake, 0.4 g kg−1 day−1; n = 41), starting on day 2 after major digestive-tract surgery. Primary endpoints were intestinal permeability, as assessed by the urinary excretion ratio of lactulose and rhamnose (weeks 1 through 4); nitrogen balance (days 4 through 6), and urinary 3-methylhistidine excretion (day 5). Secondary endpoints were mortality, length of stay in the ICU and the hospital, number of septic episodes, and usage of antibiotics and ICU resources.
Results:
Glutamine intake plateaued at 90% of the target on day 4. No differences were found between patients assigned sPN and patients assigned GlnPN regarding any of the endpoints. Glutamine supplementation was not associated with adverse effects.
Conclusions:
In newborns and infants after major digestive-tract surgery, we did not identify beneficial effects of isonitrogenous, isocaloric glutamine supplementation of parenteral nutrition. Glutamine supplementation in these patients therefore is not warranted until further research proves otherwise.
Eighty newborns and infants who underwent major digestive-tract surgery were included in a double-blind, randomized trial that compared standard parenteral nutrition with glutamine-supplemented parenteral nutrition. No differences were identified regarding intestinal permeability, nitrogen loss, mortality, length of ICU and hospital stay, septic episodes, or usage of antibiotics and ICU resources.
Glutamine is the most abundant free amino acid of the body and has many essential metabolic functions. During critical illness, demands are increased and may not be met by endogenous supply and dietary intake.1 In critically ill adults, placebo-controlled randomized studies have shown that glutamine supplementation normalizes intestinal permeability, attenuates nitrogen losses, and enhances immunologic defenses against infection.1–7 Related clinical effects include improved survival, a lower incidence of infections, shorter hospital stay, and lower ICU and hospital costs.1,3,5,8,9 Placebo-controlled randomized studies in critically ill children have so far been limited to very-low-birthweight infants.10–13 Lacey et al10 studied 78 preterm infants and concluded that parenteral glutamine reduced the time on total parenteral nutrition (PN) and on the mechanical ventilator and accelerated the transition to full enteral feeding in a subgroup of infants weighing less than 800 g. Neu et al11 studied 67 very-low-birthweight infants and concluded that enteral glutamine lowered the incidence of sepsis, improved the tolerance to enteral feeding, and lowered hospital costs.14 A subsequent multicenter trial in 649 very-low-birthweight infants, however, failed to show an effect of enteral glutamine on the incidence of blood-culture-proven nosocomial sepsis.13
We hypothesized that glutamine supplementation might be of benefit to newborns and infants who needed intensive care and PN after major digestive-tract surgery. The primary endpoints of this study were the effect of isocaloric isonitrogenous parenteral glutamine supplementation on intestinal permeability and nitrogen loss in this population.
MATERIALS AND METHODS
Study Outline
The study protocol was approved by the institutional review board. The study was designed as a double-blind randomized controlled trial. In newborns and infants who needed intensive care after digestive-tract surgery, we compared standard PN (sPN) with isonitrogenous, isocaloric, glutamine-supplemented PN (GlnPN). The primary endpoints of the study were intestinal permeability, as measured by sugar absorption tests; nitrogen balance; and urinary 3-methylhistidine (3MH) excretion. Secondary endpoints were mortality and morbidity. Measures of morbidity assessed were length of ICU stay and length of hospital stay; number of septic episodes; usage of antibiotics; usage of ICU resources, as assessed by the therapeutic intervention scoring system 76 (TISS); and renal and hepatic damage, as assessed by renal and liver function tests.
Patients
Our pediatric surgical ICU is part of a tertiary referral university children's hospital. Provided informed parental consent was obtained, patients admitted to our ICU were enrolled if they fulfilled all of the inclusion criteria and none of the exclusion criteria. The inclusion criteria were gestational age >30 weeks and age ≤2 years and not expected to tolerate enteral nutrition for at least 4 days following digestive-tract surgery. Exclusion criteria were simultaneous participation in another trial or previous participation in the current trial, preexistent renal or hepatic dysfunction that precluded the use of sPN, an inborn error of metabolism, an immunodeficiency or a disease that entailed impaired growth (other than dysmaturity), the use of corticosteroid drugs, preexistent life expectancy less than 6 months.
Baseline data included demographic data, index diagnosis, operative procedure, and the Pediatric Risk of Mortality II score (PRISM) and Surgical Stress Score (SSS). PRISM, obtained on the day of admission to the ICU, was used to assess the severity of illness;15 SSS, to assess the severity of the postoperative stress response.16
Stratification, Randomization, Blinding
The patients were divided into 4 strata according to age: I, gestational age ≥30 weeks and postconceptional age <37 weeks; II, postconceptional age ≥37 weeks and age ≤0.5 year; III, age >0.5 and ≤1 year; IV, age >1 and ≤2 years. We used a computer-generated randomization schedule to assign patients sPN or GlnPN. Patients were randomized blockwise in groups of 4. The randomization schedule was made available only to the pharmacist that supervised the processing of PN. Glutamine-enriched and standard nutrition bags were labeled identically. All physicians and nurses involved in the patients’ care were blinded to nutrition assignments.
PN
On a daily basis, PN was custom made for each patient. A 2-bag system was used, with one bag containing amino acids (Vaminolact; Pharmacia, Stockholm, Sweden), carbohydrates (dextrose; Fresenius, Bad Homburg, Germany), minerals, trace elements, carnitene, water-soluble vitamins, and water, and the other bag containing lipids (Intralipid 20%; Pharmacia) and fat-soluble vitamins. Total PN was started on day 2 after surgery (designated day 0), according to hospital guidelines, stating that amino acid and lipid intake start at approximately 50% of the recommended intake in the first 24 hours of parenteral feeding and reach the recommended intake in the following 24 hours. Depending on weight, recommended intakes were 1.5 to 2.5 g/kg/24 hours of amino acids and 85 to 100 kcal/kg/24 hours, with carbohydrates providing approximately 65% of nonprotein calories and fat approximately 35%. Provided the patient's condition and the surgical procedure would allow it, tapering of PN and reintroduction of enteral nutrition was started on or after day 6. PN was withheld once patients received more than 75% to 80% of the recommended intake via the enteral route. If patients needed to revert to parenteral feeding, they were assigned the same PN (sPN or GlnPN) as before. If patients were still being fed parenterally at day 31, the study nutrition would be replaced by sPN.
L-Glutamine was purchased as a 2.5% solution in sterile water from Oxford Nutrition (Oxford, UK) and stored at −20°C. Isonitrogenous isocaloric supplementation was achieved by substituting predefined volumes of amino acids and water with L-glutamine. Based on a study by Allen et al,17 we aimed to administer 0.4 g of glutamine per kilogram body weight per day to patients fully dependent on PN. Parenteral glutamine intake and nonprotein caloric intake were calculated in the same fashion as nitrogen intake (see below).
Sugar Absorption Tests
A sugar absorption test was performed on the fifth day of the first through fourth week after surgery, unless the patient had already been discharged from the hospital. The test solution was prepared by the hospital pharmacy. One hundred forty milligrams of glucose, 140 mg of rhamnose, and 70 mg of xylose were dissolved in 50 mL demineralized water, 8.6 g of lactulose was added, and demineralized water was added up to a total volume of 100 mL. Patients were given 1 mL of this solution per kilogram body weight, via nasogastric tube. Urine passed in the next 4 hours was collected; if the collection failed, the test was repeated once within the next 24 hours. One milliliter was frozen at −80°C until thawing and analysis. We have described the details of the analytical procedure elsewhere.18 The sugars’ urinary excretions were expressed as a percentage of the dose ingested and the lactulose-rhamnose ratio as the ratio of these percentages.
Nitrogen Balance
Nitrogen balance data were acquired on days 4, 5, and 6. The nitrogen-excretion data were also used to study the contribution of nonurinary nitrogen loss to total nitrogen loss and have recently been published.19 All excreta, with the exception of bronchopulmonary secretions and 1 patient's decubitus wound exudate, were collected, stored, and analyzed.19 Nitrogen intake was calculated as the product of the volumes and the nitrogen contents of the administered amino acid solutions. The nitrogen content was calculated for each solution separately, based on the product information and the patients’ PN datasheets. Nitrogen balance was calculated as the difference between nitrogen intake and nitrogen excretion and expressed as milligrams nitrogen per kilogram body weight per 24 hours.
Urinary 3MH Excretion
An aliquot of the urine collected on day 5 was used to calculate 3MH excretion. Urinary 3MH content was measured by ion exchange chromatography on a Biochrome 20 amino acid analyser with ninhydrin detection (Biochrome, Cambridge, UK). Twenty-four-hour urinary 3MH excretion was calculated in the same fashion as 24-hour urinary nitrogen excretion19 and expressed as micromoles per kilogram body weight per day. The urinary 3MH-creatinine ratio was expressed as micromoles per millimole.
Mortality, Length of Stay in the ICU and in the Hospital
A distinction was made between 31-day mortality (ie, mortality during the interval that the study nutrition was used as PN) and all-cause in-hospital mortality. Length of stay in the ICU and in the hospital was expressed in days and calculated as the difference between the date of discharge and the start of surgery.
Septic Episodes, Antibiotic Usage
The Centers for Disease Control and Prevention criteria for nosocomial infections were used to identify septic events.20 For septic events to be taken into account, positive blood cultures were mandatory. Primary and secondary bloodstream infections were grouped together as “sepsis.” Standard 48-hour perioperative antibiotic prophylaxis consisted of amoxicillin and cefotaxime or cefoxitin and tobramycin, depending on age. Conversion to a therapeutic regimen was based on the underlying disease and the intraoperative findings. Empirical therapy for nosocomial infections was directed by hospital guidelines. During the study period, the antibiotic policy did not change. As part of standard patient care, medication prescription data were entered into the hospital information system by the hospital pharmacy. We retrieved these data to calculate the number of antibiotic doses prescribed to each trial patient on each day, from day 0 through day 31.
TISS
As part of standard ICU patient care, TISS data were prospectively entered into the hospital information system by the ICU nurses during each night shift.21 We retrieved the TISS data for each patient from day 0 through day 31. A TISS score of 0 was assigned each day a patient was not in the ICU. As a result of a problem with electronic storage of TISS data in the hospital information system, not all TISS data were retrievable. We did not attempt to reconstruct missing data but analyzed the data that were available.
Renal and Liver Function Tests
Serum urea and creatinine were determined on days 4 through 6 and on day 7 of the second through fourth week after surgery; total and direct bilirubin, aspartate and alanine aminotransferases, γ-glutamyltransferase, alkaline phosphatase, lactate dehydrogenase, and ammonia were determined on day 7 of the first through fourth week. Blood samples were not taken if the patient did not receive any PN.
Statistical Analyses
Normal lactulose-rhamnose ratios in newborns and infants are approximately 0.05 ± 0.02 (mean ± 1 SD).22 With 2-sided tests, α = 0.05 and β = 0.20, we calculated that inclusion of 80 patients should allow us to detect a between-group difference of 0.015. At the time the study was planned, nitrogen balance and 3MH excretion data were not available for similar populations of surgical infants. Power calculations were therefore restricted to lactulose-rhamnose ratios.
Analyses were by intention-to-treat. Differences in development over time in the 2 treatment groups were analyzed with repeated-measures analysis of variance (analysis of variance proc mixed; v8.2, SAS Institute Inc, Cary, NC). We used random-intercept models that allowed inclusion of all available data. The models comprised main effects of treatment and time and the interaction between treatment and time. Time was included as a factor with a number of levels equal to the maximum number of measurements available per subject to account for possible nonlinearities over time. Sugar absorption data were log-transformed before analysis because of a skewed distribution. All other analyses were performed with StatView (v4.5; SAS Institute Inc). In view of the relatively small patient numbers, we did not perform subgroup analyses. A P value < 0.05 was considered statistically significant.
RESULTS
From January 1, 1997, through December 31, 1999, 108 newborns and infants fulfilled the inclusion criteria (Fig. 1). Three patients were excluded because they had participated in the trial before. In 25 patients, parental consent was not obtained. Of the 80 patients randomized, 39 were assigned sPN and 41 were assigned GlnPN. One of the patients assigned sPN was withdrawn from the study on day 3 when it became obvious that enteral feeding would be tolerated.
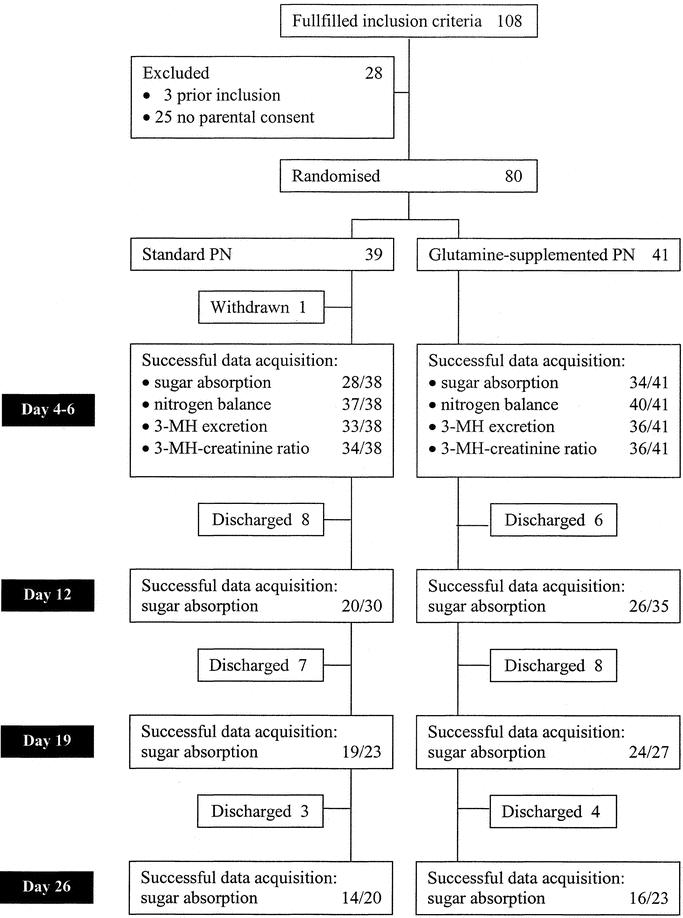
FIGURE 1. Flow chart including primary endpoints. PN, parenteral nutrition; 3-MH, 3-methylhistidine.
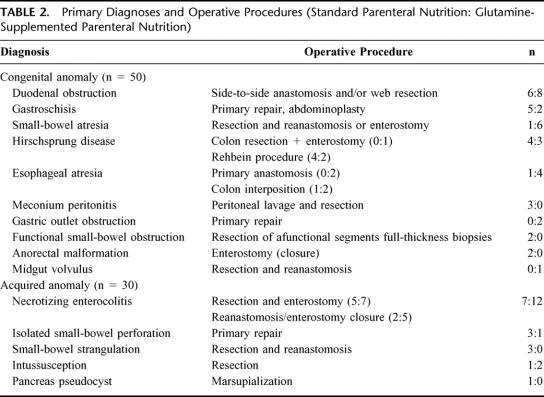
Baseline data are summarized in Table 1 and Table 2. Demographic data, PRISM, SSS, and nitrogen and caloric intake in the first week after surgery of patients receiving sPN and of those receiving GlnPN were similar (Table 1, Fig. 2). Glutamine intake reached a plateau of well over 90% of the target (0.4 g kg−1 day−1) on day 4 (Fig. 2). In univariate correlation analyses, we found no relation between PRISM or SSS and glutamine intake. Caloric intake also reached a plateau on day 4 of approximately 80% of the target (Fig. 2).
TABLE 1. Baseline Data
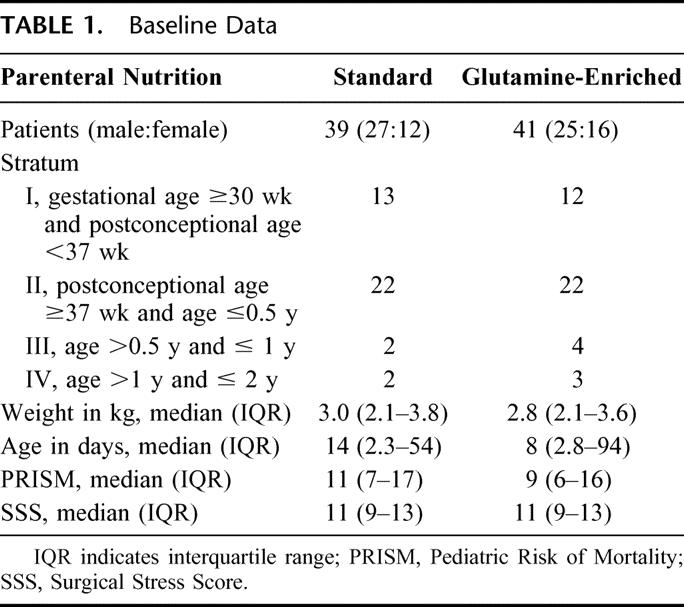
TABLE 2. Primary Diagnoses and Operative Procedures (Standard Parenteral Nutrition: Glutamine-Supplemented Parenteral Nutrition)
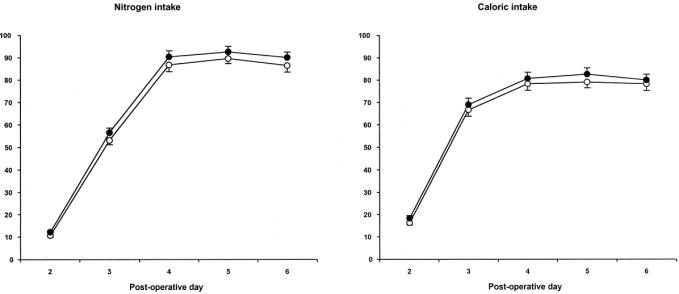
FIGURE 2. Intake of nitrogen (left panel) and nonprotein calories (right panel) on days 2 to 6 after surgery, as a percentage of the target (mean ± SEM). Open circles, patients assigned sPN; closed circles, patients assigned GlnPN. Note that in patients assigned GlnPN, nitrogen intake values equal glutamine intake values.
Primary Endpoints
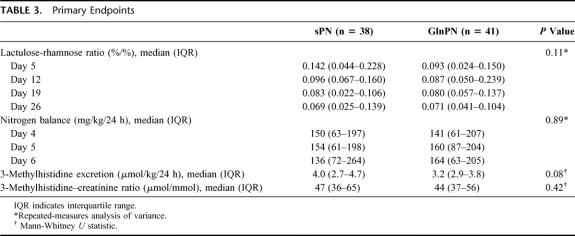
In patients assigned sPN, 81 of 111 planned tests succeeded (73%), and in patients assigned GlnPN, 100 of 126 tests succeeded (79%; Fig. 1). Repeated-measures analysis showed that the urinary excretion ratio of lactulose and rhamnose (Table 3) and the excretion of any of the 4 sugars tested (data not shown) did not differ between study groups at any time after surgery.
TABLE 3. Primary Endpoints
Nitrogen excretion data were acquired in 37 patients assigned sPN and in 40 patients assigned GlnPN (Fig. 1). Overall, 76 valid collections of excreta were obtained on day 4, 68 on day 5, and 68 on day 6. Repeated-measures analysis of variance did not show significant differences between the nitrogen balance data of sPN and GlnPN patients (Table 3).
3MH-creatinine excretion ratios were obtained in 34 sPN patients and 36 GlnPN patients. In 1 sPN patient, urine volume was not measured reliably, so that 24-hour 3MH excretion was determined only in 33 sPN patients (Fig. 1). Twenty-four-hour 3MH excretion was higher in sPN patients than in GlnPN patients, but the difference was not statistically significant (Table 3). The 3MH-creatinine ratios did not differ significantly (Table 3).
In univariate analyses, both PRISM and SSS exerted a statistically significant but small influence on nitrogen excretion and nitrogen balance (P < 0.01; r2 ≤ 0.09 for all analyses), but not on nitrogen intake and the 3MH-creatinine excretion ratio. We also found a statistically significant inverse correlation between nitrogen balance and the 3MH-creatinine ratio (P = 0.015; r2 = 0.084).
Secondary Endpoints
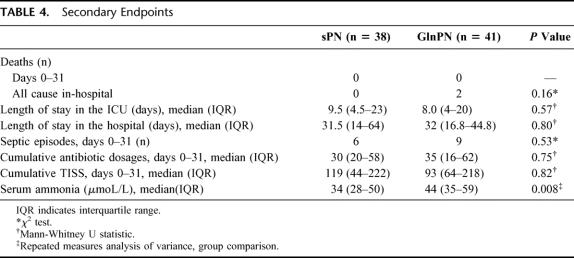
We found a similar 31-day mortality rate, length of stay in the ICU, and length of stay in the hospital in the 2 groups (Table 4). Two patients in the GlnPN group died after the first month (ie, after the period of study nutrition).
TABLE 4. Secondary Endpoints
In 38 sPN patients, 6 culture-proven septic episodes were identified from days 1 through 31 after surgery and 9 in 41 GlnPN patients (P = 0.53; Table 4). The number of antibiotic dosages prescribed was very similar in the 2 groups (Table 4). Cumulative TISS scores were retrieved in 32 patients assigned sPN and in 37 patients assigned GlnPN. Cumulative TISS scores did not differ between groups (Table 4; Fig. 3). Repeated-measures analysis of variance also did not show significant differences of the TISS scores between groups. With the exception of ammonia, laboratory tests did not reveal any differences between the 2 groups at any point in time (data not shown). Repeated-measures analysis of ammonia data showed a small but statistically significant difference between patients assigned sPN and those assigned GlnPN (overall median 34 versus 44 μmol/L; Table 4), and between points in time (data not shown).
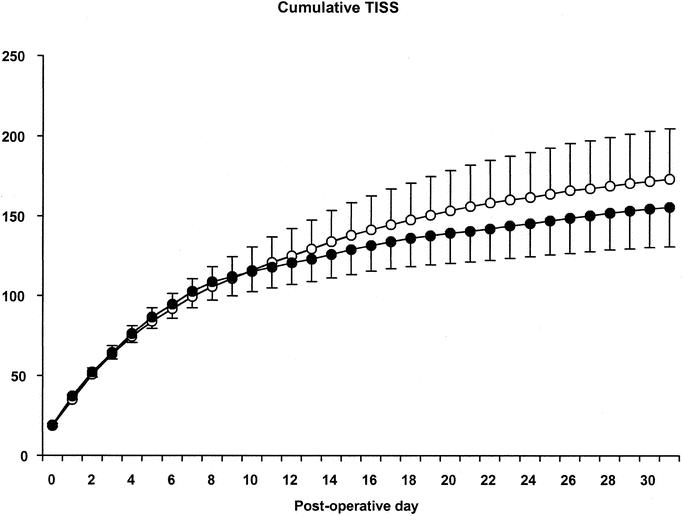
FIGURE 3. Cumulative TISS (mean ± SEM) from days 0 through 31 after surgery. Open circles, patients assigned sPN; closed circles, patients assigned GlnPN. Note that patients discharged from the ICU have a daily TISS of zero.
DISCUSSION
In a population of newborns and infants receiving PN after major digestive-tract surgery, isonitrogenous isocaloric glutamine supplementation of PN did not affect intestinal permeability or nitrogen balance. Mortality, length of ICU and hospital stay, and usage of antibiotics and ICU resources also were not affected. We did not identify adverse effects of parenteral glutamine supplementation.
A recent systematic review of glutamine supplementation in critically ill and surgical adults concluded that the greatest benefit was to be expected from parenteral administration in doses higher than 0.20 g kg−1 day−1.1 Whether children might benefit from glutamine has not been established.12,13,23 We planned to administer a parenteral glutamine dose of 0.4 g kg−1 day−1, the highest dose tried in critically ill infants.12,13 Further increasing the dose would have implied further lowering the amount of essential and other amino acids provided. Within 2 days after starting PN, we realized an average glutamine intake of 0.36 g kg−1 day−1. Realized intake did not depend on the severity of the surgical stress or of the underlying disease. Nitrogen intake also plateaued at approximately 90% of the target, which is lower than the recommended daily allowance for low-birthweight infants, but allowed nitrogen retention to approach intrauterine rates.24,25 Caloric intake plateaued at approximately 80% of the target and thus equaled or exceeded the expected total energy expenditure.26,27 Whereas daily intakes should have allowed glutamine supplementation to have an effect, cumulative intake may have been too low, as studies in adults suggest that glutamine may exert its effects only after a minimum of 5 to 6 days of supplementation.1,28 In the present study, tapering of PN, and therefore of glutamine intake, was allowed to start on day 6 (ie, 2 days after a satisfactory glutamine intake had been reached). Thus, the isonitrogenous design of this study—and of most other studies—may obscure potential benefits of glutamine supplementation by limiting the intake of glutamine and of essential and other amino acids.29
The choice of which patients to feed parenterally and how to taper parenteral feeding is closely linked to the type of surgical procedure and based on clinical judgment. We realize that the potential effects of glutamine supplementation may be obscured by loose criteria for PN and that PN should be used cautiously in ICU patients.30 In particular, some might prefer transanastomotic tube feeding for patients with a congenital foregut anomaly. This, however, is not standard practice, and we were not willing to accept the risks of transanastomotic feeding. SSS in most patients was higher than 8, indicating “major surgery.”27 PRISM was high in comparison with values observed in surgical patients in a Dutch PRISM validation study.31 Still, our patients constituted a heterogeneous population (cf. Table 2).
In adults, increased intestinal permeability has been observed in association with sepsis and organ dysfunction and after 2 weeks of standard but not glutamine-supplemented PN.1,4,32 In newborns older than 2 days, normal intestinal permeability is not related to gestational age or birth weight.33 We have previously reported increased intestinal permeability in critically ill preterm infants operated because of necrotizing enterocolitis.18 Permeability normalized in several of these infants, even though total PN was continued for 3 weeks.18 In newborns on extracorporeal membrane oxygenation, we found intestinal permeability to be increased, irrespective of whether they received parenteral or enteral nutrition.34 In the present study, lactulose-rhamnose ratios remained elevated for up to 4 weeks in the majority of patients. Also, intestinal permeability in patients who received total PN was not statistically different from that in patients who received enteral nutrition (data not shown). These findings suggest that not PN per se but rather the underlying disease, alone or in combination with surgery, causes increased intestinal permeability.
Resting energy expenditure and (nor)epinephrine levels in newborn patients increase in response to the stress of surgery but normalize again within 24 hours.27,35 Preterm infants may retain nitrogen in the first 72 hours after surgery.36 In the present study, the severity of the surgical stress and the underlying disease had limited effects on nitrogen excretion and nitrogen balance, and most patients retained adequate amounts of nitrogen on days 4 to 6.24 These findings add to the evidence that the stress response in young infants may be short-lived. Nevertheless, the urinary 3MH-creatinine ratio and 3MH excretion were increased, suggesting increased breakdown of muscle protein.37 Increased breakdown of muscle protein in the face of nitrogen retention may indicate rerouting of nitrogen fluxes for wound healing or for the defense against infection.38 Alternatively, persistently increased breakdown of muscle protein might be accompanied by increased synthesis and thus reflect increased turnover.
In adults with the systemic inflammatory response syndrome, but not in very-low-birthweight infants, enteral glutamine supplementation reduced the number of nosocomial infections.9,13 In our population, parenteral glutamine supplementation did not change the incidence of culture-proven sepsis, nor did it affect the incidence or severity of any bacterial infection, as judged by antibiotic usage and cumulative TISS scores. It should, however, be noted that TISS does not assess resource usage outside the ICU.
Studies in critically ill adults and children suggest that glutamine supplementation is safe.1,10,11,13,39–41 Glutamine is unlikely to have played a role in the 2 in-hospital deaths that occurred after the study nutrition had been replaced by sPN. It should be noted that our study did not have sufficient power to detect differences in mortality, which generally is very low in pediatric surgical intensive care populations.31 Serum ammonia was higher in glutamine-supplemented infants, but the difference was not clinically relevant (Table 4).
In view of the promising results in adults, more research into the effects of glutamine supplementation in infants and children seems justified.23,29 Currently however, our findings suggest that parenteral glutamine supplementation for surgical infants is not warranted.
ACKNOWLEDGMENTS
This study was funded by an educational grant from the Erasmus Medical Center's Clinical Research Board (Commissie Patient-gebonden Onderzoek) and by a grant from Pharmacia & Upjohn BV, Woerden, The Netherlands. The authors thank the staff of the pediatric surgical intensive care unit and the hospital pharmacy for their help in carrying out the study.
Footnotes
Reprints: M. J. I. J. Albers, Department of Pediatrics, Groningen University Hospital, X4.127, PO Box 30001, 9700 RC Groningen, The Netherlands. E-mail: m.albers@bkk.azg.nl.
REFERENCES
- 1.Novak F, Heyland DK, Avenell A, et al. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med. 2002;30:2022–2029. [DOI] [PubMed] [Google Scholar]
- 2.Stehle P, Zander J, Mertes N, et al. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989;1:231–233. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler TR, Young LS, Benfell K, et al. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. Ann Intern Med. 1992;116:821–828. [DOI] [PubMed] [Google Scholar]
- 4.van der Hulst RRWJ, van Kreel BK, von Meyenfeldt MF, et al. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363–1365. [DOI] [PubMed] [Google Scholar]
- 5.Karwowska KA, Dworacki G, Trybus M, et al. Influence of glutamine-enriched parenteral nutrition on nitrogen balance and immunologic status in patients undergoing elective aortic aneurysm repair. Nutrition. 2001;17:475–478. [DOI] [PubMed] [Google Scholar]
- 6.Boelens PG, Houdijk AP, Fonk JC, et al. Glutamine-enriched enteral nutrition increases HLA-DR expression on monocytes of trauma patients. J Nutr. 2002;132:2580–2586. [DOI] [PubMed] [Google Scholar]
- 7.Piccirillo N, De Matteis S, Laurenti L, et al. Glutamine-enriched parenteral nutrition after autologous peripheral blood stem cell transplantation: effects on immune reconstitution and mucositis. Haematologica. 2003;88:192–200. [PubMed] [Google Scholar]
- 8.Schloerb PR, Amare M. Total parenteral nutrition with glutamine in bone marrow transplantation and other clinical applications (a randomized, double-blind study). J Parenter Enteral Nutr. 1993;17:407–413. [DOI] [PubMed] [Google Scholar]
- 9.Conejero R, Bonet A, Grau T, et al. Effect of a glutamine-enriched enteral diet on intestinal permeability and infectious morbidity at 28 days in critically ill patients with systemic inflammatory response syndrome: a randomized, single-blind, prospective, multicenter study. Nutrition. 2002;18:716–721. [DOI] [PubMed] [Google Scholar]
- 10.Lacey JM, Crouch JB, Benfell K, et al. The effects of glutamine-supplemented parenteral nutrition in premature infants. JPEN J Parenter Enteral Nutr. 1996;20:74–80. [DOI] [PubMed] [Google Scholar]
- 11.Neu J, Roig JC, Meetze WH, et al. Enteral glutamine supplementation for very low birth weight infants decreases morbidity. J Pediatr. 1997;131:691–699. [DOI] [PubMed] [Google Scholar]
- 12.Tubman TRJ, Thompson SW. Glutamine Supplementation for Prevention of Morbidity in Preterm Infants (Cochrane Review): The Cochrane Library. Vol. 3. Oxford: Update Software; 2003. [DOI] [PubMed] [Google Scholar]
- 13.Vaughn P, Thomas P, Clark R, et al. Enteral glutamine supplementation and morbidity in low birth weight infants. J Pediatr. 2003;142:662–668. [DOI] [PubMed] [Google Scholar]
- 14.Dallas MJ, Bowling D, Roig JC, et al. Enteral glutamine supplementation for very-low-birth-weight infants decreases hospital costs. JPEN J Parenter Enteral Nutr. 1998;22:352–356. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MM, Ruttimann UE, Getson PR. Pediatric Risk of Mortality (PRISM) score. Crit Care Med. 1988;16:1110–1116. [DOI] [PubMed] [Google Scholar]
- 16.Anand KJ, Aynsley-Green A. Measuring the severity of surgical stress in newborn infants. J Pediatr Surg. 1988;23:297–305. [DOI] [PubMed] [Google Scholar]
- 17.Allen SJ, Pierro A, Cope L, et al. Glutamine-supplemented parenteral nutrition in a child with short bowel syndrome. J Pediatr Gastroenterol Nutr. 1993;17:329–332. [DOI] [PubMed] [Google Scholar]
- 18.Piena-Spoel M, Albers MJ, Ten Kate J, et al. Intestinal permeability in newborns with necrotizing enterocolitis and controls: does the sugar absorption test provide guidelines for the time to (re-)introduce enteral nutrition? J Pediatr Surg. 2001;36:587–592. [DOI] [PubMed] [Google Scholar]
- 19.Albers MJ, Steyerberg EW, Rietveld T, et al. Clinical relevancy of non-urinary nitrogen excretion in newborns and infants after digestive tract surgery. J Parent Enteral Nutr. 2003;27:327–332. [DOI] [PubMed] [Google Scholar]
- 20.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988 [erratum appears in Am J Infect Control. 1988;16:177]. Am J Infect Control. 1988;16:128–140. [DOI] [PubMed] [Google Scholar]
- 21.Keene AR, Cullen DJ. Therapeutic Intervention Scoring System: update 1983. Crit Care Med. 1983;11:1–3. [DOI] [PubMed] [Google Scholar]
- 22.Beach RC, Menzies IS, Clayden GS, et al. Gastrointestinal permeability changes in the preterm neonate. Arch Dis Child. 1982;57:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ball PA, Hardy G. Glutamine in pediatrics: where next? Nutrition. 2002;18:451–454. [DOI] [PubMed] [Google Scholar]
- 24.Anderson MS, Hay Jr WW. Intrauterine growth restriction and the small-for-gestational-age infant. In: Avery GB, Fletcher MA, MacDonald MG, eds. Neonatology: Pathophysiology and Management of the Newborn. Philadelphia: Lippincott Williams & Wilkins; 1999:411–444. [Google Scholar]
- 25.Georgieff MK. Nutrition. In: Avery GB, Fletcher MA, MacDonald MG, eds. Neonatology: Pathophysiology and Management of the Newborn. Philadelphia: Lippincott Williams & Wilkins; 1999:363–394. [Google Scholar]
- 26.Pierro A, Carnielli V, Filler RM, et al. Partition of energy metabolism in the surgical newborn. J Pediatr Surg. 1991;26:581–586. [DOI] [PubMed] [Google Scholar]
- 27.Jones MO, Pierro A, Hammond P, et al. The metabolic response to operative stress in infants. J Pediatr Surg. 1993;28:1258–1262. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen PA. A minimum of 5 days of feeding. Nutrition. 2002;18:715. [DOI] [PubMed] [Google Scholar]
- 29.Neu J. Glutamine supplements in premature infants: why and how. J Pediatr Gastroenterol Nutr. 2003;37:533–535. [DOI] [PubMed] [Google Scholar]
- 30.Varga P, Griffiths R, Chiolero R, et al. Is parenteral nutrition guilty? Intensive Care Med. 2003;29:1861–1864. [DOI] [PubMed] [Google Scholar]
- 31.Gemke RJ, Bonsel GJ, van Vught AJ. Effectiveness and efficiency of a Dutch pediatric intensive care unit: validity and application of the Pediatric Risk of Mortality score. Crit Care Med. 1994;22:1477–1484. [DOI] [PubMed] [Google Scholar]
- 32.Johnston JD, Harvey CJ, Menzies IS, et al. Gastrointestinal permeability and absorptive capacity in sepsis. Crit Care Med. 1996;24:1144–1149. [DOI] [PubMed] [Google Scholar]
- 33.van Elburg RM, Fetter WP, Bunkers CM, et al. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed. 2003;88:F52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piena M, Albers MJ, Van Haard PM, et al. Introduction of enteral feeding in neonates on extracorporeal membrane oxygenation after evaluation of intestinal permeability changes. J Pediatr Surg. 1998;33:30–34. [DOI] [PubMed] [Google Scholar]
- 35.Bouwmeester NJ, Anand KJ, van Dijk M, et al. Hormonal and metabolic stress responses after major surgery in children aged 0–3 years: a double-blind, randomized trial comparing the effects of continuous versus intermittent morphine. Br J Anaesth. 2001;87:390–399. [DOI] [PubMed] [Google Scholar]
- 36.Duffy B, Pencharz P. The effects of surgery on the nitrogen metabolism of parenterally fed human neonates. Pediatr Res. 1986;20:32–35. [DOI] [PubMed] [Google Scholar]
- 37.Parvy PR, Bardet JI, Rabier DM, et al. Age-related reference values for free amino acids in first morning urine specimens. Clin Chem. 1988;34:2092–2095. [PubMed] [Google Scholar]
- 38.Patterson BW, Nguyen T, Pierre E, et al. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46:573–578. [DOI] [PubMed] [Google Scholar]
- 39.Thompson SW, McClure BG, Tubman TR. A randomized, controlled trial of parenteral glutamine in ill, very low birth-weight neonates. J Pediatr Gastroenterol Nutr. 2003;37:550–553. [DOI] [PubMed] [Google Scholar]
- 40.Coghlin Dickson TM, Wong RM, Offrin RS, et al. Effect of oral glutamine supplementation during bone marrow transplantation. JPEN J Parenter Enteral Nutr. 2000;24:61–66. [DOI] [PubMed] [Google Scholar]
- 41.Long CL, Nelson KM, DiRienzo DB, et al. Glutamine supplementation of enteral nutrition: impact on whole body protein kinetics and glucose metabolism in critically ill patients. JPEN J Parenter Enteral Nutr. 1995;19:470–476. [DOI] [PubMed] [Google Scholar]