Abstract
Summary Background Data:
The intestine has been more difficult to transplant than other solid organs. We analyzed registry data to determine the scope and success of intestine transplantation in the current era.
Methods:
All known intestinal-transplant programs participated. Patient- and graft-survival estimates were obtained using the Kaplan-Meier product limit method and were analyzed with the Wilcoxon statistic.
Results:
Sixty-one programs provided data on 989 grafts in 923 patients. Four patients were lost to follow-up. The short-gut syndrome was the most common primary indication for transplantation. Sixty-one percent of the recipients were ≤18 years. Proportionally more combined intestinal and liver transplants were performed in this group. More than 80% of all current survivors had stopped parenteral nutrition and resumed normal daily activities. A multivariate analysis of cases within the last 5 years revealed that transplantation of patients waiting at home, recipient age, antibody induction immune suppression, and center experience with at least 10 cases were associated with improved patient survival. One-year graft survival rates of 81% were achieved in patients who were induced with antithymocyte globulin and maintained on tacrolimus.
Conclusions:
Transplantation is an effective therapy for the treatment of patients with end-stage intestine failure who cannot tolerate parenteral nutrition. With newer immune suppressive protocols, 1-year graft and patient survival rates approach the results of liver transplantation. Further improvement in survival are expected with early referral since suitable donor organs are scarce and survival rates are better when patients are well enough to wait at home for their transplant.
Based upon the intestinal transplant registry data, the authors report the cumulative improvement in the survival outcome after intestinal and multivisceral transplantation. With the recent introduction of innovative immunosuppressive protocols, the 1-year patient and graft survival approaches the results of liver transplantation.
The intestine is more difficult to transplant than other solid organs due to its strong expression of histocompatibility antigens, large numbers of resident leukocytes, and colonization with microorganisms.1 Early efforts to transplant the small bowel failed due to refractory graft rejection and sepsis.2 Outcomes improved during the early 1990s, but survival rates were still inferior to other organ transplants.3,4 Over the past 5 years, individual centers have reported improved outcomes with better long-term intestinal engraftment.5–8 Herein, we analyze registry data to determine the scope and success of intestine transplantation in the current era.
METHODS
Standardized report forms were sent to all known intestinal transplant programs, asking for information on intestine transplants performed between April 1985 and May 31, 2003. The report forms can be viewed at www.intestinetransplant.org. The data were entered into a Microsoft Access database and analyzed with SPSS and SAS.
Graft and patient survival estimates were obtained using the Kaplan-Meier product limit method. Any graft that included all or part of the stomach was considered a multivisceral trans. Cases since 1998 were analyzed to determine factors affecting the outcome of intestine grafting in the modern era. Variables were included in the analyses if complete data were available on at least 85% of the cases. For the survival analysis, failure was defined as occurring on graft removal or death date. A P value of < 0.05 was considered significant. The Wilcoxon test was performed to examine the effect of the following variables on survival: center size (performance of ≤10 transplants versus >10 transplants in total, an arbitrary cutoff that was used in a previous publication4 and was initially chosen based on sample size considerations and expert opinions about the number of cases that constituted a moderate clinical experience), donor type (cadaveric versus living-related), donor pretreatment (graft irradiation versus no irradiation), immunoprophylaxis (no induction versus recipient pretreatment with antithymocyte antibodies (rATG), Campath-1H, anti-IL-2 receptor antibodies, or OKT3 induction therapy), indication for transplant, year of transplant, organs transplanted (small intestine, intestine plus liver, or multivisceral transplant), effect of adding a colon segment, and age of the patient (≤18 years or >18 years of age), and recipient gender. Regression models were tested.9 The hazards were not proportional; accelerated failure time models fit the data best. Dummy variables were created for factors coding k-1 (k = number of categories in variable) indicator variables per factor as 1 or 0. The reference category included all indicator variables equal to 0. Recipient age was represented as a linear variable initially but reached significance only when included as a squared term in the final model. The final models included variables with an acceleration factor (AF) significantly different from unity, which significantly decreased the minus 2 log likelihood statistic (−2 log L). The fit of the final model was confirmed by cumulative −log plots and analyses of plots of the residuals and influential observations.
RESULTS
Overall Registry Review
Sixty-one programs provided data on 989 grafts in 923 patients who were transplanted between April 1, 1985, and May 31, 2003, in 19 countries. The participation rate was 100%; to the best of our knowledge, all intestinal transplants performed in the world since April 1985 are captured in the registry database. Four patients were lost to follow-up. The distribution of cases between the centers was highly skewed. Only 28 programs had performed a transplant within the past 2 years. The majority of cases (83%) were performed by 10 centers.
The number of cases per year increased over time from 11 in 1990 to a current rate of 140 cases per year, and 75.5% of all transplants were performed in the United States; 19.1% in Europe; 1.4% in Asia; and 4.0% in Canada, South and Central America, and the Middle East.
The causes of intestinal failure were different among both the adult and pediatric population (Fig. 1), with short-gut syndrome being the most common indication for transplantation. Sixty-one percent of the recipients were ≤18 years of age; the rest (39%) were adults. The youngest recipient was 1.2 months; the oldest was 67.8 years.
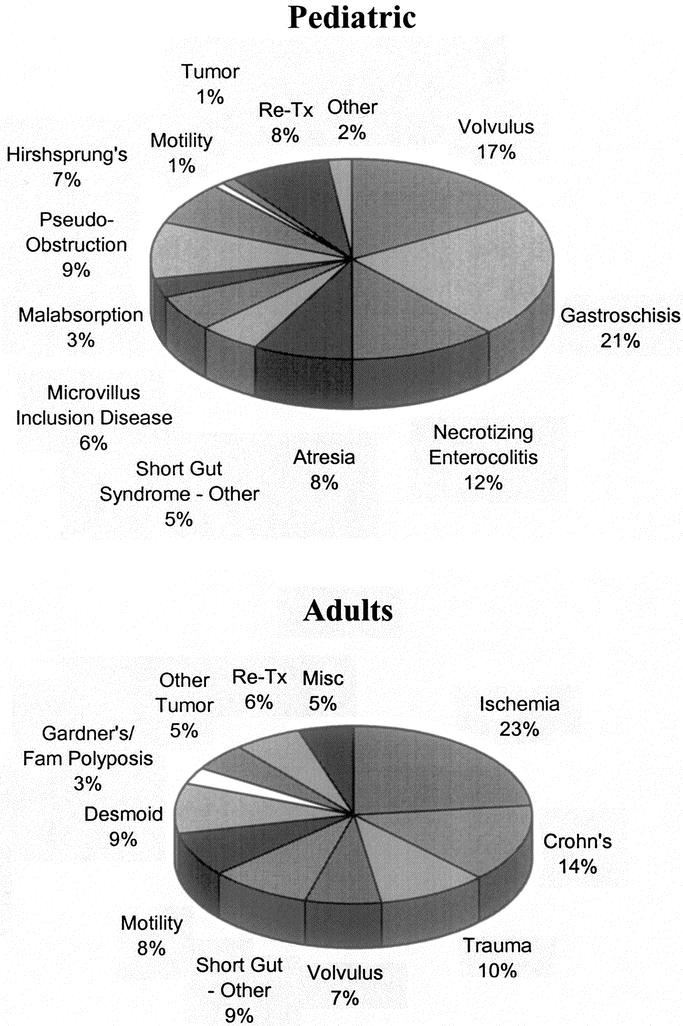
FIGURE 1. Pretransplant diagnoses in both the pediatric and adult population.
Intestine transplants alone occurred more frequently in adults (210 grafts; 55%) as compared with pediatric (≤18 years) recipients (223 grafts; 37%). Proportionally more combined intestinal and liver transplants were performed in pediatric recipients as compared with adults; 306 grafts (50%) versus 80 grafts (21%), respectively. More multivisceral grafts, 93 (24%) occurred in the adult group as compared with 77 (13%) in the pediatric recipient. Thirty-two grafts were obtained from living donors, including an identical twin and a triplet. The 1-year overall graft/patient survival rates were 57.6%/64.7% for cadaveric grafts versus 59.3%/66.7% for living donor grafts (P = 0.5322/0.9301).
Graft survival steadily improved over time (P < 0.001; Fig. 2). Four hundred thirty-five of the 919 recipients died (47.6%). Causes of death included sepsis (n = 202; 46.0%), multiorgan system failure (n = 11; 2.5%), graft thrombosis (n = 14; 3.2%), graft rejection (n = 49; 11.2%), posttransplant lymphomas (n = 27; 6.2%), respiratory causes (n = 29; 6.6%), technical reasons (n = 27; 6.2%), and other causes (n = 76; 17.3%). Of the 406 patients who were alive for more than 6 months at the time of data collection, 328 (81%) were off total parenteral nutrition (TPN); 16 (3.9%) required IV fluids only, 26 (6.4%) required partial TPN with their graft in place, and 32 (7.9%) were on TPN after graft enterectomy (Fig. 3A).
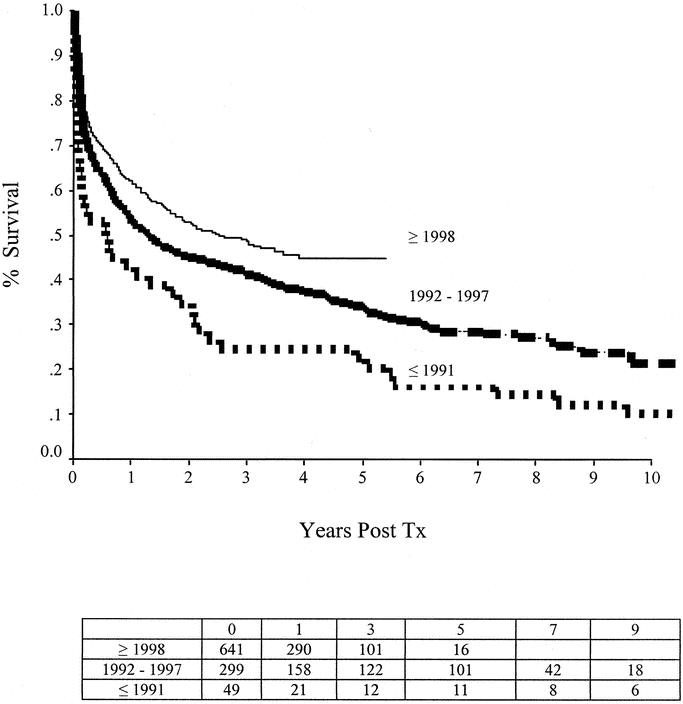
FIGURE 2. Graft-survival rates after intestine transplantation have significantly improved over time (P < 0.001).
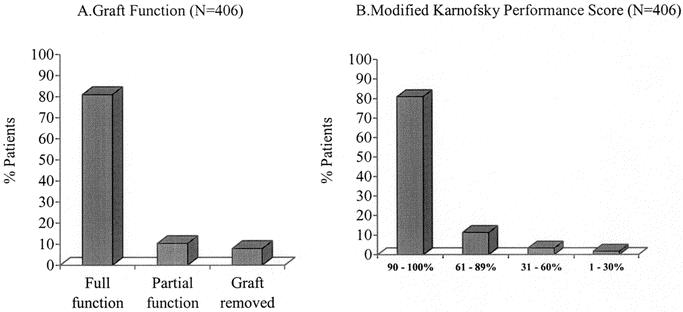
FIGURE 3. Freedom from parenteral nutrition (A) and modified Karnofsky performance scores (B) in all patients who are currently alive more than 6 months after intestine transplantation. Most recipients are completely free of parenteral supplementation and are performing most daily activities of daily living.
There were 484 patients alive after intestinal transplantation. The longest survivor had been on an oral diet for 14.2 years after receiving an intestinal transplant for a volvulus. The performance scores of survivors who were alive more than 6 months after transplantation are shown in Figure 3B.
Analysis of Transplants Completed ≥1998
The 1-year graft/patient survival rates were 65%/77% for intestinal grafts, 59%/60% for small-bowel and liver grafts, and 61%/66% for multivisceral grafts. Survival rates were significantly higher in patients who were called in from home for their transplant. One-year graft survival rates in home versus hospitalized patients were 70%/51% for intestine grafts (P < 0.001), 64%/50% for intestine and liver grafts (P = 0.018), and 71%/48% (P = 0.010) for multivisceral grafts. One-year patient survival rates in home versus hospitalized patients were 78%/72% for intestine grafts (P = 0.03), 67%/51% for intestine and liver grafts (P = 0.008), and 76%/54% (P = 0.011) for multivisceral grafts. There was a shift in practice with proportionately more patients transplanted who were waiting at home instead of being in the hospital. Prior to 2001, 52% of patients received their graft after being called in from home as compared with 71% in the last 3 years.
The median hospital stay for discharged patients was 42 days for intestine grafts, 51 days for combined intestine and liver grafts, and 51 days for multivisceral grafts. Graft-rejection rates were 57% for intestine grafts, 39% for combined intestine and liver grafts, and 48% for multivisceral grafts. Posttransplant lymphoproliferative rates for children ≤18 years and adults were 11.1%/3.4% for intestine grafts, 10.4%/2.9% for small-bowel and liver grafts, and 18.6%/6.0% for multivisceral grafts, respectively.
Factors not associated with differences in patient and graft survival included primary diagnosis, donor type, transplant type, and donor graft irradiation and maintenance immunosuppression.
Factors associated with improved graft survival in the log-logistic model included transplantation of patients waiting at home compared with patients waiting in hospital (68% versus 50% at 1 year; AF, 0.33; confidence interval (CI), 0.20–0.55; P < 0.001) and the use of induction antibody therapies directed against interleukin-α 2 receptors; (AF, 0.38; CI, 0.20–0.70; P = 0.002) and T/B lymphocytes (AF, 0.24; CI, 0.09–0.61; P = 0.0028) (75% versus 46% at 1 year). Factors associated with improved patient survival in the log-logistic model included transplantation of patients waiting at home compared with patients waiting in hospital (75% versus 60% at 1 year; AF, 0.39; CI, 0.23–0.67; P < 0.001); centers that had performed >10 transplants compared with those that had performed ≤10 cases in total (70% versus 50% at 1 year; AF, 2.73; CI, 1.25–5.96; P = 0.0118); the use of induction antibody therapies directed against interleukin-α 2 receptors (AF, 0.46; CI, 0.24–0.88; P = 0.0182), T/B lymphocytes (AF, 0.22; CI, 0.07–0.67; P = 0.0077) (81% versus 56% at 1 year; Fig. 4); and recipient age alone (AF, 0.90; CI, 0.85–0.95; P < 0.001) and as a squared (nonlinear) continuous variable (AF, 1.002; CI, 1.001–1.003; P < 0.001).
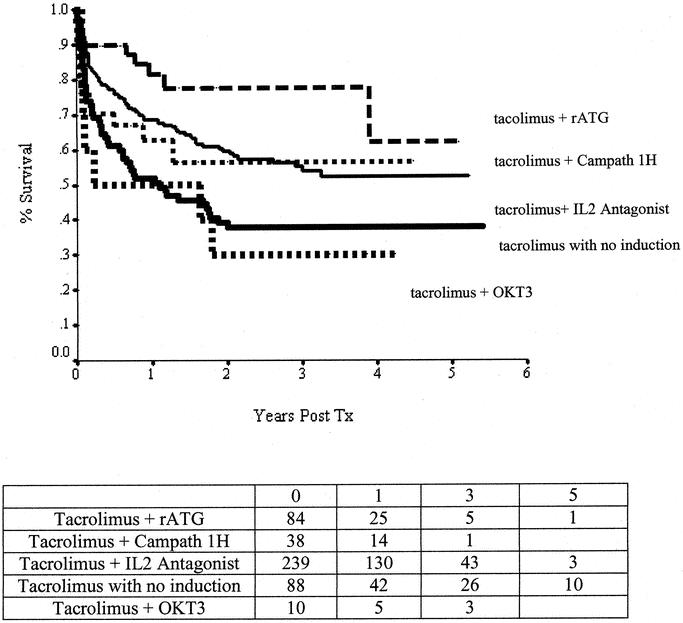
FIGURE 4. Graft-survival rates plotted according to the type of immunosuppressive protocol with particular reference to induction therapy.
DISCUSSION
Transplantation has become the definitive treatment of patients with end-stage intestinal failure who cannot tolerate parenteral nutrition. This therapy is being provided with increasing frequency and success (Fig. 2). Early referral and listing are important for successful outcomes (Fig. 5). One-year patient survival rates of more than 80% (comparable to those after liver transplantation) are being achieved using antibody-based pretreatment/induction therapy and tacrolimus-based maintenance immunosuppression (Fig. 4). Most intestine recipients (>90%) stop parenteral nutrition, resume oral nutrition, and return to normal daily activities (Fig. 3A).
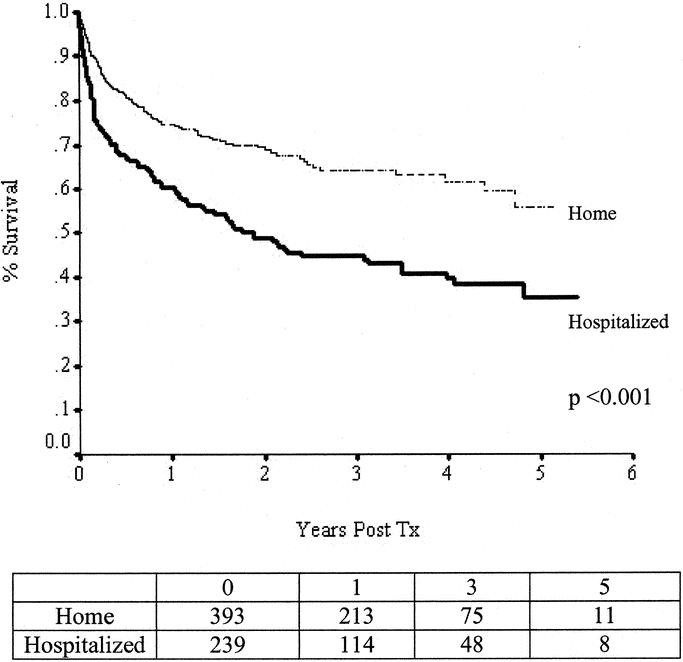
FIGURE 5. Patient survival rates plotted by status at the time of transplantation show significantly higher survival rates in patients who are waiting at home.
Intestine transplantation is being offered for all of the conditions that lead to chronic intestinal failure requiring prolonged treatment with parenteral nutrition. The spectrum of pretransplant diagnoses is shown in Figure 1. The short-gut syndrome is the most common pretransplant diagnosis. Combined liver and intestine grafts are the most frequent procedure in infants and children, who accounted for half of the cases; proportionally more isolated intestine transplants are being performed in teenagers and adults. Isolated intestine transplants, the preferred procedure, are offered to patients with limited intravenous access or recurrent line infections. Combined liver-intestine transplants are performed for treatment of irreversible liver disease caused by the parenteral nutrition solutions. Multivisceral transplants are recommended for the treatment of severe gastrointestinal motility disorders; locally aggressive, nonmetastasizing tumors; and severe abdominal injuries resulting in gastrointestinal tracts that cannot be otherwise reconstructed. Presently, because of the risks, transplants are rarely offered for quality-of-life reasons alone, but this indication will undoubtedly become more common over time as the results of intestine transplantation continue to improve.
The scope of intestine transplantation is steadily growing, with a total of 989 transplants performed as of May 31, 2003. The total number of the intestinal-transplant programs worldwide is 61, with a significant increase (>5 times) in the annual transplant rate over the last decade. However, most of the clinical experience remains concentrated in a few centers, with 82.8% of the total cases being performed by 10 centers, and only 28 programs performed a transplant within the past 2 years. As anticipated, 75.5% of the total cases have been performed in the United States, with a recent increase (50%) in the annual case volume after the Centers for Medicare and Medicaid Services approved funding of intestinal, liver-intestinal, and multivisceral transplants in April 2001.10
Because of the 100% participation rate and the relatively large numbers of patients available to study, the registry provides a unique opportunity to examine the factors contributing to variability in outcomes. We studied all cases performed since the last report of the Intestine Registry in 1998 to determine the factors associated with patient- and graft-survival rates in the modern era. A multivariate analysis identified 2 significant variables associated with increased graft survival: (1) transplants performed in patients waiting at home versus hospital; and (2) antibody induction immune suppression with monoclonal IL-α 2 receptor blockers or polyclonal antilymphocyte agents. Four significant variables were associated with improved patient survival: transplants performed in patients waiting at home versus waiting in hospital, antibody induction immunosuppression with monoclonal IL-α 2 receptor blockers or polyclonal antilymphocyte agents, center experience with 10 or more total cases, and age as a continuous variable when included with age squared. Factors that did not reach significance in these analyses included primary diagnosis, recipient gender, donor type, transplant type, retransplantation, portal venous drainage, and donor-graft irradiation.
Early referral and listing are important for successful outcomes with intestinal grafting (P < 0.001; Fig. 5). Patients who are called in from home for their intestine transplant have significantly higher survival rates, irrespective of the type of transplant that is performed. The trend to transplant proportionately more patients who are waiting at home in recent years appears to be a major factor contributing to the recently improved survival rates.
Guidelines for the timing of intestine-transplant assessments have recently been published.11,12 Urgent transplant assessments are recommended for patients with gut failure who are doing poorly on parenteral nutrition due to loss of more than 50% of the major intravenous access site; recurrent severe intravenous catheter sepsis; progressive liver dysfunction; impaired renal function due to massive gastrointestinal fluid losses; or locally aggressive, nonmetastasizing desmoid tumors that can only be removed by a massive evisceration. Preemptive assessments are recommended, even for patients doing well on parenteral nutrition, for infants and adults with an ultrashort gut (less than 20–30 cm of small intestine) or for infants with total intestinal aganglionosis or microvillus inclusion disease, since patients with these findings have very poor survival rates on parenteral nutrition.
In North America, the highest death rates on the intestine transplant waiting list occur in young children who need combined intestine/liver transplants.13,14 Two strategies have been proposed to alleviate the excess mortality in this group: assignment of a higher allocation scores to patients waiting for combined liver-intestine grafts and implantation of reduced-size grafts from adults.15,16
The use of antibody induction therapy appears to be particularly important for the success of small-bowel transplantation, possibly due to the large lymphoid mass of this graft. In this analysis, improved induction immune suppression had a strong association with higher survival rates (Fig. 4). These findings are consistent with single centers’ reports of high success rates using induction therapy with antithymocyte globulin17,18 and Il-2 antagonists.19–22 In the present analysis, the highest survival rates were associated with rATG and posttransplant tacrolimus monotherapy, a treatment regimen recently advocated by Starzl and his associates.17 Although the initial results with newer immune-suppression regimens are very encouraging, more follow-up is needed to determine if these regimens will also provide consistently high long-term survival.
Most intestinal recipients are being maintained on tacrolimus and steroids (90%). Some centers have recently advocated adding rapamycin to this regimen.6 We did not find a survival advantage in patients maintained on rapamycin and tacrolimus, but benefits of induction with rapamycin may have been obscured by patients who received rapamycin as a rescue therapy.
Intestine transplantation allows patients to resume a more normal lifestyle. Most recipients (81%) stop parenteral supplementation, eat, and resume normal activities (Fig. 3). These data are consistent with center reports showing that a well-functioning intestinal transplant provides an excellent quality of life23–26 and is highly cost-effective after 2 years in comparison to the costs of long-term parenteral nutrition.27,28
Despite refinements, intestine transplantation remains a challenging undertaking. Thus, it is not surprising that centers with more experience (arbitrarily defined in this analysis as those centers that had performed more than 10 cases in total) had better survival rates in the cases performed during the past 5 years (graft: P = 0.0192; patient: P = 0.0108). The positive effects of center experience are well documented for other complex surgical procedures.29 An analysis of the United Network of Organ Sharing database, which contains more extensive clinical information than the Intestine Transplant Registry, is planned to determine whether there are key threshold case volumes required to performed intestinal transplants with a high rate of success and/or to determine if there are associations between center resources, clinical experience, and clinical outcomes that are unique to intestine transplantation.
Registry databases provide a valuable tool to define the scope of practice, to document general outcomes of procedures, and to facilitate analyses for associations that may be difficult to discern when single-center case volumes are low or when the frequency of an event is low.30 However, registry analyses also have potential limitations. First, data collection may be subject to reporting biases or undetected problems with data quality. Second, associations detected by registry analyses require prospective, randomized trials to prove causation. Third, if key data have not been collected, important associations may be missed by the analyses.
Refinements that were not recorded in the registry database but undoubtedly contributed to improved outcomes in the modern era include integration of transplantation into comprehensive, multidisciplinary programs that can provide the entire continuum of care for the treatment intestine failure;31–33 improved methods to detect and treat posttransplant infections;34–36 better methods to diagnose graft rejection;37,38 early recognition and prompt treatment of posttransplant lymphoproliferative disorders;39 surgical innovations, including techniques for combining abdominal wall tissue transplants with intestine grafts;40 and techniques to reduce the size of adult organs for implantation into children.15,16
In conclusion, intestine transplantation has become the definitive treatment of patients with chronic intestinal failure who cannot be maintained on parenteral nutrition. Proof of efficacy has been attained,6 and the objective of becoming the preferred treatment of gut failure is within sight.
ACKNOWLEDGMENTS
The authors acknowledge the effort made by the contributing centers to ensure accurate, complete capture of all intestine transplant cases. Robert Smith, database manager, performed an outstanding job collecting and analyzing the data and preparing the figures for this manuscript. Statistical advice was provided by Carlos Gutierrez.
PARTICIPANTS
Participants: Asia: Dr. K. Tanaka, Kyoto University Hospital, Kyoto; Dr. T. Hasegawa, Osaka University Medical School, Osaka; Prof. J. Li, Jinling Hospital, Nanjing; Dr. S. Xia, Tongji Hospital, Wuhan; Dr. P. Wang, Tianjin University Medical Hospital, Tianjin; Dr. G. Wu, Xijing Hospital, Xi'an; Canada: Dr. D. Grant, Toronto General Hospital and Hospital for Sick Children, Toronto, Ontario; Dr. D. Bigam, University of Alberta Hospital, Edmonton; Dr. P. Atkison, London Health Sciences Center; Europe: Prof. J. Pirenne, University of Leuven, Leuven; Prof. R. Reding and Prof. J. de Ville de Goyet, St-Luc University Clinics, Brussels; Dr. M. Pakarinen, University of Helsinki, Helsinki; Dr. T. Beckurts, Universität zu Köln, Cologne; Dr. Allers, Johann Wolfgang Goethe University, Frankfurt; Dr. A. Mueller, University of Schleswig-Holstein, Kiel; Dr. A. Pascher, Humboldt University of Berlin, Berlin; Dr. E. Deltz, Friedrich-Ebert Hospital, Neumunster; Dr. H. D. Becker, University of Tubingen, Tubingen; Prof. Dr. R. Ploeg and Dr. G. Dijkstra, University Hospital Groningen, Groningen; Dr. E. Vicente, Hospital Ramon y Cajal and Dr. M. Lopez-Santamaria, Hospital Universitario La Paz, Madrid; Dr. R. Margreiter, University Hospital, Innsbruck; Dr. G. Rossi, Ospedale Maggiore, Milan; Dr. B. Gridelli, Ospedali Riuniti, Bergamo; Dr. A. Pinna, Universita’ di Modena e Reggio Emilia, Modena; Dr. P. Bruzzone, University La Sapienza, Policlinico Umberto 1, Rome; Dr. S. Meurling and Dr. F. Duraj, Uppsala University Hospital, Uppsala; Dr. M. Olausson and Dr. G. Herlenius, Sahlgrenska University Hospital, Goteborg; Prof. P. Morel and Dr. T. Berney, Hopitaux Universitaires de Geneve, Geneva; Dr. D. Azoulay, Hopital Paul Brousse, Courtier; Dr. O. Goulet, Hopital Necker Enfants Malades, Paris; Prof. A. Linhares Furtado, Hospitais da Universidade de Coimbra, Coimbra; Dr. R. Charco, Hospital General Universitario Vall d'Hebron, Barcelona; Mexico: Dr. F. Juarez de la Cruz, Hosp de Especialdades 71, Torreon; Middle East: Dr. I. Fazel and Dr. B. Nikoumaram, Shahid Beheshti Medical University Taleghani Hospital, Tehran; South America: Dr. M. Iasi and Dr. A. Bakonyi, Santa Casa School of Medicine, Sao Paulo; Prof. P. Argibay, Hospital Italiano de Buenos Aires, Buenos Aires; United Kingdom: Sir R. Calne, Dr. S. Middleton, Addenbrooke's Hospital, Cambridge; Dr. S. Beath, The Birmingham Children's Hospital NHS Trust, Birmingham; Mr. S. Pollard, St. James's University Hospital, Leeds; Dr. P. Muiesan, King's College Hospital, London; United States: Dr. B. Cosimi, Massachusetts General Hospital, Boston; Dr. C. Lillihei, Children's Hospital, Boston; Dr. P. Baliga, Medical University of South Carolina, Charleston; Dr. P. Foster, Rush-Presbyterian-St. Luke's Medical Center, Chicago; Dr. J. Fryer, Northwestern Memorial Hospital, Chicago; Dr. E. Benedetti, University of Illinois at Chicago, Chicago; Dr. M. Levy, Baylor University Medical Center, Dallas; Dr. D. Farmer, The Dumont-UCLA Transplant Center, Los Angeles; Dr. A. Tzakis, University of Miami School of Medicine, Miami; Dr. A. D'Alessandro, University of Wisconsin Hospital, Madison; Dr. A. Langnas, University of Nebraska Medical Center, Omaha; Dr. K. Abu-Elmagd and Dr. J. Reyes, Thomas E. Starzl Transplantation Institute, Pittsburgh; Dr. C. Esquivel, Stanford University Medical Center, Palo Alto; Dr. J. Lowell, St. Louis Children's Hospital, St. Louis; Dr. W. Andrews, The Children's Mercy Hospital, Kansas City; Dr. B. Jaffe, Tulane University School of Medicine, New Orleans; Dr. R. Gruessner, University of Minnesota, Minneapolis; Dr. Y. Wu, University of Iowa Hospitals and Clinics, Iowa City; Dr. L. Cicalese, University of Massachusetts, Worcester; Dr. S. Byron Jr., University of Alabama, Birmingham; Dr. T. Fishbein (currently at Georgetown University Hospital, Washington) and G. Gondolesi, Mt. Sinai Medical Center, New York; Dr. A. Bozorgzadeh, University of Rochester Medical Center, Rochester; Dr. B. Nour, Oklahoma Transplant Institute, Oklahoma.
Footnotes
The Registry is supported by an unconditional educational grant from Fujisawa Canada, Inc, who had no input into the registry design, analysis, or report writing.
A preliminary report was presented at the Eighth International Symposium on Small Bowel Transplantation in Miami, Florida, on September 13th, 2003.
Dr. Tzakis is supported by NIH 1 R03 DK061445-01A2.
This paper was written on behalf of the Registry participants, by the Registry Director (David Grant) and representatives from the 6 intestine transplant programs contributing the most patients to the database, listed in descending order according to the number of cases each center contributed to the Intestine Transplant Registry. Authorship by the University of Pittsburgh program is shared by 2 surgeons (KA-E, JR) representing the adult and pediatric programs, respectively.
Reprints: David Grant, MD, The University Health Network, The Toronto General Hospital, 200 Elizabeth Street, NCSB 11C-1248, Toronto, ON M5G 2C4, Canada. E-mail: david.grant@uhn.on.ca.
REFERENCES
- 1.Newell KA. Transplantation of the intestine: is it truly different? Am J Transplant. 2003;3:1–2. [DOI] [PubMed] [Google Scholar]
- 2.Kirkman RL. Small bowel transplantation. Transplantation. 1984;37:429–433. [DOI] [PubMed] [Google Scholar]
- 3.Grant D. Current results of intestinal transplantation: the International Intestinal Transplant Registry. Lancet. 1996;347:1801–1803. [DOI] [PubMed] [Google Scholar]
- 4.Grant D. Intestinal transplantation: 1997 report of the international registry: Intestinal Transplant Registry. Transplantation. 1999;67:1061–1064. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: a decade of experience at a single center. Ann Surg. 2001;234:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishbein TM, Kaufman SS, Florman SS, et al. Isolated intestinal transplantation: proof of clinical efficacy. Transplantation. 2003;76:636–640. [DOI] [PubMed] [Google Scholar]
- 7.Goulet O, Revillon Y. Intestinal transplantation. Indian J Pediatr. 2003;70:737–742. [DOI] [PubMed] [Google Scholar]
- 8.Tzakis AG, Kato T, Nishida S, et al. Preliminary experience with campath 1H (C1H) in intestinal and liver transplantation. Transplantation. 2003;75:1227–1231. [DOI] [PubMed] [Google Scholar]
- 9.Collet D. Modeling Survival Data in Medical Research. London: Chapman & Hall; 1994. [Google Scholar]
- 10.Abu-Elmagd K, Bond G, Reyes J, et al. Intestinal transplantation: a coming of age. Adv Surg. 2002;36:65–101. [PubMed] [Google Scholar]
- 11.American Gastroenterological Association. American Gastroenterological Association medical position statement: short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1105–1110. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman SS, Atkinson JB, Bianchi A, et al. Indications for pediatric intestinal transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant. 2001;5:80–87. [DOI] [PubMed] [Google Scholar]
- 13.Fryer J, Pellar S, Ormond D, et al. Mortality in candidates waiting for combined liver-intestine transplants exceeds that for other candidates waiting for liver transplants. Liver Transpl. 2003;9:748–753. [DOI] [PubMed] [Google Scholar]
- 14.Fecteau A, Atkinson P, Grant D. Early referral is essential for successful pediatric small bowel transplantation: the Canadian experience. J Pediatr Surg. 2001;36:681–684. [DOI] [PubMed] [Google Scholar]
- 15.Reyes J, Fishbein T, Bueno J, et al. Reduced-size orthotopic composite liver-intestinal allograft. Transplantation. 1998;66:489–492. [DOI] [PubMed] [Google Scholar]
- 16.de Ville dG, Mitchell A, Mayer AD, et al. En block combined reduced-liver and small bowel transplants: from large donors to small children. Transplantation. 2000;69:555–559. [DOI] [PubMed] [Google Scholar]
- 17.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzakis AG, Kato T, Nishida S, et al. Alemtuzumab (Campath-1H) combined with tacrolimus in intestinal and multivisceral transplantation. Transplantation. 2003;75:1512–1517. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Elmagd K, Fung J, McGhee W, et al. The efficacy of daclizumab for intestinal transplantation: preliminary report. Transplant Proc. 2000;32:1195–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer DG, McDiarmid SV, Yersiz H, et al. Outcomes after intestinal transplantation: a single-center experience over a decade. Transplant Proc. 2002;34:896–897. [DOI] [PubMed] [Google Scholar]
- 21.Goulet O, Lacaille F, Colomb V, et al. Intestinal transplantation in children: Paris experience. Transplant Proc. 2002;34:1887–1888. [DOI] [PubMed] [Google Scholar]
- 22.Carreno MR, Kato T, Weppler D, et al. Induction therapy with daclizumab as part of the immunosuppressive regimen in human small bowel and multiorgan transplants. Transplant Proc. 2001;33:1015–1017. [DOI] [PubMed] [Google Scholar]
- 23.Rovera GM, Sileri P, Rastellini C, et al. Quality of life after living related small bowel transplantation. Transplant Proc. 2002;34:967–968. [DOI] [PubMed] [Google Scholar]
- 24.Sudan D HS, Botha J, Grant W, et al. Quality of life after pediatric intestinal transplantation: the perception of pediatric patients and their parents. Am J Transplant. 2004;4:407–413. [DOI] [PubMed] [Google Scholar]
- 25.Sudan D, Iyer K, Horslen S, et al. Assessment of quality of life after pediatric intestinal transplantation by parents and pediatric recipients using the child health questionnaire. Transplant Proc. 2002;34:963–964. [DOI] [PubMed] [Google Scholar]
- 26.DiMartini A, Rovera GM, Graham TO, et al. Quality of life after small intestinal transplantation and among home parenteral nutrition patients. JPEN J Parenter Enteral Nutr. 1998;22:357–362. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Elmagd KM, Reyes J, Fung JJ, et al. Evolution of clinical intestinal transplantation: improved outcome and cost effectiveness. Transplant Proc. 1999;31:582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schalamon J, Mayr JM, Hollwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol. 2003;17:931–942. [DOI] [PubMed] [Google Scholar]
- 29.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan B, Schold J, Meier-Kriesche HU. Overview of large database analysis in renal transplantation. Am J Transplant. 2003;3:1052–1056. [DOI] [PubMed] [Google Scholar]
- 31.Fishbein TM, Schiano T, LeLeiko N, et al. An integrated approach to intestinal failure: results of a new program with total parenteral nutrition, bowel rehabilitation, and transplantation. J Gastrointest Surg. 2002;6:554–562. [DOI] [PubMed] [Google Scholar]
- 32.Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1111–1134. [DOI] [PubMed] [Google Scholar]
- 33.Goulet O, Ruemmele F, Lacaille F, et al. Irreversible intestinal failure. J Pediatr Gastroenterol Nutr. 2004;38:250–269. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman SS, Chatterjee NK, Fuschino ME, et al. Calicivirus enteritis in an intestinal transplant recipient. Am J Transplant. 2003;3:764–768. [DOI] [PubMed] [Google Scholar]
- 35.Cicalese L, Sileri P, Green M, et al. Bacterial translocation in clinical intestinal transplantation. Transplantation. 2001;71:1414–1417. [DOI] [PubMed] [Google Scholar]
- 36.Delis S, Kato T, Ruiz P, et al. Herpes simplex colitis in a child with combined liver and small bowel transplant. Pediatr Transplant. 2001;5:374–377. [DOI] [PubMed] [Google Scholar]
- 37.Ding J, Guo CC, Li CN, et al. Postoperative endoscopic surveillance of human living-donor small bowel transplantations. World J Gastroenterol. 2003;9:595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gondolesi GE, Kaufman SS, Sansaricq C, et al. Defining normal plasma citrulline in intestinal transplant recipients. Am J Transplant. 2004;4:414–418. [DOI] [PubMed] [Google Scholar]
- 39.McGhee W, Mazariegos GV, Sindhi R, et al. Rituximab in the treatment of pediatric small bowel transplant patients with posttransplant lymphoproliferative disorder unresponsive to standard treatment. Transplant Proc. 2002;34:955–956. [DOI] [PubMed] [Google Scholar]
- 40.Levi DM, Tzakis AG, Kato T, et al. Transplantation of the abdominal wall. Lancet. 2003;361:2173–2176. [DOI] [PubMed] [Google Scholar]


