Abstract
Objective:
The surgical margin status after breast-conserving surgery is considered the strongest predictor for local failure. The purpose of this study is to survey how radiation oncologists in North America (NA) and Europe define negative or close surgical margins after lumpectomy and to determine the factors that govern the decision to recommend reexcision based on the margins status.
Methods:
A questionnaire was sent to active members of the European Society of Therapeutic Radiation Oncology and the American Society for Therapeutic Radiology and Oncology who had completed training in radiation oncology. Respondents were asked whether they would characterize margins to be negative or close for a variety of scenarios. A second survey was sent to 500 randomly selected radiation oncologists in the United States to assess when a reexcision would be recommended based on surgical margins.
Results:
A total of 702 responses were obtained from NA and 431 from Europe to the initial survey. An additional 130 responses were obtained from the United States to the second survey regarding reexcision recommendations. Nearly 46% of the North American respondents required only that there be “no tumor cells on the ink” to deem a margin negative (National Surgical Adjuvant Breast and Bowel Project definition). A total of 7.4% and 21.8% required no tumor cells seen at <1 mm and <2 mm, respectively. The corresponding numbers from European respondents were 27.6%, 11.2%, and 8.8%, respectively (P <0.001). Europeans more frequently required a larger distance (>5 mm) between tumor cells and the inked edges before considering a margin to be negative.
Conclusion:
This study revealed significant variation in the perception of negative and close margins among radiation oncologists in NA and Europe. Given these findings, a universal definition of negative margins and consistent recommendations for reexcision are needed.
This study presents the results of a survey to radiation oncologists in North America and Europe regarding their perception of negative and close margins after lumpectomy in patients with early breast cancer. It revealed significant variation in the definition of negative/close margins. Given these findings, a universal definition of negative margins and consistent recommendations for reexcision are needed.
Following the National Institutes of Health (NIH) Consensus Development Conference on the treatment of patients with stage I and II breast cancer, there has been an overall national increase in the rate of breast-conserving therapy (BCT).1 The NIH recommendations regarding BCT for early-stage cancer rested on the results of 6 major prospective, randomized control trials in Europe and North America (NA): the National Surgical Adjuvant Breast and Bowel Project (NSABP) (1976–1984),2 National Cancer Institute (NCI) (1979–1987),3 the European Organization for Research and Treatment of Cancer (EORTC) (1980–1986),4 Danish (1983–1989),5 French (1972–1980),6 and Italian (1973–1980)7 trials.
In these trials, there was little consensus regarding the definition of negative margins after conservative surgery. The NSABP B-06 study8,9 defined the margin as negative if there were no tumor cells found at the ink of the surgical specimen on pathology review. In the NCI trial, patients assigned to breast conservation were required to have all gross tumor removed at the time of surgery but were not required to have negative margins on microscopic examination.3 The Danish trial was similar to the NCI in that it only required obtaining clean margins at gross examination for the BCT arm.5 The EORTC 10801 trial sought a 1-cm gross margin, but patients with microscopically incomplete excision of the tumor were not excluded from BCT.10 In France, the Institut Gustave-Roussy defined a “tumorectomy” as a 2-cm margin of normal tissue around the mass11 and the Milan study was even more aggressive in requiring a “rim of extra normal tissue around the tumor at the time of excision,” and quadrantectomy was the surgical procedure performed in that trial. The Italian investigators defined quadrantectomy as excision of 2 to 3 cm of normal tissue around the tumor plus the removal of a sufficiently large portion of overlying skin and underlying fascia.12,13 Thus, at the time of these randomized trials, there was wide variation in the requirements for surgical margins, even though all 6 trials showed the equivalence of BCT (local excision plus radiation therapy) as compared with mastectomy in terms of disease-free and overall survival. Even now that BCT has become widely adopted, it is unclear which requirements for surgical margins are necessary.
A number of factors affect the outcome of BCT, including patient age, tumor stage, multicentric and multifocal disease, and surgical margins.14–18 Of these, surgical margins have proven to be the strongest predictor of local recurrence.19–22 Therefore, the primary goal of surgeons and radiation oncologists is to obtain adequate negative margins of excision.23 The definition of a positive margin can vary greatly, being either gross assessment at surgery or microscopically determined as the presence of tumor cells at a fixed distance from the cut edge of the surgical specimen. Therefore, at present, the definition of a negative margin is not well established in the existing literature. This study was therefore conducted to determine the self-reported practice patterns and perceptions of radiation oncologists in the United States, Canada, and Europe about how they define negative and close margins, as well as to evaluate the decision for reexcision based on margin status. We hypothesized that the perception of negative and close margins might vary widely in this controversial area. A further hypothesis was that physicians might accord preferential weight to evidence from studies and habits in their own geographic region over those conducted in countries further away, thereby causing perception and practice patterns to diverge in a predictable fashion.
MATERIALS AND METHODS
The questionnaire used in this study was designed after a thorough review of the literature on breast cancer management to identify potential areas of controversy. The questionnaire was piloted with a small number of oncologists with experience in breast cancer management to ensure that the content was unambiguous and covered an appropriate range of topics. The final version included questions examining the respondents’ management of both invasive breast cancer and ductal carcinoma in situ (DCIS). The results from these 2 questions as well as the detailed description of the survey were reported elsewhere.24–26
In brief, a postal survey was conducted of active physician members of the American Society for Therapeutic Radiology and Oncology (ASTRO) and the European Society of Therapeutic Radiation Oncology (ESTRO). Questionnaires were mailed to 3401 ASTRO members and 2680 ESTRO members from European countries included in this analysis. The 2 lists were crossreferenced to avoid sending out duplicate surveys. Although the lists included only physician members, ASTRO and ESTRO were unable to exclude from the lists nonactive physicians and resident members. Therefore, physicians were asked not to respond if they were currently in residency training or saw fewer than 5 patients with breast cancer per month, so that the responses would reflect the practice of physicians who actively manage patients with breast cancer. The termination date for all responses was March 1, 2002. A business reply envelope was enclosed for response and full anonymity was maintained so that it was not possible to contact nonrespondents.
Contained in the instrument were questions describing type of practice facility (academic, community, private, or other), location (city, state, and country), number of patients with breast cancer seen or treated per week, and number of years since completing training in radiation oncology. The questionnaire also asked respondents how they would define negative margins after local excision of breast cancer from the following choices: 1) no tumor cells are seen on the ink margins, 2) no tumor cells are seen within 1 mm from inked margins, 3) no tumor cells are seen within 2 mm from inked margins, 4) no tumor cells are seen within 3 mm from inked margins, 5) no tumor cells are seen within 5 mm from inked margins, and 6) no tumor cells are seen within 10 mm from inked margins. Respondents were then asked to define close margins after local excision as no tumor cells seen on the inked margins and within 1 mm, 2 mm, 3 mm, or 5 mm from the inked margins.
To evaluate the recommendation for reexcision based on margins for invasive breast cancer, grade 3 DCIS, and low-grade DCIS (grades 1 and 2), a second survey was sent to 500 randomly selected radiation oncologists in the United States. They were asked whether they “always” or “sometimes” recommend reexcision based on the definition of close margins. Again, a business reply envelope was enclosed for response and full anonymity was maintained. Physicians were again asked not to complete the questionnaires if they were currently in training or saw fewer than 5 patients with breast cancer per month.
To evaluate intra-European variations, Europe was divided into 7 regions based on geography, language, and cultural clustering. This was as follows: Eastern Europe; United Kingdom; France and Belgium; Germany, Austria, and Switzerland; Italy and Greece; Spain and Portugal; and The Netherlands, Finland, Sweden, and Denmark. The United States was divided into 6 regions to assess for any regional differences. Regional definitions were chosen based on a previous study predicting the use of BCT in stage I and II breast cancer.27 Canada was considered a separate, seventh North American region.*
All responses were tabulated and examined for significant differences between types of institution (academic vs. nonacademic) and region using SAS System 8.1 software. A 2-tailed test was used to compare training years and number of patients with breast cancer.28 Fisher exact test was used for the frequency analysis in 2 × 2 tables. For analysis of tables with 2 columns and r rows, when the response variable was nominal, the chi-squared statistic was used, and when the response variable was ordinal, the Kendal correlation was used.28–30 P values less then 0.05 were considered statistically significant in this analysis.
RESULTS
Demographics and Response Rates
A total of 1137 responses were obtained to the first questionnaire: 431 from Europe and 702 from NA. The European respondents included 45 from Eastern Europe; 36 from the United Kingdom; 80 from France and Belgium; 95 from Germany, Austria, and Switzerland; 46 from Italy and Greece; 50 from Spain and Portugal; and 79 from The Netherlands, Sweden, Finland, and Denmark. The American respondents included 667 from the United States and 35 from Canada. An additional 130 responses were obtained from the second survey, which was only administered in the United States. Only fully licensed physicians who saw at least 5 new patients with breast cancer per month were asked to respond.
As noted previously, the first survey was mailed to the full physician membership of ASTRO and ESTRO (3401 and 2680 individuals, respectively), including a number of recipients who were not part of the true target population (residents, retirees, and physicians who saw fewer than 5 patients with breast cancer per week). According to records from the 2 associations, 20% of ASTRO members and 26% of ESTRO members are nonpracticing or in training (ASTRO, personal communication, 2004; ESTRO, personal communication, 2004), leaving the total estimated target population as 2724 possible North American respondents and 1975 possible European respondents. This leads to an estimated response rate of 26% for NA and 22% for Europe. For the second survey, the corresponding number was 32.5%. These figures are conservative insofar as it is impossible to estimate and exclude from the denominator the number of recipients of the mailing who were not part of the true target population because they did not see an adequate volume of patients with breast cancer.
The majority of American respondents—74.1%—described their practice as community-based or private, whereas 25.2% were from academic institutions. The remaining 0.7% described their practice as “other,” including military and locum tenens postings. A significantly larger proportion of European respondents practiced in academic institutions—62.1%—whereas 37.4% were community-based or private and 0.5% “other” (P <0.01). The mean number of years since completion of training in radiation oncology was 13.7 for American respondents and 12.9 for European respondents with no statistically significant difference. The mean number of patients with breast cancer seen per month was 16.
Negative Margins After Local Excision
The results regarding the definition of negative margins within NA are illustrated in Figure 1. The comparison between NA and Europe is shown in Figure 2. Among North American respondents, 45.9% defined a negative margin as no tumor cells seen on the ink, whereas 7.4% required no tumor cells to be seen within 1 mm of the ink and 21.8% required no tumor cells to be seen within 2 mm of the inked margins. Approximately one fourth required no tumor cells to be seen from 3 to 10 mm from inked margins before deeming the margin negative. This contrasted with the findings in Europe, where respondents were more likely to require larger tumor-free distances, with a majority requiring no tumor cells from 3 to 10 mm from the inked margins to consider the margin negative (P <0.001). Only 27.6% of European respondents were willing to deem a margin negative when no tumor cells were seen on the inked margins.
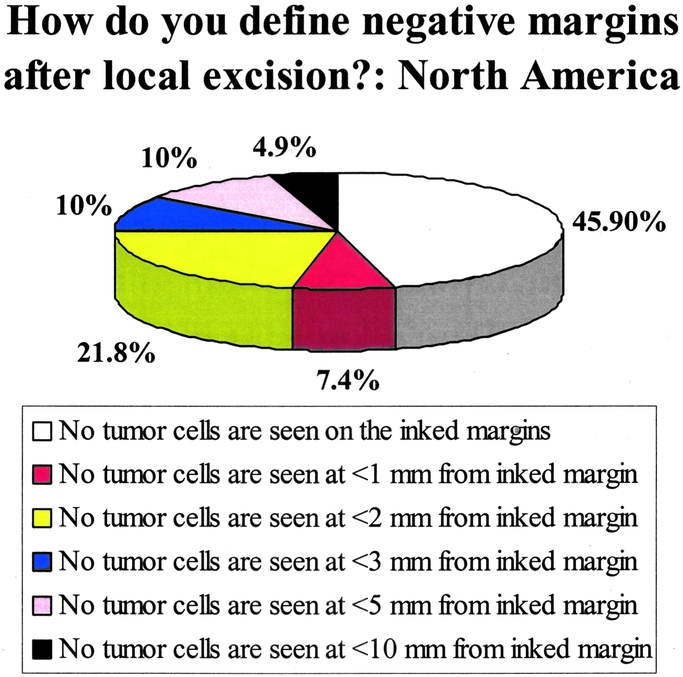
FIGURE 1. Responses regarding the definition of negative margins in North America (United States and Canada). Results from 702 respondents. The question asked was: “How do you define negative margins after local excision?”
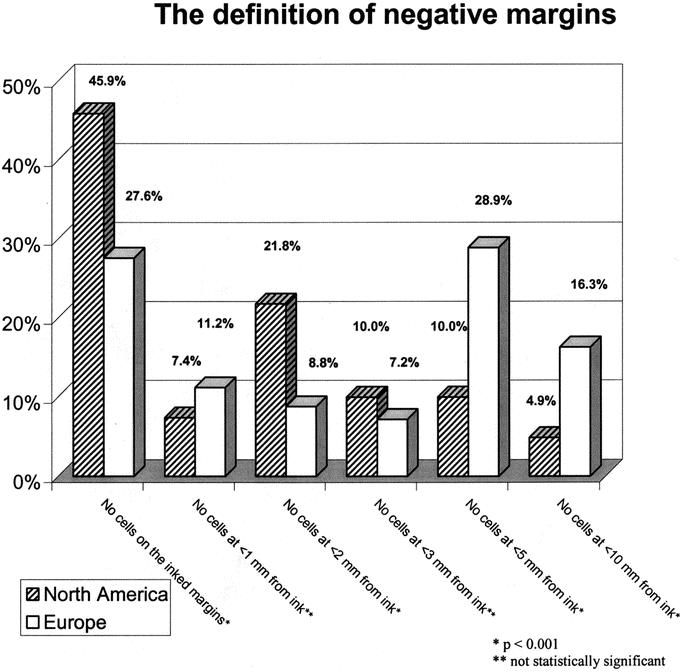
FIGURE 2. Responses regarding the definition of negative margins (comparison between North America and Europe). The question asked was: “How do you define negative margins after local excision?”
Close Margins After Local Excision
The results regarding the definition of close margins are shown in Figure 3. North Americans were more likely to consider a close margin as no cells seen on the inked margins but within 2 mm of the ink (38.0% vs. 21.0% for Europeans, P <0.001). Europeans were divided in their responses, most often considering tumor cells within 5 mm of the ink to be close (31.8% vs. 14.6% for North Americans, P <0.001). Sixty-nine percent of North Americans chose close margins as either <1 or <2 mm compared with 50.3% from Europe (P <0.001).

FIGURE 3. Responses regarding the definition of close margins (comparison between North America and Europe). The question asked was: “How do you define close margins after local excision?” All options had “no tumor cells are seen on the ink margins.” NA, North America, including the United States and Canada.
American Regional Variations
To evaluate potential intra-American variations, the United States was divided into 6 regions as described in the Methods section. There was no significant difference in the definition of negative or close margins between the different regions with the United States or between the United States and Canada. The NSABP definition for negative margins (no tumor cells seen on the ink) was used by 52.5%, 51.2%, 41.6%, 31.8%, 50%, 44%, and 54.3% of respondents from Midwest, Mountain, Northwest, Pacific, South, South Atlantic regions, and Canada, respectively (P = more than nonsignificant [NS]). For close margins, a range of 30.4% to 45.7% used the definition of no tumor cells seen at <2 mm from inked margins (P = more than NS).
European Regional Variations
Overall, Europeans were more likely to require larger distances of 5 mm or even 10 mm between tumor cells and ink to consider a margin to be negative (combined percentage of 45.2% compared with 14.9% in NA, P <0.001). However, in contrast to the lack of regional variation in NA, there were significant differences in the perception of negative or close margins within the European countries. The eastern (32.0%) and the northern (46.8%) regions generally favored the least stringent definition for negative margins, which required only no tumor cells seen on the inked margin, similar to the preferred American definition. However, the most popular response from British respondents (35.6%) was to define negative margins as no tumor cells seen at within 1 mm. Respondents from Germany–Austria–Switzerland (34.7%) and Italy–Greece (32.6%) most frequently required the minimum distance between tumor and ink to be 5 mm. A substantial proportion of respondents from Spain–Portugal and other eastern regions also preferred a minimum distance of 5 mm or 10 mm from the ink to deem a margin negative (Table 1).
TABLE 1. Results of an International Survey (European Countries): Locoregional Differences in Europe (for definition of regions, see Materials and Methods): “How do you define negative margins after local excision?”
Similarly, the definition of close margins for all categories of margin status differed significantly between the different European countries. Respondents from The Netherlands, Sweden, Finland, and Denmark, as well as respondents from France and Belgium most often defined close margins as <1 mm (47.5% and 31.3%, respectively). Those from Spain and Portugal (45%), Italy and Greece (37.7%), and Germany (30.7%) considered any distance less than 5 mm to be close. The United Kingdom was evenly divided for all margin categories (Table 2)
TABLE 2. Results of International Survey (European Countries): Locoregional Differences in Europe (for definition of regions, see Materials and Methods): How do you define close margins after local excision? All options had “No tumor cells are seen on the ink margins”
Academic versus Nonacademic Institutions
Respondents from academic and nonacademic institutions did not differ in their recommendations in Europe for either negative (P = 0.29) or close (P = 0.65) margins. There were also no differences within North America between respondents from academic and nonacademic institutions for negative (P = 0.97) or close (P = 0.31) margins. When academic centers were considered alone, there was a statistically significant difference between NA and Europe for negative (P <0.0001) and close (P <0.0001) margins. The results for each margin category are shown in Table 3.
TABLE 3. Results of International Survey (North America and Europe): Negative and Close Margins in Europe as Compared With North America in the Academic or Nonacademic Setting
Reexcision for Invasive Tumor and Ductal Carcinoma In Situ Based on Margin Status
U.S. respondents’ recommendations for reexcision based on margin status are shown in Table 4. The percentage of radiation oncologists responding “always reexcise” if tumor cells were found on the ink were 93.7%, 92.9%, and 84.1% for invasive, grade 3 DCIS, and grade 1/2 DCIS, respectively. “Always” scores for tumor cells within 1 mm, but not at the ink, were nearly halved, at 38.9%, 46.8% and 28.6%, respectively. The “sometimes” responses for within 1 mm were 47.6%, 38.9%, and 49.2% and for within 1 to 2 mm were 40.5%, 42.1%, and 38.9%, respectively. As the margin became larger, the “always” response became smaller, and even the “sometimes” response was relatively small. For example, for tumor cells within 3 to 5 mm of the margin, the reexcision recommendation rates were 13.5%, 12.7%, and 14.3% again for the categories of invasive cancer, grade 3 DCIS, and grade 1/2 DCIS, respectively. Reexcisions were rarely recommended for tumor cells within 5 to 10 mm of the inked margin.
TABLE 4. Results of Practice Patterns Survey (United States)
DISCUSSION
Importance of Margin Status in Local Control
The importance of determining margin status has been reviewed frequently. In 30 of 34 studies reviewed by Singletary,23 the local recurrence rate was increased in the cases that had persistent “positive” margins compared with “negative” margins. This effect appeared to be independent of the size of the margin. For example, when comparing recurrence rates in patients with negative versus positive margins, van Dongen et al (using a “gross” margin) reported local recurrence rates of 9% versus 17%,31 Recht et al (defining negative margins as no tumor cells within 1 mm of ink) found rates of 3% versus 22%,32 Wazer et al (defining negative margins as no tumor cells within 2 mm of ink) found rates of 4% versus 16%,33 Pittinger et al (defining negative margins as no tumor cells within 3 mm of ink) found rates of 3% versus 25%,34 and Horiguchi et al (defining negative margins as no tumor cells within 5 mm of ink) found rates of 1% versus 11%.35
To confound the issue further, “positive” margins are not necessarily all the same, because the extent of margin involvement may affect the risk of local recurrence. In 1 study from Thomas Jefferson,36 the surgeon shaved arcs of extra tissue along the edges of the cavity contour after removal of the primary tumor. If tumor involved this additionally excised tissue, then the margin was deemed positive. The authors found that patients with 1 microscopically positive margin had a risk of local recurrence equal to that of patients with negative margins, but patients with 2 or more positive margins had worse local control and overall survival.36 In another study, Schnitt et al37 found that a focally positive margin (tumor at margin in 3 or fewer low-power fields) had 15 times the risk compared with a negative margin.37 Park et al38 reported that focally positive margins had an intermediate local recurrence rate (14%) compared with extensively positive margins (27%) and negative ones (7%).38 Wazer et al33 grouped margins into 4 categories after tumor resection: “focal” involvement was defined as margin involvement by a single microscopic focus in 1 histologic section; “minimal” was in less than or equal to 1 low-power field and/or limited to involvement in 2 to 4 sections at 1 geographic edge of the specimen; “moderate” was margin involved in 2 to 4 low-power fields and/or present in 5 to 7 sections; and “extensive” was margin involved in greater than or equal to 5 low-power fields and/or present in greater than or equal to 8 sections. “Moderate/extensive” margins were predictive of worse local control as compared with “focal/minimal”(22.2% vs. 2.8%).33 Although this extensive categorization seems somewhat labor-intensive, it does give significant separation between a high and low risk of local failure. The remaining question is which categories give the best discrimination with the easiest reproducibility between pathologists.
Significant Variation in the Definition of “Close Margins”
In light of the several studies reporting similar local recurrence (LR) rates in patients with close margins and patients with negative margins,34,38,39 it may seem unclear why oncologists might define an additional category of close margins at all. Still, there is considerable uncertainty over the definition of negative and positive margins that make the definition of a third category valuable. Two studies that defined negative margins as no tumor cells within 2 mm of ink demonstrated that close margins were equivalent to positive margins.22,40,41 However, 4 additional studies showed that the LR in that group was intermediate between groups of patients with negative and positive margins.32,42–44 The unclear relationship between focally positive or close margins and the risk of local recurrence has forced many oncologists to take a cautious approach to BCT and advocate greater tissue resection at the time of the primary procedure (eg, the use of shaved additional margins after gross total resection) or to advise reexcision as a second operation. In a study published by the Milan group comparing lumpectomy with quadrantectomy,13,45 the authors defined lumpectomy as limited to the tumor mass with a narrow margin (1 cm of normal tissue and no skin excision). Although there was no difference in the rate of distant metastasis or the probability of survival, lumpectomy had a higher 10-year crude cumulative rate of LR (7.4% vs. 18.6%)45 and frequency of margin positivity (16% vs. 3%)13 compared with quadrantectomy. Accordingly, a wider margin of tissue resected around the tumor appears to help decrease local recurrence, but this concern needs to be weighed against achieving poor cosmesis and the effect on the patient's quality of life. Indeed, to address the cosmetic defect of quadrantectomy, concomitant plastic surgery reconstruction was encouraged.46
Perception of Negative Margins Among Radiation Oncologists
Despite guidelines for BCT and multiple studies on the importance of margins and the risk of local recurrence, there is no clear consensus regarding the ideal margin after wide local resection. This study sought to assess the self-reported practice patterns of radiation oncologists in NA and Europe. Based on the survey data reported here, nonacademic and academic institutions within each continent appear to be consistent in using the same definitions for close and negative margins. However, the survey findings strongly indicate that North American and European radiation oncologists differ in their definitions of negative and close margins.
In NA, oncologists appear to be heavily swayed by the NSABP B-06 definition of the tumor margin, which deems a margin to be positive only if carcinoma cells are found at the inked margin of the specimen (Fig. 1). Slightly less than half of the North Americans used the B-06 definition as compared with roughly one fourth of Europeans (Fig. 2). The next most frequent definition of a negative margin among North Americans was the 2-mm margin chosen by nearly one fifth of the respondents. In terms of defining close margins, most North Americans chose either <1 mm or <2 mm (Fig. 3). There was no significant regional variability found within the North American continent, and there was no difference between respondents from academic and nonacademic institutions regarding margin definition (Table 3).
Radiation oncologists in Europe chose a wider excision margin and appeared, in part, to be influenced by the experience of the Institut Gustave-Roussy, France,6,11 EORTC31 and the Milan quadrantectomy12,13,45 studies. Slightly more than half required a distance of 3 mm, 5 mm, or 10 mm between tumor cells and ink to consider a margin negative. Comfort with a margin of <5 mm ranged from 22.8% to 33.7% and was consistent for all geographic regions in this category. For defining close margins, Europeans showed more widespread variation across all the categories of answer. Unlike the United States, there were statistically significant variations noted between different geographic regions of Europe for both negative and close margins. For example, respondents from Germany–Austria–Switzerland and Italy–Greece required larger distances between tumor cells and ink to consider a margin negative and also considered even the most generous response category (<5 mm) to be a close margin. Although most European trials do not specify an ideal margin,47 the Italian studies recommend a minimum margin of 1 to 2 cm.48 Marked variation in compliance (37–89%) with the breast cancer treatment guidelines in Italy has been noted.49 Respondents from Northern Europe, including The Netherlands, Sweden, Finland, and Denmark, however, more often chose a definition like the NSABP B-06 for negative margins and most often considered only <1 mm to be a close margin. This is consistent with the Danish Breast Cancer Group trial5 requiring only free gross margins, similar to the American NCI trial.3 The Dutch also used a less stringent definition, in which margins were considered to be involved only if there was DCIS or invasive ductal carcinoma microscopically present at the surface of the specimen. The Dutch study reported a very low rate of local recurrence, however, which may be secondary to the wider excision of 1- to 2-cm margins of macroscopically normal tissue and radiation doses of 75 Gy, compared with the more standard 50 to 65 Gy given in the United States.50 Thus, comparisons between these countries and the United States are difficult. Regional variability in Europe has also been reported in an EORTC study. Seven EORTC centers, consisting of hospitals from The Netherlands, Belgium, Denmark, Italy, and the United Kingdom, were analyzed with the intent of standardizing surgical reporting for BCT. Variation in practice was seen in terms of the likelihood of 1-step excision, amount of skin resection, size of excision specimen, and the expected tumor-free margins. Six of the 7 centers aimed at a minimal 1 to 2 cm macroscopic margin without tumor, although 1 center used <1 cm in 80% of cases. Thus, although all participating centers performed a “wide local excision,” the volume of tissue removed and the extent of surgical margins were considerably different. The surgeries were not guided by a fixed margin or by the tumor size.51
Recommendations for Reexcision
The decision to recommend reexcision is difficult and can depend on the pathologist's handling of the tumor specimen. Proper orientation of the specimen is key to guiding reexcision or even the radiation boost, and many pathologists will ink all 6 sides separately. Reexcision recommendations were not evaluated in the initial survey, and therefore no European data were available for when a reexcision was recommended. Data from the EORTC suggest that reexcision is less commonly undertaken, because more tissue is taken at the first excision.51 Recommendations for reexcision by American radiation oncologists were relatively consistent in this study. The presence of high-grade DCIS at or near a margin was viewed as equally concerning as invasive carcinoma for all margin categories. The great majority would recommend reexcision if there were positive margins with invasive cancer or DCIS. However, less than 40% would always recommend reexcision if tumor cells were at least 1 mm from inked margins and less than 20% if tumor cells were present at least 2 mm from the inked margins.
Strengths and Limitations of This Study
This study is, to our knowledge, the first international survey attempting to document the perception of negative and close margins among radiation oncologists practicing BCT. A large number of responses were obtained from diverse regions of Europe and NA and from physicians practicing in a variety of institutional settings. This allowed a unique insight into the ways that practices may vary and helped to identify issues that require further clinical investigation. One potential limitation is the possibility that the self-reported data collected in this study may not correspond to actual physician practices. Nevertheless, physician surveys have often been used to identify controversial issues and regional variations, not only in other oncologic areas,52–54 but also in other medical and surgical fields,55 and they remain a useful, if incomplete, tool for illuminating the complex nature of medical decision-making and practice.56
Another concern is the possibility that those who responded to this survey were not representative of the broader population of physicians in their respective countries. Particularly when considering certain regions of Europe from which only low numbers of responses were obtained, there may be concern that some of the variations observed may have been the result of chance or selection bias. Several factors help to mitigate this concern. First, it is reasonable to assume that the true response rate was much higher than the modest 24% we conservatively calculated for the first survey and the 32.5% for the second survey, because it was impossible to exclude from the denominator those physicians who were asked not to respond because they saw fewer than 5 patients with breast cancer per month. Second, any selection bias would most likely act in the same direction in all countries and thus would be an unlikely explanation for the key finding of this study, which is the international divergence. Third, demographic characteristics of respondents were broadly similar between the different regions of the United States, and there were no indications of an unrepresentative sample when one compares demographic characteristics such as the mix of academic and private practitioners responding to internal ASTRO and ESTRO membership information or published demographic data.57 Finally, because the margins questions were part of a more general survey of practice patterns with respect to breast cancer treatment,24–26 it is unlikely that respondents self-selected based on their feelings toward the issue of margins in particular, and yet this was 1 of the areas that demonstrated a great international variation, as originally hypothesized.
Internal ASTRO (personal communication, 2004) and ESTRO (personal communication, 2004) membership information indicates that approximately one fourth of members who indicated an area of specialization were particularly interested in breast cancer—very close to the number of respondents to our survey. It is reasonable to assume that members with an interest or expertise in breast cancer are the most likely to have responded to this survey, and to the extent that this is the case, it is perhaps even more striking to note the extent of variability in responses regarding the perception of negative or close margins, because respondents most likely represent those most informed about breast cancer research and treatment. Thus, we feel that the findings of this study were a reliable representation of the diversity of clinical practice in the radiotherapeutic management of early-stage breast cancer.
CONCLUSIONS
Therefore, the results from this survey showed that there are significant differences in the perception of negative and close margins among radiation oncologists in NA and Europe. Overall, the Europeans favored a larger margin width (5 mm). Interestingly, however, only 46% of respondents in NA considered the NSABP definition of no tumor cells seen at the inked margins as sufficient for defining negative margins, yet this is considered the dominating definition, because other definitions using a distance from the inked margins were used by 4.9% (<10 mm) to 21.8% (<2 mm) of respondents. Regional variations within Europe demonstrate the importance of cultural predispositions, which may themselves be rooted in physicians’ tendency to rely more heavily on data and instructions generated in studies close to home. In general, “grossly negative” margins predict for a low local recurrence rate that is further decreased when microscopic criteria are applied. However, given these large differences in self-reported practice patterns and the importance of margin status in local control in BCT, a universal definition of negative margins and recommendations for reexcision are needed.
ACKNOWLEDGMENTS
The authors thank those physicians who took the time to complete this comprehensive survey and Dr. Lisa Kachnic for discussion. The authors also acknowledge the valuable effort of Todd Karstaedt, ASTRO Membership Coordinator, and Lean Minnen, ESTRO Membership Coordinator. The authors are also grateful to Rebecca Gelman of the Dana Farber Cancer Center and to Nancy Briton, PhD, from the Survey and Measurement Core, Dana Farber/Harvard Cancer Center, for their statistical advice.
Footnotes
This work was partially supported by the Susan and Michael Schechter Research Fund.
The study was presented at the American Society of Therapeutic Radiology and Oncology (ASTRO) meeting in Salt Lake City, October 18–23, 2003.
*The following regional definitions were used within North America: Can, Canada; Northeast, Maine, Vermont, New Hampshire, Massachusetts, Rhode Island, Connecticut, New York, Pennsylvania, and New Jersey; South Atlantic, Delaware, District of Columbia, Maryland, West Virginia, Virginia, North Carolina, South Carolina, Georgia, and Florida; Midwest, Wisconsin, Michigan, Illinois, Indiana, Ohio, Minnesota, North Dakota, South Dakota, Iowa, Nebraska, Kansas, and Missouri; South, Kentucky, Tennessee, Mississippi, Alabama, Oklahoma, Arkansas, Texas, and Louisiana; Mountain, Montana Idaho, Wyoming, Nevada, Utah, Colorado, Arizona, and New Mexico; Pacific, Washington, Oregon, California, Alaska, and Hawaii.
Reprints: Alphonse Taghian, MD, PhD, Massachusetts General Hospital, Department of Radiation Oncology, Cox 302, 100 Blossom Street, Boston, MA 02114. E-mail: ataghian@partners.org.
REFERENCES
- 1.National Institutes of Health Consensus Development Panel Consensus Statement. Treatment of early-stage breast cancer. J Natl Cancer Inst Monogr. 1992;11:11–15. [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson J, Danforth D, Cowan K, et al. Ten-year results of the National Cancer Institute's randomized trial of breast conservation versus mastectomy for stage I and II breast cancer. N Engl J Med. 1995;332:907–911. [DOI] [PubMed] [Google Scholar]
- 4.van Dongen J, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. [DOI] [PubMed] [Google Scholar]
- 5.Blichert-Toft M, Rose C, Anderson JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life table analysis. J Natl Cancer Inst Monogr. 1992;11:19–26. [PubMed] [Google Scholar]
- 6.Arriagada R, Le MG, Rochard F, et al., for the IGR Breast Cancer Group. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with fifteen years of follow-up. J Clin Oncol. 1996;14:1558–1564. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 8.Fisher E, Anderson S, Tan-Chiu E, et al. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer. 2001;91:1679–1687. [PubMed] [Google Scholar]
- 9.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. [DOI] [PubMed] [Google Scholar]
- 10.van Dongen J, Bartelink H, Fentiman I, et al. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Inst Monogr. 1992;11:15–18. [PubMed] [Google Scholar]
- 11.Sarrazin D, Le M, Arriagada R, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol. 1989;14:177–184. [DOI] [PubMed] [Google Scholar]
- 12.Veronisi U, Salvadori B, Luini A, et al. Conservative treatment of early breast cancer: long-term results of 1232 cases treated with quadrantectomy, axillary dissection, and radiotherapy. Ann Surg. 1990;211:250–256. [PMC free article] [PubMed] [Google Scholar]
- 13.Veronesi U, Volterrani F, Luini A, et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer. 1990;26:671–673. [DOI] [PubMed] [Google Scholar]
- 14.Fowble B, Schultz D, Overmoyer B, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys. 1994;30:23–69. [DOI] [PubMed] [Google Scholar]
- 15.Fowble B, Schultz DJ, Overmoyer B, et al. The influence of young age on outcome for life, relapse, and second primary tumors. Int J Radiat Oncol Biol Phys. 1992;23:969–975.1322389 [Google Scholar]
- 16.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. [DOI] [PubMed] [Google Scholar]
- 17.Recht A, Connolly JL, Schnitt, et al. The effect of young age on tumor recurrence in the treated breast after conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1988;14:3–10. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz J, Jacquiemier J, Amalric R, et al. Breast-conserving therapy for macroscopically multiple cancers. Ann Surg. 1990;212:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedwinek JM, Perez CA, Kramer S, et al. Irradiation as the primary management of stage I and II adenocarcinoma of the breast. Cancer Clin Trials. 1980;3:11–18. [PubMed] [Google Scholar]
- 20.Chu A, Cope O, Russo R, et al. Patterns of locoregional recurrence and results in stages I and II breast cancer treated by irradiation following limited surgery. Am J Clin Oncol. 1984;7:221–229. [DOI] [PubMed] [Google Scholar]
- 21.Harris JR, Botnick L, Bloomer WD, et al. Primary radiation therapy for early breast cancer: the experience at the Joint Center for Radiation Therapy. Int J Radiat Oncol Biol Phys. 1981;7:1549–1552. [DOI] [PubMed] [Google Scholar]
- 22.Smitt M, Nowels K, Carlson R, et al. Predictors of reexcision findings and recurrence after breast conservation. Int J Radiat Oncol Biol Phys. 2003;57:979–985. [DOI] [PubMed] [Google Scholar]
- 23.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–393. [DOI] [PubMed] [Google Scholar]
- 24.Taghian A, Jagsi J, Makris A, et al. Results of a survey regarding irradiation of the internal mammary chain in patients with breast cancer: practice is culture-driven rather than evidence-based. Int J Radiat Oncol Biol Phys. 2004;60:706–714. [DOI] [PubMed] [Google Scholar]
- 25.Ceilley E, Jagsi R, Goldberg S, et al. The radiotherapeutic management of invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61:365–373. [DOI] [PubMed] [Google Scholar]
- 26.Ceilley E, Jagsi R, Goldberg S, et al. The management of ductal carcinoma in-situ in North America and Europe: results of a survey. Cancer. 2004;101:1958–1967. [DOI] [PubMed] [Google Scholar]
- 27.Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol. 2001;19:2254–2262. [DOI] [PubMed] [Google Scholar]
- 28.Lee A, Gurland J. Size and power of tests for equality of means of two normal populations with unequal variances. J Am Stat Assoc. 1975;70:933–941. [Google Scholar]
- 29.Mantel N. Chi-square tests with one degree of freedom; extensions of the Mantel-Haenszel procedure. J Am Stat Assoc. 1963;58:690–700. [Google Scholar]
- 30.Andersen E. The Statistical Analysis of Categorical Data, 2nd ed. Berlin: Springer-Verkag; 1991. [Google Scholar]
- 31.van Dongen J, Bartelink H, Fentiman IS, et al. Factors influencing local relapse and survival and results of salvage treatment after breast-conserving therapy in operable breast cancer: EORTC trial 10801, breast conservation compared with mastectomy in TNM stage I and II breast cancer. Eur J Cancer. 1992;28A:801–805. [DOI] [PubMed] [Google Scholar]
- 32.Recht A, Come S, Henderson I, et al. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996;334:1356–1361. [DOI] [PubMed] [Google Scholar]
- 33.Wazer D, Jabro G, Ruthazer R, et al. Extent of margin positivity as a predictor for local recurrence after breast conserving irradiation. Radiat Oncol Investig. 1999;7:111–117. [DOI] [PubMed] [Google Scholar]
- 34.Pittinger T, Maronian NC, Poulter CA, et al. Importance of margin status in outcome of breast-conserving surgery for carcinoma. Surgery. 1994;116:605–608; discussion 608–609. [PubMed]
- 35.Horiguchi J, Lino Y, Takei H, et al. Surgical margin and breast recurrence after breast-conserving therapy. Oncol Rep. 1999;6:135–138. [PubMed] [Google Scholar]
- 36.DiBiase S, Komarnicky LT, Schwartz GF, et al. The number of positive margins influences the outcome of women treated with breast preservation for early stage breast carcinoma. Cancer. 1998;82:2212–2220. [PubMed] [Google Scholar]
- 37.Schnitt S, Abner A, Gelman R, et al. The relationship between microscopic margins of resection and the risk of local recurrence in breast cancer patients treated with conservative surgery and radiation therapy. Cancer. 1994;74:1746–1751. [DOI] [PubMed] [Google Scholar]
- 38.Park C, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18:1668–1675. [DOI] [PubMed] [Google Scholar]
- 39.Gage I, Schnitt S, Nixon A, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer. 1996;78:1921–1928. [DOI] [PubMed] [Google Scholar]
- 40.Freedman G, Fowble B, Hanlon A, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys. 1999;44:1005–1015. [DOI] [PubMed] [Google Scholar]
- 41.Smitt M, Nowels K, Zdeblick M, et al. The importance of the lumpectomy surgical margin status in long term results of breast conservation. Cancer. 1995;76:259–264. [DOI] [PubMed] [Google Scholar]
- 42.Slotman B, Meyer OW, Njo KH, et al. Importance of timing of radiotherapy in breast conserving treatment for early stage breast cancer. Radiother Oncol. 1994;30:206–212. [DOI] [PubMed] [Google Scholar]
- 43.Borger J, Kemperman H, Hart A, et al. Risk factors in breast conservation therapy. J Clin Oncol. 1994;12:653–660. [DOI] [PubMed] [Google Scholar]
- 44.Ryoo M, Kagan AR, Wollin M, et al. Prognostic factors for recurrence and cosmesis in 393 patients after radiation therapy for early mammary carcinoma. Radiology. 1989;172:555–559. [DOI] [PubMed] [Google Scholar]
- 45.Mariani L, Salvadori B, Marubini E, et al. Ten year results of a randomised trial comparing two conservative treatment strategies for small size breast cancer. Eur J Cancer. 1998;34:1156–1162. [DOI] [PubMed] [Google Scholar]
- 46.Petit J, Garusi C, Greuse M, et al. One hundred and eleven cases of breast conservation treatment with simultaneous reconstruction at the European Institute of Oncology (Milan). Tumori. 2002;88:41–47. [PubMed] [Google Scholar]
- 47.Bartelink H, Garavaglia G, Johansson KA, et al. Quality assurance in conservative treatment of early breast cancer. Report on a consensus meeting of the EORTC Radiotherapy and Breast Cancer Cooperative Groups and the EUSOMA (European Society of Mastology). Radiother Oncol. 1991;22:323–326. [DOI] [PubMed] [Google Scholar]
- 48.Valdagni R, Amichetti M, Ciocca M. Patterns of radiotherapy for early breast cancer in Northern Italy compared with European and national standards. Radiother Oncol. 1999;51:79–85. [DOI] [PubMed] [Google Scholar]
- 49.Grilli R, Apolone G, Marsoni S, et al. The impact of patient management guidelines on the care of breast, colorectal, and ovarian cancer patients in Italy. Med Care. 1991;29:50–63. [DOI] [PubMed] [Google Scholar]
- 50.Harris J, Gelman R. What have we learned about risk factors for local recurrence after breast-conserving surgery and irradiation? J Clin Oncol. 1994;12:647–649. [DOI] [PubMed] [Google Scholar]
- 51.Christiens M, Cataliotti L, Fentiman I, et al. Comparison of the surgical procedures for breast conserving treatment of early breast cancer in seven EORTC centres. Eur J Cancer. 1996;32A:1866–1875. [DOI] [PubMed] [Google Scholar]
- 52.Fowler F, McMaughton-Collins M, Albertson PC, et al. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283:3217–3222. [DOI] [PubMed] [Google Scholar]
- 53.Joffe S, Weeks JC. Views of American oncologists about the purposes of clinical trials. J Natl Cancer Inst. 2002;94:1847–1853. [DOI] [PubMed] [Google Scholar]
- 54.Emanuel EJ, Fairclough D, Clarridge BC, et al. Attitudes and practices of US oncologists regarding euthanasia and physician-assisted suicide. Ann Intern Med. 2000;133:527–532. [DOI] [PubMed] [Google Scholar]
- 55.Meier D, Emmons CA, Wallenstein S, et al. A national survey of physician-assisted suicide and euthanasia in the United States. N Engl J Med. 1998;338:1193–1201. [DOI] [PubMed] [Google Scholar]
- 56.Greenwald H, Hart LG. Issues in survey data on medical practice: some empirical comparisons. Public Health Rep. 1986;101:540–546. [PMC free article] [PubMed] [Google Scholar]
- 57.ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56:309–318. [DOI] [PubMed] [Google Scholar]






