Abstract
Objective:
To conduct a retrospective study of 39 patients with papillary carcinoma of the thyroid with histologic vascular invasion (VI+) and 361 patients without any sign of vascular invasion (VI−).
Summary Background Data:
In the present study, we undertook a retrospective analysis of papillary carcinoma of the thyroid to assess whether histologically determined vascular invasion can be considered a predictive factor for prognosis.
Methods:
By means of a retrospective study, we evaluated the department's database of patients with papillary thyroid carcinoma who had undergone total thyroidectomy from January 1993 to December 1999.
Results:
Group I consisted of papillary carcinoma without any sign of vascular invasion (VI−) comprising 361 patients. Group II consisted of papillary carcinoma with vascular invasion (VI+) comprising 39 patients. At the time of diagnosis, we observed no metastases in patients with VI−, whereas a pulmonary metastasis was observed in 1 patient with VI+ (P = 0.0023). In 3.6% patients with VI− and in 20.5% patients with VI+, we observed recurrences in the regional lymph nodes (P < 0.001); we observed 6 (1.66%) distant metastases in patients with VI− and in the 12.8% patients with VI+ (P < 0.001). Three patients with VI+ (7.7%) and 2 patients with VI− (0.6%) died of tumor-related causes; these figures were found to be statistically significant (P < 0.001).
Conclusions:
In papillary carcinoma, it should be noted that histologic vascular invasion may be considered as a sign of an increased tendency toward hematogenic invasion and consequent increase in the relative percentage of metastases; ultimately, this means a poorer prognosis. In the presence of risk factors indicating a possible increase in biologic aggressiveness, adequate postoperative treatment and close follow up become essential.
A retrospective analysis of thyroid papillary carcinomas to assess whether histologically determined vascular invasion could be considered a predictive factor for prognosis is presented. In papillary carcinoma, it should be noted that histologic vascular invasion may be taken as a sign of an increased tendency toward hematogenic invasion, consequent increase of metastases, and of a poorer prognosis.
Approximately 75% of thyroid cancers are papillary carcinomas. A number of authors have analyzed the prognostic factors that can affect survival. The prognosis for thyroid carcinoma has been reported previously,1–4 and much attention has been focused on the influence of such variables as tumor stage and patient sex and age.
Papillary thyroid carcinoma tends to metastasize through the lymphatic system, with cervical metastasis occurring in 40% to 60% of the patients diagnosed.5 Despite this high frequency, cervical nodal metastasis has been shown to be only a minor predictor of prognosis, affecting recurrence rather than mortality.6–10 Histologic vascular invasion is more frequently associated with follicular thyroid carcinoma, where it has been found to be a negative prognostic indicator.11,12
Although histologic vascular invasion occurs in 2% to 14% of papillary carcinomas,5 its prognostic significance has not been analyzed thoroughly.11 Gardner5 recently postulated that vascular invasion, either intrathyroidal or extrathyroidal, might be associated with metastasis at initial diagnosis and with a higher incidence of tumor recurrence.
In the present study, we conduct a retrospective analysis of papillary carcinomas of the thyroid to assess whether histologically determined vascular invasion can be considered a predictive factor for prognosis.
MATERIALS AND METHODS
We carried out a retrospective study of patients with papillary thyroid carcinoma who had undergone total thyroidectomy at the Surgical Science Department of “La Sapienza” University of Rome from January 1, 1993, to December 31, 1999.
The patients were assigned to 2 groups on the basis of clinical–pathologic features. The first group was made up of patients who had been classified after histologic examination as being affected by papillary carcinoma with vascular invasion (VI+). This group was distinguished from the second group, which was made up of patients histologically classified as having papillary carcinoma without any sign of vascular invasion (VI−).
In this work, histologic vascular invasion is defined as groups of tumor cells attached to the blood vessel wall and projecting into the lumen of blood vessels in the capsule or outside the tumor. The intravascular portion of tumor cells is often covered by a layer of endothelial cells (Fig. 1).
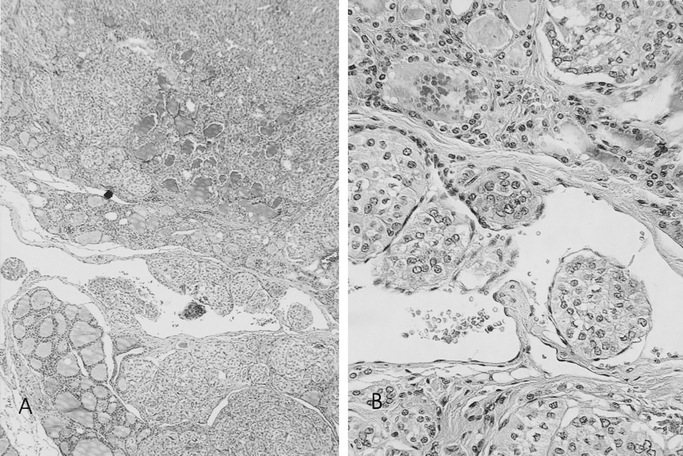
FIGURE 1. Characteristic histologic features of vascular invasion. Hematoxylin–eosin stains elastic fiber around capillary vessels. Papillary carcinomas show blood vessel. (A, original magnification ×5; B, original magnification ×20).
Thyroid tests were carried out using 3-mm histologic sections microscopically and then histologically by hematoxylin–eosin staining. All the histologic specimens were analyzed by the same pathologist (MA).
We also analyzed the various histologic subtypes of papillary carcinoma: pure, sclerosing, tall cell, follicular variant, and oxyphil, with VI+ and VI−.
Information regarding background variables was included for the 2 groups, VI+ and VI−: sex, age, mean neoplasm diameter (largest dimension in centimeters), multifocality, primary tumor extension (intrathyroidal growth, tumor growth in the thyroid capsule, and extrathyroidal extension), positive lymph nodes, as confirmed by postoperative histologic examination, and metastases at initial diagnosis.
All the patients underwent total thyroidectomy. Patients with preoperative fine needle aspiration (FNA) diagnosis of carcinoma also underwent central lymphectomy (perirecurrential lymphectomy and anterior superior mediastinum lymphectomy). This occurs also in the case of laterocervical lymph nodes positive for carcinoma, laterocervical lymphectomy. The patients were also subsequently subjected to l-thyroxin therapy at thyroid-stimulating hormone (TSH)-suppressive doses.
The neoplasm was staged both as a function of histologic vascular invasion and of the various different histologic subtypes. The mean follow-up period was 6.6 years (8.4 ± 6.5).
During follow up, an assessment was made of laterocervical metastases (lymph node recurrences), distant metastases, and death resulting from cancer-related causes.
Moreover, recurrences were postulated in cases with thyroglobulin values >5 μg/L (as measured by the immunoradiometric method) or of whole-body scan (WBS) positivity, subsequently confirmed by standard x-ray, computed tomography (CT), and nuclear magnetic resonance (NMR). Surgically untreatable metastases were treated with 131I or by radiotherapy.
A statistical method was used to estimate the presence of lymph node recurrences in the 2 groups (VI− and VI+), as well as distant metastases and mortality.
Statistical analyses were performed using the chi-squared test and Kaplan-Meier actuarial survival curves were generated for each group. For the chi-squared test, significance at the levels of P < 0.05 and P < 0.001 was considered. Levels of P > 0.05 were considered nonsignificant (NS). The cumulative rates of metastases and disease-specific deaths were compared using Kaplan-Meier plots. The log-rank test was used to evaluate the differences between curves. Data analysis was performed using the SPSS statistical package for Windows (release 10.0; SPSS, Inc., Chicago, IL).
The effect of histologic vascular invasion (VI+), and other variables, on survival was considered in our analysis. Furthermore, using the Cox model, we produced a more precise estimate of the prognostic variables on the survival effect through the confidence interval.
The Cox model (Cox, 1972, Esplace), as used in the paper, is the following: h(t) = h0 (t)exp βT ×, where the baseline hazard function h0(t) is modified multiplicatively by covariates (including the group indicator). The parameter vectorβ, which gives the proportional change that can be expected in the hazard related to changes in the explanatory variables, is estimated by maximizing a partial likelihood.
The Cox model included “independent variables”: histologic vascular invasion (group), age, sex, histology, diameter (>1.5 cm) of the cancer, lymph nodal and distant metastasis at diagnosis, multifocality, forms locally invasive to extrathyroidal soft tissue (pT4), infiltration capsule, infiltration prethyroidal soft tissues, infiltration muscles, and stage of cancer.
An approximate test of significance for each variable was carried out by dividing the regression estimate (β) by its standard error (SE) and comparing the result with the standard normal distribution.
RESULTS
During the period 1993–1999, 421 patients underwent surgery for papillary thyroid carcinoma; 21 patients were excluded from the present study because they were lost to follow up.
The 2 groups were formed as follows: group I, with 361 patients with no histologically confirmed vascular invasion (VI−), and group II with 39 patients showing histologic signs of vascular invasion (VI+).
The female/male ratio in group I was 2.9 (268 females and 93 males) and 2.5 in group II (28 females and 11 males).
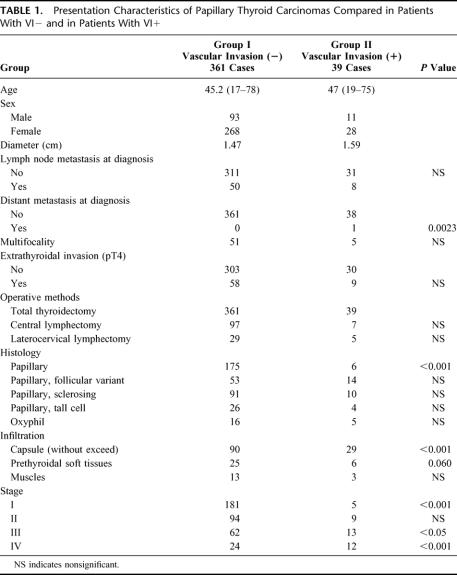
Significant age-related differences between the 2 groups and mean neoplasm diameters differences were not found. Laterocervical lymph node pathology at diagnosis, as confirmed by postoperative histologic examination, was found in 50 VI− patients (13.9%) and in 8 VI+ patients (20.6%) (P = NS). At the time of diagnosis, we observed no distant metastases in patients with VI−, whereas a pulmonary metastasis was observed in 1 patient with VI+ (P = 0.0023).
Multifocality was observed in 51 patients with VI− and in 5 patients with VI+ (P = NS). Forms locally invasive to extrathyroidal soft tissue (pT4) were found in 58 patients with VI− (16.1%) and in 9 patients with VI+ (23.1%) (P = NS).
All the patients underwent total thyroidectomy. Systematic lymph node dissection of the central compartment (perirecurrential lymph nodes and upper anterior mediastinum lymph nodes) was carried out in 97 patients with VI− (26.9%) and in 7 patients with VI+ (17.9%) (P = NS); ipsilateral laterocervical lymph node dissection was performed in 29 patients with VI− (8%) and in 5 patients with VI+ (12.8%) (P = NS).
The characteristics of papillary thyroid carcinoma in patients VI− and VI+ are shown in Table 1. Infiltration, without exceeding the capsule, in patients with VI− was found in 90 patients (24.9%) and in 29 cases in patients with VI+ (74.4%) (P < 0.001).
TABLE 1. Presentation Characteristics of Papillary Thyroid Carcinomas Compared in Patients With VI− and in Patients With VI+
Invasion of the prethyroidal soft tissues in patients VI− was observed in 25 cases (6.9%) and in 6 cases VI+ patients (12.8%) (P = NS). Infiltration of the prethyroid muscles was found in 13 VI− patients (3.6%) and in 3 VI+ patients (7.7%) (P = NS).
Carcinomas with VI− and VI+ at stage III were observed, respectively, in 62 and 13 cases (P < 0.05) and at stage IV in 24 and 12 cases, respectively (P < 0.001).
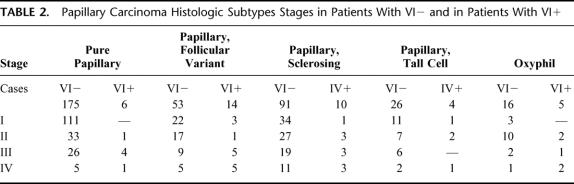
Table 2 shows the histologic subtype stages in patients with VI− and in patients with VI+. All the patients underwent TSH suppressive therapy after surgery. Fifteen percent of the patients with VI− and 23% of the patients with VI+ received postoperative radioiodine ablative therapy. Twenty-one percent of the patients with VI− and the 27% of the patients with VI+ received radioiodine for prophylaxis.
TABLE 2. Papillary Carcinoma Histologic Subtypes Stages in Patients With VI− and in Patients With VI+
No local recurrences in either group were observed. In 13 patients (3.6%) with VI− and in 8 patients (20.5%) with VI+, we observed recurrences in the regional lymph nodes (P < 0.001) (Fig. 2). We found 6 (1.66%) distant metastases in patients with VI−: 5 cases affecting the lungs and 1 case the bones. We found 5 (12.8%) distant metastases in patients with VI+: 3 cases affecting the lungs, 1 case the bones, and 1 case the liver (P < 0.001) (Fig. 3). During follow up, 2 patients with VI− (0.6%) and 3 patients with VI+ (7.7%) died of tumor-related causes (P < 0.001) (Fig. 4).
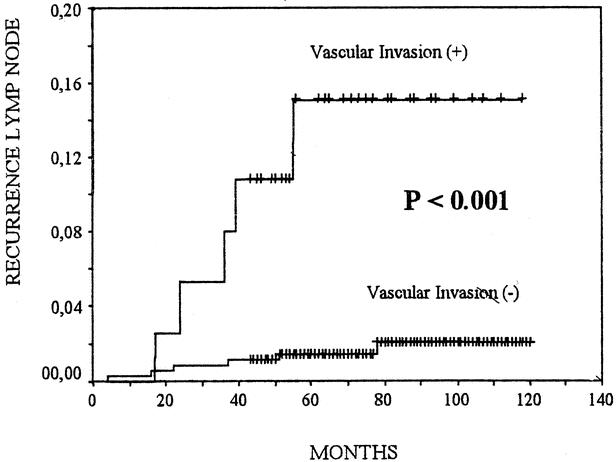
FIGURE 2. Cumulative risk of lymph node metastases of patients with vascular invasion (+) or (−) papillary thyroid carcinomas. The Kaplan-Meier method is used. The P value between patients with vascular invasion (+) and those with vascular invasion (−) was computed with the log-rank test.
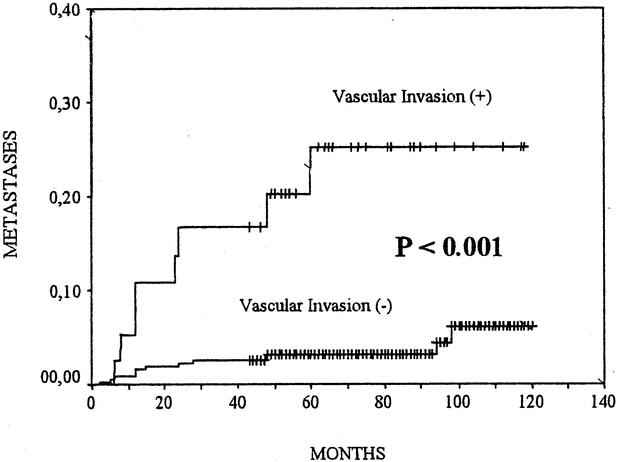
FIGURE 3. Cumulative risk of distant metastases of patients with vascular invasion (+) or (−) papillary thyroid carcinomas. The Kaplan-Meier method is used. The P value between patients with vascular invasion (+) and those with vascular invasion (−) was computed with the log-rank test.
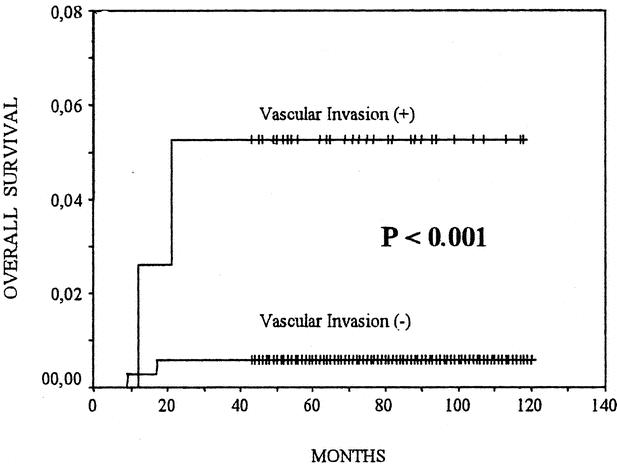
FIGURE 4. Postoperative survival of patients with vascular invasion (+) or (−) papillary thyroid carcinomas. The Kaplan-Meier method for postoperative overall survival is used. The P value between patients with vascular invasion (+) and those with vascular invasion (−) was computed with the log-rank test.
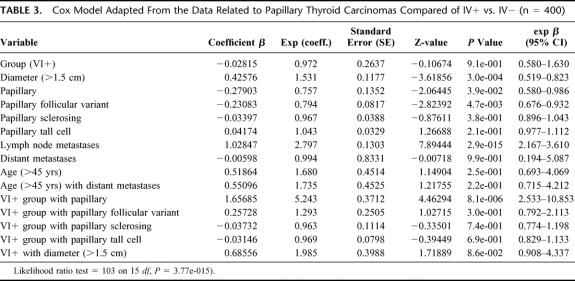
Multivariate analysis (test di Cox) demonstrated that the variable VI+ is not an independent predictor of survival. The statistically significant variables are: diameter >1.5 cm (P = 0.001) and lymph node metastases (P = 0) characterized by significant worsening of the prognosis (β = 10.2). Variable histology papillary (P = 0.039) and variable histology papillary follicular variant (P = 0.0047) are associated with a favorable prognosis.
The VI+ variable is statistically significant and means an unfavorable prognosis in association with the variable histology papillary (P < 0.001) and the variable diameter >1.5 cm (P = 0.009). (For example, the estimated hazard in the second case is 0.686, indicating a 31.4% decrease in the risk of death) (Table 3).
TABLE 3. Cox Model Adapted From the Data Related to Papillary Thyroid Carcinomas Compared of IV+ vs. IV− (n = 400)
DISCUSSION
Vascular invasion of the lymph vessels and veins is associated with a high incidence of locoregional and lymph node metastases in gastrointestinal and breast cancer.13–15 Only a few studies have been carried out regarding problems related to vascular invasion in thyroid carcinoma. Various factors are known to play a prognostic role such as sex, age, tumor diameter, capsule invasion, regional and distant metastases at initial diagnosis.6,7,10
Follicular carcinoma of the thyroid is also known to metastasize mainly through the blood, whereas papillary carcinoma does so through the lymph system. In a review of our clinical histories, we found that 45% of follicular carcinomas displayed histologic characteristics typical of vascular invasion. Papillary carcinoma prognostic significance is related to distant metastases at initial diagnosis.10,16 Metastases at initial diagnosis may also be related to histologic vascular invasion.5
In our study, vascular invasion was found in 9.75% of cases, comparable with 7.6% found by Gardner.5 Mai17 found vascular invasion in 17% of the 99 papillary carcinoma cases; Nishida18 reported a total of 46.9% in a study carried out in 256 patients (dropping to 33% for papillary carcinomas with vascular invasion). Paessler19 reported 13% in a study of 253 papillary thyroid carcinomas. These differences may be related to histologic analysis criteria. Our definition of histologic vascular invasion is “groups of tumor cells attached to the blood vessel wall and projecting into the lumen of blood vessels in the capsule or outside the tumor. The intravascular portion of tumor cells is often covered by a layer of endothelial cells.” Nishida18 defined VI as either: “1) tumor cell thrombi in vessels or 2) direct tumor involvement in vessel walls.”
In our study, histologically detected vascular invasion correlates positively with distant metastases at initial diagnosis. This is in line with the findings of Gardner5 who nevertheless differentiated between intra- and extrathyroidal vascular invasion, obtaining significant results, particularly regarding the association with intrathyroidal vascular invasion. In our study, we did not make this distinction because Gardner's findings5 indicated a substantial agreement regardless of the extent of vascular invasion.
One significant factor is the evaluation of the various different histologic subtypes. Although agreeing with the importance of the individual prognostic factors, several authors20–22 claim that prognosis is determined by the greater biologic aggressiveness of the neoplasm and that this greater aggressiveness is expressed through a wide range of histopathologic characteristics. Factors such as nuclear atypia, tumor necrosis, and histologic vascular invasion are considered to be important aspects that determine greater aggressiveness and a greater tendency to metastasize in patients with differentiated thyroid carcinomas.
We have observed how vascular invasion is significantly lower in the less aggressive histologic forms. Signs of vascular invasion were found in 3.31% of pure papillary carcinoma. This percentage is statistically significant compared with sclerosing and tall cell papillary carcinoma (P < 0.05). Vascular invasion is found in 20.9% of cases of the follicular variant of papillary carcinoma. This percentage is statistically significant compared with pure papillary carcinoma (P < 0.01) and with sclerosing papillary carcinoma (P < 0.05); this result confirms the tendency of the follicular forms to invade vascular tissue.
Our results agree with the findings of Asklen and Li Volsi22 who reported histologic vascular invasion in 14% of pure papillary carcinoma, in 30% of tall cell variant, in 33% of “complex solid variant,” and in 75% of solid variants. Multivariate analysis allowed us to show an aspect as the association between pure papillary carcinoma and vascular invasion to date not published in the literature; whereas the histologic pure papillary alone is associated with a significantly favorable prognosis, the same variable (histologic pure papillary) with vascular invasion (VI+) is characterized by significant worsening of the prognosis (Table 3).
In our study, histologic vascular invasion is associated with increased regional lymph node recurrences (P < 0.001) and distant metastases (P < 0.001).
Regarding the increased incidence of distant metastases seen in the presence of intrathyroidal vascular invasion, our results are in general agreement with the findings of Gardner5 and Nishida.18
In addition, our results regarding lymph node recurrences agree with the findings of Nishida.18 He observed a higher percentage of lymph node recurrences in differentiated carcinomas with vascular invasion. Gardner,5 although noting an increase in local and distant recurrences, found that this was not a statistically significant factor.
The greater biologic aggressiveness of papillary carcinoma with vascular invasion was demonstrated in our study by the increased number of forms locally invasive to extrathyroidal soft tissues (pT4) and stage IV tumors. In contrast, a significantly higher incidence of stage I carcinomas was observed in the VI− group.
In our study, a diameter >1.5 cm is associated with a worse prognosis. The association of a tumor with a diameter >1.5 cm and vascular invasion leads to a significant worsening of the prognosis.
The higher incidence of lymph node and distant metastases in patients with vascular invasion can account for the significant increase in mortality associated with the tumor. In our study, we found 3 cases of death of VI+ patients (7.7%) and 2 of VI− patients (0.6%); the mortality comparison was found to be statistically significant (P < 0.001). This finding is in agreement with those of Nishida18 and those of certain previous authors.14,21,23
Univariate analysis showed that vascular invasion provokes a significant increase of lymph node recurrences, of distant metastases, and a worse prognosis. Multivariate analysis demonstrated that vascular invasion is not a single statistically variant, but in association with the variant histologic pure papillary or a tumor with a diameter >1.5 cm, impacts significantly on the prognosis.
Our results show the importance of the concept of differentiated thyroid carcinoma having greater biologic aggressiveness characterized by the overlapping of several different risk factors. Among the latter, in papillary carcinoma, it should be noted that histologic vascular invasion may be considered a sign of an increased tendency toward hematogenic invasion and subsequent increase in the relative percentage of distant metastases and ultimately of a poorer prognosis. The presence of risk factors indicating a possible increase in biologic aggressiveness necessitates adequate postoperative treatment and careful follow up.
Footnotes
Reprints: Laura Falvo, MD, PhD, Via A. Musa n. 5/a 00161 Rome, Italy. E-mail: laurafalvo@mclink.it.
REFERENCES
- 1.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma. A study of the EORTC Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033–1041. [DOI] [PubMed] [Google Scholar]
- 2.Tubiana M, Schlumberger M, Rougier P, et al. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer. 1985;55:794–804. [DOI] [PubMed] [Google Scholar]
- 3.Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–1095. [PubMed] [Google Scholar]
- 4.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 5.Gardner RE, Tuttle RM, Burman KD, et al. Prognostic importance of vascular invasion in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:309–312. [DOI] [PubMed] [Google Scholar]
- 6.Treseler PA, Clark OH. Prognostic factors in thyroid carcinoma. Surg Oncol Clin N Am. 1997;6:555–598. [PubMed] [Google Scholar]
- 7.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. [PubMed] [Google Scholar]
- 8.Akslen LA, Myking AO, Salvesen H, et al. Prognostic importance of various clinicopathological features in papillary thyroid carcinoma. Eur J Cancer. 1992;29A:44–51. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham MP, Duda RB, Recant W, et al. Survival discriminants for differentiated thyroid cancer. Am J Surg. 1990;160:344–347. [DOI] [PubMed] [Google Scholar]
- 10.Hay ID. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1990;19:545–576. [PubMed] [Google Scholar]
- 11.Jorda M, Gonzalez-Campora R, Mora J, et al. Prognostic factors in follicular carcinoma of the thyroid. Arch Pathol Lab Med. 1993;117:631–635. [PubMed] [Google Scholar]
- 12.Donohue JH, Goldfien SD, Miller TR, et al. Do the prognoses of papillary and follicular thyroid carcinomas differ? Am J Surg. 1984;148:168–173. [DOI] [PubMed] [Google Scholar]
- 13.Maehara Y, Orita H, Okuyama T, et al. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245–247. [DOI] [PubMed] [Google Scholar]
- 14.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Kimura T, Miyakawa R, et al. Clinicopathologic study associated with long-term survival in Japanese patients with node-negative breast cancer. Br J Cancer. 2000;82:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. [DOI] [PubMed] [Google Scholar]
- 17.Mai KT, Khanna P, Yazdi HM, et al. Differentiated thyroid carcinomas with vascular invasion: a comparative study of follicular, Hurthle cell and papillary thyroid carcinoma. Pathology. 2002;34:239–244. [DOI] [PubMed] [Google Scholar]
- 18.Nishida T, Katayama S, Tsujimoto M. The clinicopathological significance of histological vascular invasion in differentiated thyroid carcinoma. Am J Surg. 2002;183:80–86. [DOI] [PubMed] [Google Scholar]
- 19.Paessler M, Kreisel FH, LiVolsi VA, et al. Can we rely on pathologic parameters to define conservative treatment of papillary thyroid carcinoma? Int J Surg Pathol. 2002;10:267–272. [DOI] [PubMed] [Google Scholar]
- 20.Chang TC, Kuo SH, How SW. Coefficient of variation of nuclear diameters as a prognostic factor in papillary thyroid carcinoma. Anal Quant Cytol Histol. 1991;13:403–406. [PubMed] [Google Scholar]
- 21.Akslen LA, Myking AO. Differentiated thyroid carcinomas: the relevance of various pathological features for tumor classification and prediction of tumor progress. Virchows Arch A Pathol Anat Histopathol. 1992;421:17–23. [DOI] [PubMed] [Google Scholar]
- 22.Akslen LA, LiVolsi VA. Prognostic significance of histological grading compared with subclassification of papillary thyroid carcinoma. Cancer. 2000;88:1902–1908. [PubMed] [Google Scholar]
- 23.Sakuragi N, Takeda N, Hareyama H, et al. A multivariate analysis of blood vessel and lymph vessel invasion as predictors of ovarian and lymph node metastases in patients with cervical carcinoma. Cancer. 2000;88:2578–2583. [PubMed] [Google Scholar]