Abstract
Background:
Pulmonary complications remain the major cause of postoperative mortality in patients with esophageal cancer undergoing esophagectomy. It was unclear whether this dismal complication has a genetic predisposition. We therefore investigated the role of an angiotensin-converting enzyme (ACE) insertion/deletion polymorphism in developing these complications.
Methods:
We conducted a prospective study including 152 patients with esophageal cancer who underwent esophagectomy in National Taiwan University Hospital between 1996 and 2002. The ACE genotype was determined by polymerase chain reaction amplification of leukocyte DNA obtained before surgery. The serum ACE concentration was determined by enzyme-linked immunosorbent assay.
Results:
Thirty-five patients (23%) developed pulmonary complications following esophagectomy. As compared with patients with the I/I and I/D genotypes, those with the D/D genotype had a higher risk for pulmonary complications (adjusted odds ratio [OR], 3.12; 95% confidence interval [CI], 1.01–9.65). The risk was additively enhanced by combination of the ACE D/D genotype with other clinical risk factors (old age, hypoalbuminemia, and poor pulmonary function). The circulating ACE level was also dose-dependently with the presence of ACE D allele. As compared with the patients with circulating ACE less than 200 ng/mL, the patients with circulating ACE of 200 to 400 ng/mL and over 400 ng/mL had ORs (95% CI) of 2.75 (1.12–6.67) and 15.00 (4.3–52.34) to present with ACE D allele, respectively.
Conclusions:
An ACE insertion/deletion polymorphism might modulate the function of ACE gene and play a role in affecting individual susceptibility to pulmonary injury following esophagectomy in patients of esophageal cancer.
The individual susceptibility to pulmonary complications after esophagectomy for esophageal cancer can be influenced by the angiotensin-converting enzyme polymorphism, which interacts with the factors of patients’ age, preoperative lung function, and serum albumin level to predict an incremental risk of these complications.
Esophageal cancer, one of the most common malignancies in men over age 60, is a major public health concern. Although multimodality treatment has been advocated to improve survival,1 surgery is still the cornerstone of treatment of potentially resectable esophageal cancer. With the advancement of surgical techniques, postoperative morbidity and mortality rates have remarkably improved.2 However, postoperative pulmonary complications develop in 20% to 35% of the patients and account for the major cause of postoperative mortality.3,4
Several preoperative factors related to the development of postoperative pulmonary complications after esophagectomy include age, nutritional status, presence of underlying disease, tumor stage, and lung function.3–7
In addition to the clinical profiles, the variation of host inflammatory response after this major surgical trauma might well play a significant role in determining the individual risk for developing postoperative pulmonary complications. van Sandick et al8 studied the immune response of patients after transhiatal or transthoracic esophagectomy and found that these patients had a profound depression in their monocyte and T-lymphocyte cytokine production. Those who had a lower preoperative interferon-γ production had a higher risk to develop major postoperative infectious complications. On the other hand, esophagectomy patients with postoperative pulmonary complications have a higher production of interleukin-8 and granulocyte elastase activity in bronchoalveolar lavage fluid after surgery.9 Given the association between postesophagectomy pulmonary complications and inflammatory responses in the lung, a genetic predisposition might exist for the risk of these complications. However, to date no genetic factors have been documented.
The angiotensin-converting enzyme (ACE) gene, located on 17q23, encodes the key enzyme to cleave angiotensin I to form angiotensin II, which in turn binds to the angiotensin II receptor and induces vasoconstriction. It is also called kininase II for its ability to degrade bradykinin. The renin-angiotensin system can modulate endothelium function through pathways involved in cellular metabolism, proliferation, or viability. It can induce apoptosis in human or rat alveolar epithelial cells.10 The pathways of inflammatory response can be also modulated by the ACE, which may work through the activation nuclear factor-κB,11 a coordinating factor that increases the gene expression of many cytokines, chemokines, or adhesion molecules.
The intronic deletion of a 287-bp Alu sequence repetitive element (D allele) detected within the ACE gene was associated with a higher serum ACE level.12 Previously, the ACE D/D genotype was found associated with higher susceptibility to and mortality rate of the acute respiratory distress syndrome.13 Individual susceptibility to asthma,14 berylliosis,15 pulmonary fibrosis,16 and sarcoidosis17 could be also modulated by this genetic polymorphism. The D/D genotype was also associated with a poor prognosis in Finnish sarcoidosis patients.18 In this study, we hypothesized that the insertion/deletion ACE polymorphism can influence the function of ACE gene and the development of pulmonary complications following esophagectomy for esophageal cancer. We therefore examined the effect of insertion/deletion ACE polymorphism on the production of circulating ACE and risk of pulmonary complications after esophagectomy for esophageal cancer. Its interaction with other clinical risk factors was also analyzed.
PATIENTS AND METHODS
This study prospectively recruited patients with esophageal cancer who underwent esophagectomy and esophageal reconstruction in National Taiwan University Hospital between November 1996 and July 2002. The patients’ blood was collected before surgery and stored at −70°C until examination. All clinical data were obtained from the medical chart review. The study was approved by the ethical committee of the hospital, and informed consent was obtained from each patient before blood collection. In our hospital, tumors located at or above the middle third thoracic esophagus were resected through right thoracotomy and reconstructed via a retrosternal conduit and left cervical esophageal anastomosis. The stomach was the first choice of organ tissue for esophageal reconstruction. The left side of colon based on the left colic artery would be used if the stomach was unsuitable for reconstruction. Tumors at the lower third thoracic esophagus or near the gastroesophageal junction were resected through a left thoracoabdominal incision and reconstructed via intrathoracic esophagogastrostomy or esophagojejunostomy. The cervical, mediastinal, and abdominal lymph nodes were routinely dissected, except for those who were receiving thoracoabdominal incision, whose nodal dissection was confined in the mediastinum and abdomen. Pulmonary complications were defined by the following criteria:
The presence of respiratory distress after operation necessitating endotracheal intubation, or trachostomy for prolonged ventilator support
Pulmonary infection with evidence by radiologic pulmonary infiltrates and/or presence of pathogenic bacteria in the sputum culture
Pulmonary atelectasis required frequent bronchoscopic toilet or prolonged ventilator support
Other surgical or medical complications recorded included cardiovascular events, anastomotic leakage, graft organ necrosis, recurrent laryngeal nerve injury, wound infections, or other infections. Postoperative 1-month mortality was defined as death occurring within 1 month of hospitalization after esophagectomy.
ACE Genotyping
Genomic DNA was prepared from the leukocytes with serial extraction by phenol and chloroform followed by ethanol precipitation. Using polymerase chain reaction (PCR) amplifications described previously with slight modification,19 the ACE genotyping was performed by staff blinded to all subject data. Briefly, we used primers of ACE-1F: 5′-GCCCTGCAGGTGTCTGCAGCATGT-3′, and ACE-1R: 5′-GGATGGCTCTCCCCGCCTTGTCTC-3′specific for ACE. The PCR mixture consisted of a 30-μL reaction volume, including 50 ng of genomic DNA, 2.5 mM of dNTPs, 3 mM of MgCl2, and 1 U of Fast-Start TaqDNA polymerase (Roche, Germany). The PCR conditions were an initial denaturation step at 94°C for 4 minutes, followed by 35 cycles of denaturation at 94°C for 40 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 60 seconds, and a final extension at 72°C for 10 minutes. The product D and I alleles resulted in 319-bp and 597-bp amplicons, respectively. Whereas the D allele was preferentially amplified, each sample found to have the D/D genotype was subjected to a second round of PCR amplification with a insertion specific primer (ACE-2F: 5′-TGGGACCACAGCGCCCGCCACTAC-3′ and ACE-2R: 5′-TCGCCAGCCCTCCCATGCCCATAA-3′), under the same PCR condition except for an annealing temperature of 60°C. Only the I allele yielded a 335-bp amplicon in this PCR reaction.
Assay of Serum ACE Level
The blood taken from the patients before operation was collected in precooled Na-EDTA tubes, centrifuged at 4°C, and stored at −70°C until analysis. ACE serum level was determined by enzyme-linked immunosorbent assay with a commercially available kit (Chemicon International, Inc, CA).
Statistical Analysis
Values were expressed as the mean ± SD. In Table 1, differences in demographic and clinical data between groups were assessed by the χ2 test. For some values, such as sex, site, N, cell type, and ACE genotype, and 25% of the cells having expected counts less than 5, χ2 may not be a valid test. We used Fisher exact test instead. Mantel-Haenszel χ2 test was used to evaluate the trend of distribution if χ2 test showed significant difference. Crude odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of pulmonary complications were calculated at first without adjusting with other variables, which was followed by multiple logistic regression, stepwise, including the possible significant factors to show the adjusted ORs and 95% CI. The continuous variables were dichotomized or trichotomized according the patient-number distribution. All the analyses were performed using Statistical Analysis Systems, version 8 (SAS Inc, Cary, NC).
TABLE 1. Patient Characteristics and Presence of Pulmonary Complications

RESULTS
Fifteen females and 137 males were enrolled in this study. The mean age of the patients was 61.0 ± 10.9, ranging from 40 to 92 years of age. Forty-one patients (27.2%) had anastomotic leakage; 35 (23.0%) had pulmonary complications; while 16 (10.5%) had other complications, including graft necrosis (1), wound dehiscence (1), wound infection (5), persistent atrial flutter or fibrillation (3), shock (1), ileus adhesions (1), hyperosmolar hyperglycemic nonketotic syndrome (1), acute renal failure (1), tracheal laceration (1), and acute cholecystitis (1).
Of the patients with pulmonary complications, 33 had persistent pulmonary infiltrate in chest image with prolonged respiratory failure, and 2 subsequently developed pleural effusion or empyema requiring further tube or surgical drainage. Patient characteristics are listed in Table 2. Of the patients, 133 (88.1%) had a pathologic diagnosis of squamous-cell carcinoma, while 16 (10.6%) had adenocarcinoma, and 2 (1.3%) patients had other cell types of small-cell carcinoma and carcinosarcoma. Most of the patients (84.9%) underwent 3-field surgery, including thoracotomy, laparotomy, and left cervical esophageal anastomosis. Twenty-one patients underwent left thoracoabdominal incision, and 1 had segmental esophageal resection and reconstruction with free jejunal flap. Eighty-seven patients received neoadjuvant treatment including chemotherapy (6), radiation alone (2), or concurrent chemoradiation (79).
TABLE 2. Clinical and Genetic Characteristics of Study Population
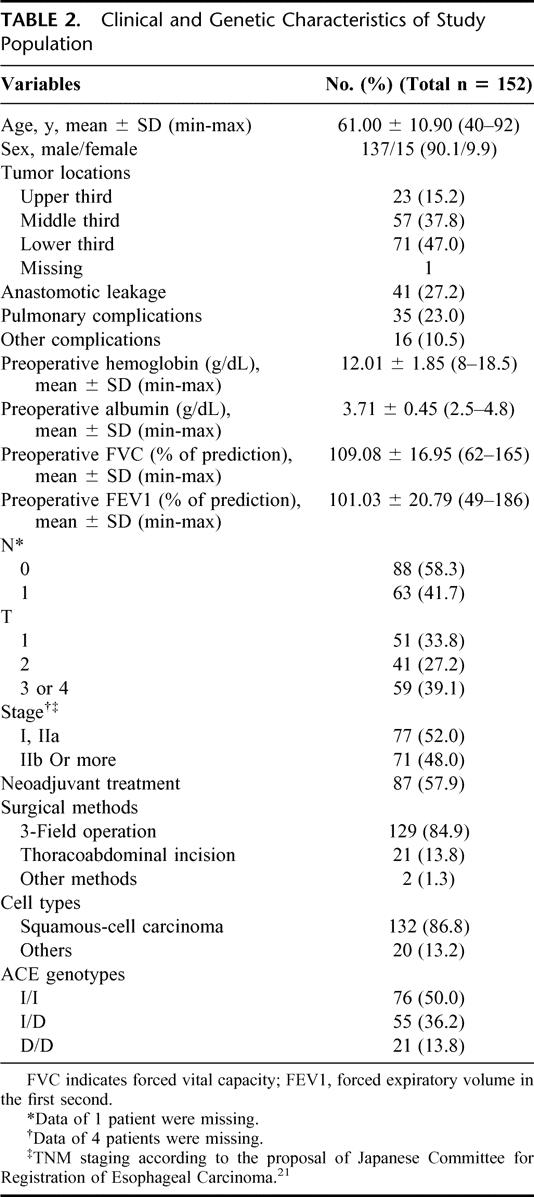
Table 2 also shows the ACE genotypes. The frequencies of the I/I, I/D, and D/D genotypes were 50.0%, 36.2%, and 13.8%, respectively.
The 1-month hospital mortality rate of esophagectomy was strongly associated with the presence of postoperative pulmonary complications, occurring in 25.7% (9/35) of the patients with pulmonary complications as compared with 4.3% (5/117) in the patients without pulmonary complications (P = 0.0001). No significant association was found between the ACE genotype and the 1-month hospital mortality rate (data not shown).
Table 1 demonstrates the preliminary association analysis for developing postoperative pulmonary complications based upon preoperative factors and the ACE polymorphism. The risk of pulmonary complications increased with the patient's age (trend of P = 0.003). A preoperative albumin level lower than 3.5 g/dL was also associated with the tendency to develop postoperative pulmonary complications (P = 0.0005).
Other possible significant factors, including preoperative forced expiratory volume in the first second (FEV1) less than 80% of prediction (P = 0.0106), preoperative forced vital capacity less than 90% of prediction (P = 0.0304), and hemoglobin less than 11 g/dL (P = 0.0393), were also demonstrated. There was a higher incidence to develop pulmonary complications in the patients with ACE D/D genotypes compared with those who had other genotypes (I/D and I/I) (P = 0.0949). Other clinical factors, including neoadjuvant therapy, surgical approaches, tumor cell type, and stage, were not determinants of pulmonary complications.
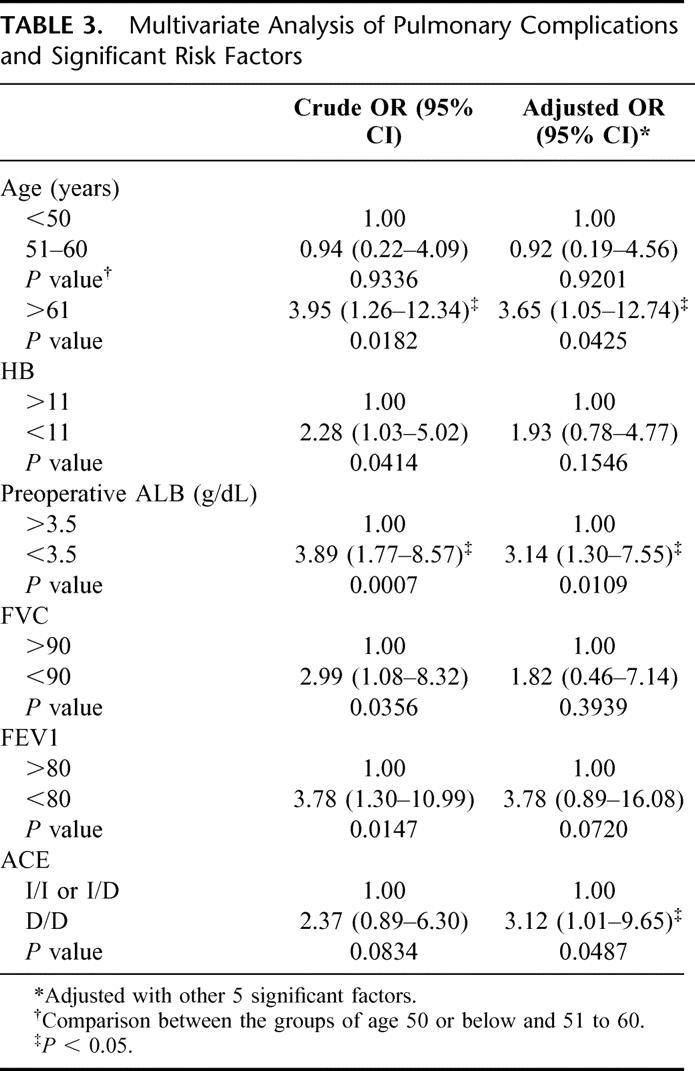
Considering the effect of ACE on vascular remodeling, we also analyzed the association of systemic hypertension with the ACE genotype. There was no significantly different distribution of the ACE genotype and incidence of pulmonary complications in the patients with or without systemic hypertension (data not shown). On the other hand, the AEC D/D genotype did not associate with a higher risk of anastomotic leakage or other complications, as did the pulmonary complications (data not shown). These possible clinical and genetic risk factors were further analyzed by multivariate analysis and are illustrated in Table 3. The patients’ age, preoperative serum albumin, preoperative FEV1 and D/D genotype were found to be significant factors under this model of analysis (Table 3). The adjusted ORs (95% CI) of old age (more than 60 years of age), hypoalbuminemia, and ACE D/D genotype were 3.65 (1.05-12.74), 3.14 (1.30-7.55), and 3.12 (1.01-9.65), respectively. The low FEV1 also had a borderline significant OR (95% CI) of 3.78 (0.89-16.08) (P = 0.072).
TABLE 3. Multivariate Analysis of Pulmonary Complications and Significant Risk Factors
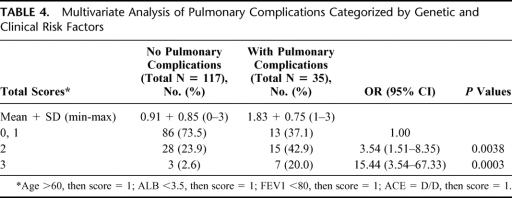
We also analyzed the interaction of ACE genetic polymorphism with other clinical risk factors in Tables 4 and 5. Using the patients with 1 or less than 1 these clinical or genetic risk factor as reference, those who had 2 or 3 of these risk factors had ORs (95% CI) of 3.54 (1.51-8.35) or 15.44 (3.54-67.33), respectively (Table 4). In patients of the ACE I/I or I/D genotypes, having at least 2 of the clinical risk factors increased their risk for pulmonary complications as compared with patients with 1 or less of the risk factors (ORs [95% CI]: 4.66 [1.91-11.38]) (Table 5). The risk would be increased further for those who simultaneously have the ACE D/D genotype and at least 2 of the clinical risk factors (OR [95% CI]: 20.5 [3.7-113.47]) (Table 5).
TABLE 4. Multivariate Analysis of Pulmonary Complications Categorized by Genetic and Clinical Risk Factors
TABLE 5. Comparison of Clinical Risk Profiles and ACE Genotype to Pulmonary Complications
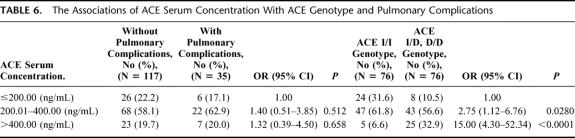
We also found that the presence of the ACE D allele was dose-dependently associated with a higher ACE serum concentration. As compared with the patients with ACE serum concentration less than 200 ng/mL, the patients with ACE serum level between 200 and 400 or more than 400 ng/mL had ORs (95% CI) of 2.75 (1.12-6.76) or 15.00 (4.30-52.34), respectively, to have the ACE D allele in genotype. Although the risk of pulmonary complication seemed to be higher in those patients with higher-level ACE serum level, a statistical significance was not achieved in this association (Table 6).
TABLE 6. The Associations of ACE Serum Concentration With ACE Genotype and Pulmonary Complications
DISCUSSION
This study represents the first report that the individual genetic variance can be a significant factor in the development of pulmonary complications following esophagectomy. An individual with the D/D genotype as compared with the I/I and I/D genotypes has a significantly higher risk for pulmonary complications, after adjustment for other significant clinical factors. The functional significance of the ACE genetic polymorphism was also demonstrated by the influence of ACE genotypes on the circulating ACE level. The presence of ACE D/D genotype was associated a higher level of circulating ACE, although a significance cannot be established between the circulating ACE and the postoperative pulmonary complications.
The effect of the ACE genotype on the risk seemed to be independent from that of the clinical factors (ie, age, nutritional status, or pulmonary function). Both in the clinical low- or high-risk patients, presence of the ACE D/D genotype can increase the risk of postoperative pulmonary complications. However, the risk was more evidently increased in the combination of the genetic and clinical risk factors, with an around 20-fold increase as compared with the both genetic and clinical low-risk patients. On the other hand, the risk was incrementally enhanced with the accumulation of any of the clinical or genetic risk factors, with a 3- to 5-fold increase by each factor. These findings suggested that the postesophagectomy-related pulmonary complications have a multifactor in etiology, including the genetic and clinical factors. This preliminary experience is required to be examined further with trials based on multi-institute collection of more patients.
The association of ACE D/D genotype with the circulating ACE level was compatible with that observed by Rigat et al,12 who found that the ACE D allele was dose-dependently accounting for the 47% variation in the serum of the healthy population. This functional alteration of ACE gene was suggested to be resulted from the linkage disequilibrium with other genes.12 Whether the variation of circulating ACE is responsible for the individual predisposition to pulmonary complications following esophagectomy is required to be clarified further. Although an increased risk of postesophagectomy pulmonary complications can also be observed in the individual with a higher circulation ACE level, a statistical significance cannot be achieved. From the independent presentation of ACE genotypes and systemic hypertension, it is also possible that the ACE insertion/deletion polymorphism influenced the other inflammation-related genes and results in a difference for the individual predisposition for pulmonary complications.
The clinical risk factors found in this study (ie, age, serum albumin, and FEV1) were consistent with that of previous studies3–5,7,20 Nagawa et al5 used a multivariate analysis and identified 3 significant risk factors: vital capacity, presence of liver cirrhosis, and tumor stage. A scoring system based on these 3 factors predicted the development of pulmonary complications with 70% accuracy. In the evaluation of a larger patient population (n = 292) using multivariate analysis, Ferguson and Durkin3 developed another scoring system based on a significant (FEV1) and 2 borderline significant risk predictors (age and performance status). They found this scoring system worked well to predict both the risk of pulmonary and cardiac complications.
In conclusion, our study for the first time demonstrated that there was a genetic predisposition for the pulmonary complications following esophagectomy for esophageal cancer. In addition to the patient's clinical condition, taking into account his or her genetic background can make a more precise prediction of the postesophagectomy pulmonary complication for esophageal cancer. This observation also implies the possibility of prevention of such complications by manipulation of the function of the susceptibility gene once its effect has been confirmed.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Science Council (NSC:91-2314-B-002-231; NSC:91-2314-B-002-233), and National Health Research Institute (NHRI-CN-IN-9007P) of the Republic of China.
Footnotes
Reprints: Jang-Ming Lee, Department of Surgery, National Taiwan University Hospital, 7 Chung-Shang South Road, Taipei, Taiwan, Republic of China. E-mail: ntuhlee@yahoo.com.
REFERENCES
- 1.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. [DOI] [PubMed] [Google Scholar]
- 2.Swisher SG, Hunt KK, Holmes EC, et al. Changes in the surgical management of esophageal cancer from 1970 to 1993. Am J Surg. 1995;169:609–614. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson MK, Durkin AE. Preoperative prediction of the risk of pulmonary complications after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2002;123:661–669. [DOI] [PubMed] [Google Scholar]
- 4.Fan ST, Lau WY, Yip WC, et al. Prediction of postoperative pulmonary complications in oesophagogastric cancer surgery. Br J Surg. 1987;74:408–410. [DOI] [PubMed] [Google Scholar]
- 5.Nagawa H, Kobori O, Muto T. Prediction of pulmonary complications after transthoracic oesophagectomy. Br J Surg. 1994;81:860–862. [DOI] [PubMed] [Google Scholar]
- 6.Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg. 2000;231:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwano H, Sumiyoshi K, Sonoda K, et al. Relationship between preoperative assessment of organ function and postoperative morbidity in patients with oesophageal cancer. Eur J Surg. 1998;164:581–586. [DOI] [PubMed] [Google Scholar]
- 8.van Sandick JW, Gisbertz SS, ten Berge IJ, et al. Immune responses and prediction of major infection in patients undergoing transhiatal or transthoracic esophagectomy for cancer. Ann Surg. 2003;237:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukada K, Hasegawa T, Miyazaki T, et al. Predictive value of interleukin-8 and granulocyte elastase in pulmonary complication after esophagectomy. Am J Surg. 2001;181:167–171. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Zagariya A, Ibarra-Sunga O, et al. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol. 1999;276:L885–889. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Ortega M, Lorenzo O, Ruperez M, et al. Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms. Circ Res. 2000;86:1266–1272. [DOI] [PubMed] [Google Scholar]
- 12.Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall RP, Webb S, Bellingan GJ, et al. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. [DOI] [PubMed] [Google Scholar]
- 14.Benessiano J, Crestani B, Mestari F, et al. High frequency of a deletion polymorphism of the angiotensin-converting enzyme gene in asthma. J Allergy Clin Immunol. 1997;99:53–57. [DOI] [PubMed] [Google Scholar]
- 15.Maier LA, Raynolds MV, Young DA, et al. Angiotensin-1 converting enzyme polymorphisms in chronic beryllium disease. Am J Respir Crit Care Med. 1999;159:1342–1350. [DOI] [PubMed] [Google Scholar]
- 16.Morrison CD, Papp AC, Hejmanowski AQ, et al. Increased D allele frequency of the angiotensin-converting enzyme gene in pulmonary fibrosis. Hum Pathol. 2001;32:521–528. [DOI] [PubMed] [Google Scholar]
- 17.Maliarik MJ, Rybicki BA, Malvitz E, et al. Angiotensin-converting enzyme gene polymorphism and risk of sarcoidosis. Am J Respir Crit Care Med. 1998;158:1566–1570. [DOI] [PubMed] [Google Scholar]
- 18.Pietinalho A, Furuya K, Yamaguchi E, et al. The angiotensin-converting enzyme DD gene is associated with poor prognosis in Finnish sarcoidosis patients. Eur Respir J. 1999;13:723–726. [DOI] [PubMed] [Google Scholar]
- 19.Lindpaintner K, Pfeffer MA, Kreutz R, et al. A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med. 1995;332:706–711. [DOI] [PubMed] [Google Scholar]
- 20.Avendano CE, Flume PA, Silvestri GA, et al. Pulmonary complications after esophagectomy. Ann Thorac Surg. 2002;73:922–926. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Committee for Registration of Esophageal Carcinoma. A proposal for a new TNM classification of esophageal carcinoma: Japanese Committee for Registration of Esophageal Carcinoma. Jpn J Clin Oncol. 1985;15:625–636. [PubMed] [Google Scholar]