Abstract
Objective:
To evaluate the influence of surgical margin status on survival and site of recurrence in patients treated with hepatic resection for colorectal metastases.
Methods:
Using a multicenter database, 557 patients who underwent hepatic resection for colorectal metastases were identified. Demographics, operative data, pathologic margin status, site of recurrence (margin, other intrahepatic site, extrahepatic), and long-term survival data were collected and analyzed.
Results:
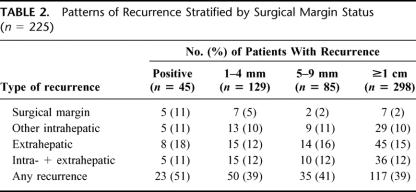
On final pathologic analysis, margin status was positive in 45 patients, and negative by 1 to 4 mm in 129, 5 to 9 mm in 85, and ≥1 cm in 298. At a median follow-up of 29 months, the 1-, 3-, and 5-year actuarial survival rates were 97%, 74%, and 58%; median survival was 74 months. Tumor size ≥5 cm, >3 tumor nodules, and carcinoembryonic antigen level >200 ng/mL predicted poor survival (all P < 0.05). Median survival was 49 months in patients with positive margins and not yet reached in patients with negative margins (P = 0.01). After hepatic resection, 225 (40.4%) patients had recurrence: 21 at the surgical margin, 56 at another intrahepatic site, 82 at an extrahepatic site, and 66 at both intrahepatic and extrahepatic sites. Patients with negative margins of 1 to 4 mm, 5 to 9 mm, and ≥1 cm had similar overall recurrence rates (P > 0.05). Patients with positive margins were more likely to have surgical margin recurrence (P = 0.003). Adverse preoperative biologic factors including tumor number greater than 3 (P = 0.01) and a preoperative CEA level greater than 200 ng/mL (P = 0.04) were associated with an increased risk of positive surgical margin.
Conclusions:
A positive margin after resection of hepatic colorectal metastases is associated with adverse biologic factors and increased risk of surgical-margin recurrence. The width of a negative surgical margin does not affect survival, recurrence risk, or site of recurrence. A predicted margin of <1 cm after resection of hepatic colorectal metastases should not be used as an exclusion criterion for resection.
The effect of surgical margin status on survival and pattern of recurrence in 557 patients who underwent hepatic resection of colorectal metastasis was analyzed. We report that a positive margin is associated with adverse biologic factors and increased risk of surgical margin recurrence. The width of a negative surgical margin does not affect survival, recurrence risk, or site of recurrence.
Liver resection currently represents the only potentially curative therapeutic option for hepatic colorectal metastasis (CRM), and 5-year survival rates of 25% to 58% have been reported.1–6 Traditionally, primary tumor stage, preoperative carcinoembryonic antigen (CEA) level, hepatic tumor size, number of hepatic metastases, time from primary tumor treatment to diagnosis of hepatic metastases, and presence of extrahepatic disease have been reported to be independent predictors of survival after resection.7,8
Surgical margin status is an additional factor that has been evaluated for its influence on long-term survival after resection of CRM, but its significance remains controversial. Several series concerning liver resection for colorectal liver metastasis have reported that surgical margins of less than 1 cm are an absolute9,10 or relative contraindication to surgery.11 Cady et al10 have reported that a surgical margin less than 1 cm was associated on univariate analysis with a significantly shorter disease-free survival. As a result, major centers have adopted a 1-cm margin as a target during resection to minimize hepatic recurrence and improve survival after resection of CRM.12,13 In fact, a 1-cm margin has been proposed as the minimally acceptable margin even for ablative techniques.14,15
Despite the emphasis on a 1-cm margin, some investigators16 have reported that the actual width of the surgical margin has no effect on survival as long as the margin is negative. Altendorf-Hofmann and Scheele16 noted that patients with a microscopically positive margin (R1) had a worse prognosis compared with patients who had a microscopically negative margin (R0), but survival was not associated with the width of the negative surgical margin. More recently, Adam et al17 reported a 5-year survival rate of 33% in 138 patients, among whom 67% had less than 1-mm surgical margins. All of these studies, however, examined only the effect of surgical margin status on survival but not local recurrence.
If margin status or width of margin is important, it has practical implications. Specifically, margin considerations may dictate which patients are resectable, the extent of resection, and the treatment of residual positive margins at the time of surgery.10,18 The objective of the current study was to evaluate the influence of surgical margin status on survival and site of recurrence in patients treated with hepatic resection for CRM at 3 hepatobiliary centers.
PATIENTS AND METHODS
Using a multicenter database, we identified 557 patients who underwent hepatic resection for CRM between September 1990 and May 2004 at 3 hepatobiliary centers: the University of Texas M. D. Anderson Cancer Center (Houston, TX), the Surgical Oncology Unit, Institute of Research and Cure of Cancer (Candiolo, Italy), and the Division of Digestive Surgery, University Hospital (Geneva, Switzerland). All 557 patients included in the study had complete follow-up radiologic imaging. Patients who did not have imaging studies to document disease status and site of recurrence were not included in the study. The institutional review board at each participating institution approved this study.
Prior to surgery, all patients were evaluated with a baseline history and physical examination, serum laboratory tests, and appropriate imaging studies (computed tomography or magnetic resonance imaging scan of the abdomen and pelvis and chest radiography or a chest computed tomography) at the discretion of the treating physician. All patients with CRM and no clinical, radiographic, or intraoperative evidence of extrahepatic disease were eligible for resection. Patients were deemed to have resectable disease only if it was anticipated that the metastasis could be completely resected, at least 2 adjacent liver segments could be spared, vascular inflow and outflow could be preserved, and the volume of the liver remaining after resection would be adequate.19,20 Patients were not excluded from surgery based on the predicted margin of resection, but in all cases the intent of the surgical procedure was curative. Patients with macroscopically incomplete resection were excluded.
After hepatic resection, all patients were regularly followed and prospectively monitored for recurrence by serum CEA levels and a computed tomography or magnetic resonance imaging scan of the abdomen every 3 to 4 months up to 2 years and then every 6 months thereafter. Chest radiography and serum laboratory tests were also performed.
The following data were collected for each patient: demographics, laboratory data (CEA level), tumor number and location, operative details, pathologic margin status, disease status, site of recurrence, date of last follow-up, and date of death. Data were recorded as follows: age, less than 60 years versus 60 years or older; CEA level, less than or equal to 200 ng/mL versus greater than 200 ng/mL; tumor number, less than 3 versus at least 3; tumor size, less than 5 cm versus at least 5 cm. The extent of the hepatic resection was categorized as less than a hemihepatectomy, hemihepatectomy, or extended hepatic resection (≥5 liver segments).21
Patients were classified according to the width of the resection margin, defined as the shortest distance from the edge of the tumor to the line of transection. A positive margin was defined as the presence of exposed tumor along the line of transection or the presence of tumor cells at the line of transection detected by histologic examination. Per these definitions, margin status was then divided into 4 subgroups: positive (<1 mm) and negative by 1 mm to 4 mm, 4 mm to 9 mm, or at least 1.0 cm. A radiologist at each hepatobiliary center reviewed all available radiologic imaging to detect the initial site(s) of recurrence following resection. Site of metastatic recurrence was categorized as surgical resection margin, other intrahepatic site, or extrahepatic. Figure 1 depicts the computed tomography appearance of surgical margin recurrence in 2 patients after right hepatectomy.
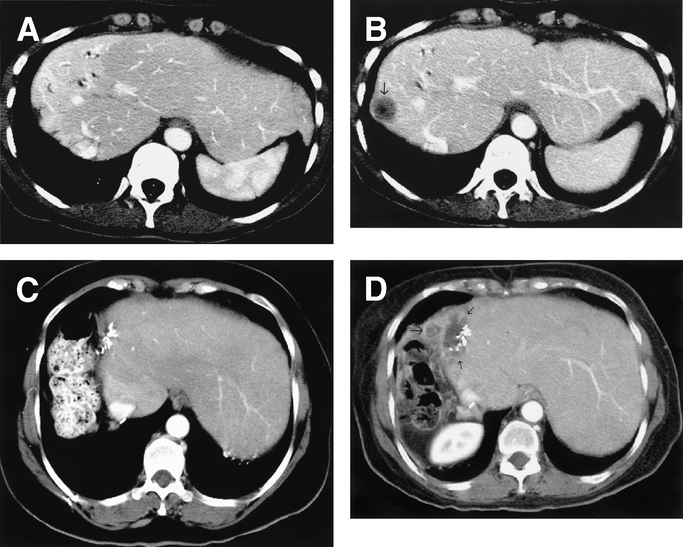
FIGURE 1. Imaging patterns of marginal recurrences in 2 patients. A, Contrast-enhanced computed tomography obtained 11 months after right hepatectomy shows perfusion changes along surgical margin but no tumor recurrence. B, Contrast-enhanced CT obtained 3 months after A shows hypoattenuating tumor nodule (arrow) abutting the surgical margin. C, Contrast-enhanced computed tomography obtained 17 months after right hepatectomy in another patient shows surgical clips and no recurrence. D, Contrast-enhanced computed tomography obtained 5 months after C shows recurrent tumor infiltrating the surgical margin and adjacent perihepatic tissues (arrows).
All data are presented as percentages of patients or the median value. Statistical analyses were performed using univariate tests (χ2, log-rank) to test for differences in variables with regard to survival. Factors that appeared to be significantly associated with survival were entered into a Cox proportional hazards model to test for significant effects while adjusting for multiple factors simultaneously. Actuarial survival was calculated using the Kaplan-Meier method. Differences in survival were examined using the log-rank test. A P value less than 0.05 was considered significant.
RESULTS
Clinicopathologic Characteristics
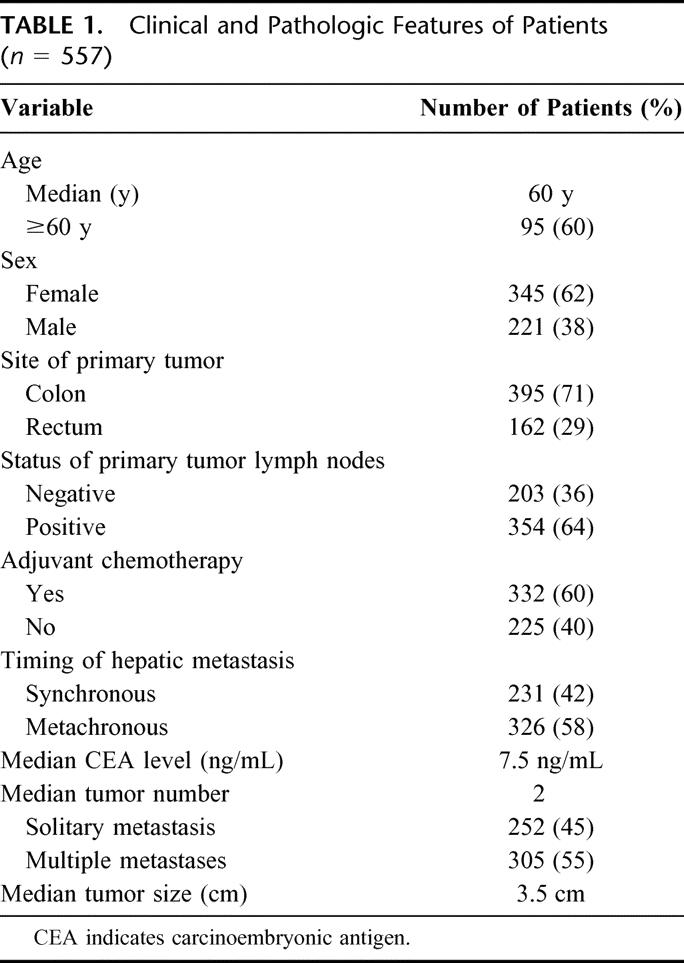
The clinical features of the 557 patients included in the study are presented in Table 1. There were 212 men and 345 women, for a male to female ratio of 0.6:1. The median patient age was 60 years (range, 19 to 88 years). The primary tumor originated from the colon in 395 (70.9%) cases and from the rectum in 162 (29.1%) cases. The majority of patients (n = 354; 63.6%) had positive lymph nodes on pathologic analysis of the primary colorectal tumor specimen, and most patients received chemotherapy (n = 332; 59.6%). Two hundred thirty-one (41.5%) patients had synchronous hepatic metastasis, while 326 (58.5%) had metachronous disease. The median time to the diagnosis of liver metastasis was 15.7 months (range, 0.5 to 204.0 months). The median number of CRM was 2 (range, 1 to 11). Three hundred five (54.8%) patients had multiple CRM, and 115 (20.6%) had more than 3 metastases. The median diameter of the CRM was 3.5 cm (range, 0.5 to 18.0 cm). The median preoperative CEA level was 7.5 ng/mL (range, 0.1 to 3692 ng/mL), and 33 (5.9%) patients had a CEA level greater than 200 ng/mL.
TABLE 1. Clinical and Pathologic Features of Patients (n = 557)
The majority of patients underwent either less than a hemihepatectomy (n = 238; 42.7%) or a hemihepatectomy (n = 216; 38.8%); only 103 patients (18.5%) underwent an extended hepatic resection. On final pathologic analysis, margin status was positive in 45 patients and negative by 1 mm to 4 mm in 129, 5 mm to 9 mm in 85, and at least 1.0 cm in 298 (Fig. 2). Among the 45 patients with positive margin, the diagnosis of positive margin was made postoperatively in 34 patients and intraoperatively in 11 patients. Among these 11 patients, 8 were treated: 1 was reresected and 7 received local therapy (radiofrequency ablation, n = 3; ablation with cautery, n = 4).
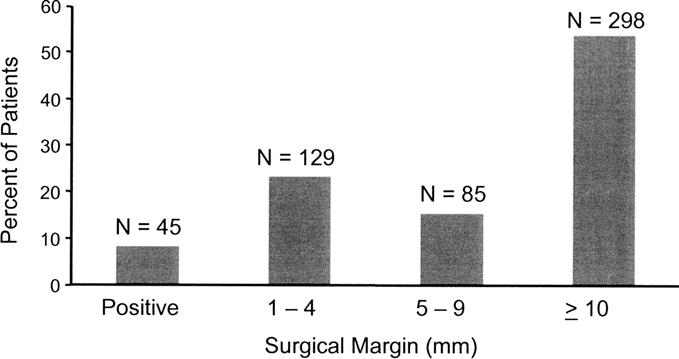
FIGURE 2. Distribution by size of the surgical resection margin (n = 557).
Factors associated with a positive surgical margin included tumor number greater than 3 (P = 0.01) and a preoperative CEA level greater than 200 ng/mL (P = 0.04).
Factors Influencing Patterns of Recurrence
With a median follow-up of 29 months, 225 of 557 patients (40.4%) developed a recurrence. The median time to recurrence was 10.5 months (range, 0.7 to 112.4 months), with the majority of patients (124 out of 225; 55.1%) recurring within 12 months after hepatic resection. Among all patients, 66 (11.8%) patients developed both intra- and extrahepatic metastases. In contrast, 82 (14.7%) patients developed extrahepatic metastasis only and 56 (10.1%) patients recurred solely at an intrahepatic site away from the surgical margin. Only 21 (3.7%) patients developed a recurrence at the site of the surgical margin. Among the 21 patients, only 4 patients (19%) had a surgical margin recurrence as the sole site of recurrence. Three of 4 patients underwent liver directed therapy (reresection, radiofrequency ablation, chemoradiation). At last follow-up, 3 patients were disease free (follow-up 26, 65, and 90 months), and 1 had recurrent disease within the liver at a site remote from the surgical margin (follow-up 26 months).
Several clinicopathologic factors predicted the pattern of recurrence following resection of CRM. A CEA greater than 200 ng/mL, tumor size of at least 5 cm, or a positive resection margin each was associated with a higher overall recurrence rate. Specifically, patients who had a preoperative CEA greater than 200 ng/mL had a rate of overall recurrence of 62.1% compared with 39.1% for patients with a lower CEA (P = 0.01). A tumor size of at least 5 cm was associated with a 55.0% rate of overall recurrence compared with 39.1% for tumors less than 5 cm (P = 0.04). Patients with a positive surgical margin also had a higher overall recurrence rate: 51.1% compared with 38.6% for patients with a negative surgical margin (P = 0.04). In contrast, patients with negative margins of 1 mm to 4 mm (38.7%), 5 mm to 9 mm (41.2%), and at least 1.0 cm (39.2%) had similar overall recurrence rates (P = 0.32) (Table 2). On multivariate analysis, only CEA level greater than 200 ng/mL remained an independent predictor of overall recurrence (hazard ratio [HR] = 2.23, 95% confidence interval [CI] = 1.08–4.95, P = 0.04).
TABLE 2. Patterns of Recurrence Stratified by Surgical Margin Status (n = 225)
Among all the variables examined, only a positive surgical margin was associated with surgical margin recurrence (P = 0.003). Surgical margin recurrence was slightly more common in patients with negative margins of 1 mm to 4 mm than with wider margins, but this difference was not significant (P = 0.25) (Table 2). Whether a patient received chemotherapy (12.6%) or not (10.4%) did not affect a patient's risk for developing intrahepatic or margin site recurrence. Similarly, no other clinicopathologic factor predicted recurrence at the surgical margin (all P > 0.05). Only tumor number greater than 3 was a significant predictor of intrahepatic recurrence away from the surgical margin site (P = 0.03).
Factors Influencing Probability of Survival
Five patients died within 30 days of resection, for a perioperative mortality rate of 0.9%. At a median follow-up of 29 months, the median survival was 74.3 months (Fig. 3). The 1-, 3-, and 5-year overall survival rates were 97%, 74%, and 58%, respectively. The longest-living survivor was alive and disease free at 11.3 years.
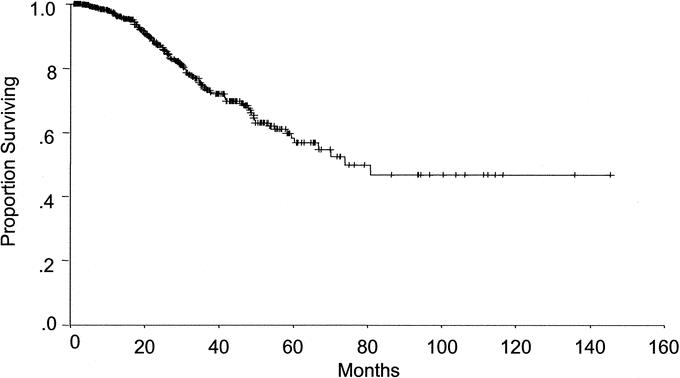
FIGURE 3. At a median follow-up of 29 months, the overall median survival for patients after resection of CRM was 74.3 months. The 1-, 3-, and 5-year survival rates were 97%, 74%, and 58%, respectively.
Statistical analysis revealed several factors that affected survival. On univariate analysis, tumor number greater than 3, tumor size of at least 5 cm, CEA greater than 200 ng/mL, and a positive surgical margin were significant predictors of poor survival. Patients with greater than 3 hepatic metastasis or tumors measuring at least 5 cm had a median survival of 53.2 months (95% CI, 40.2–66.3 months) and 54.9 months (CI, 38.7–71.2 months), respectively. In contrast, median survival had not yet been reached for patients with less than 3 metastases (P = 0.007) or those with tumors smaller than 5 cm (P = 0.015). Patients with a CEA level less than 200 ng/mL also had not yet reached median survival; however, patients with a preoperative CEA level greater than 200 ng/mL had a median survival of 49.7 months (95% CI, 17.2–82.3 months) (P = 0.006).
Median survival was 49.6 months in patients with positive margins and not yet reached in patients with negative margins (P = 0.005). The 5-year survival rate was 17.1% for patients with a positive margin compared with 63.8% for patients with a negative surgical margin (P = 0.01). The width of the surgical margin did not significantly affect survival in patients with negative margins. No significant difference in survival was seen in patients with a negative surgical margin, regardless of the width of the margin (Fig. 4). The width of the negative surgical margin did not affect the 5-year survival rate (1 mm to 4 mm: 62.3%; 5 mm to 9 mm: 71.1%; at least 1.0 cm: 63.0%) (P = 0.63). Median survival also did not differ based on the type of surgical procedure performed (less than a hemihepatectomy: 73.9 months; hemihepatectomy: not reached; extended hepatic resection: 80.7 months, months) (P = 0.56). Univariate analysis revealed no differences in survival based on age, gender, synchronous versus metachronous metastasis, disease-free interval, or nodal status of the primary colorectal tumor (all P > 0.05).
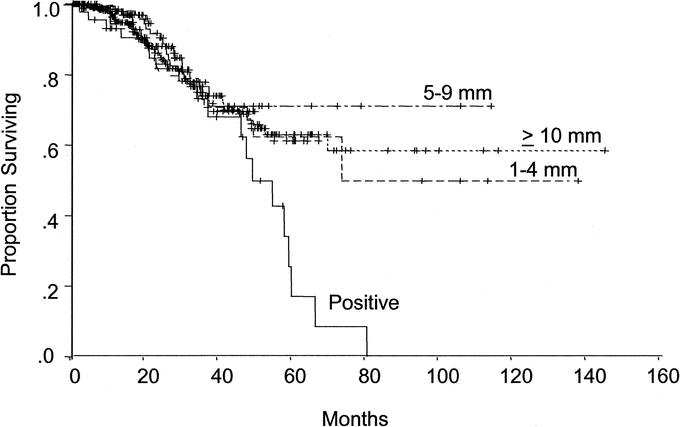
FIGURE 4. Survival stratified by margin status. Median survival was 49.6 months in patients with positive margins and not yet reached in patients with negative margins (P = 0.005). No significant difference in survival was seen in patients with a negative surgical margin, regardless of the width of the margin (all P > 0.5).
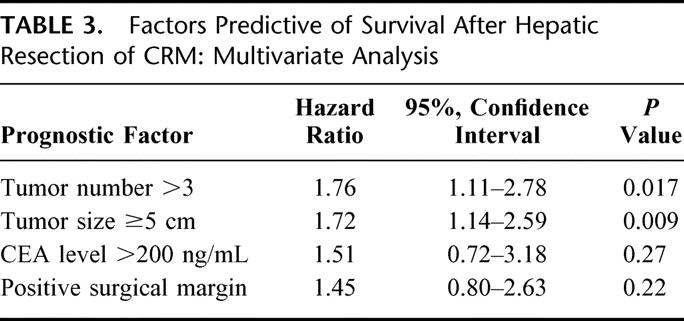
On multivariate analysis, tumor number greater than 3 and tumor size of at least 5 cm remained independent predictors of poor survival (Table 3). Patients with more than 3 colorectal hepatic metastases had a higher likelihood of death than those with fewer metastases (HR = 1.76, 95% CI = 1.11–2.78, P = 0.017). Similarly, tumor size of a least 5 cm was associated with an increased risk of death (HR = 1.72, 95% CI = 1.14–2.59, P = 0.009). Margin status was not a significant predictor of survival on multivariate analysis (HR= 1.45, 95% CI = 0.80–2.63, P = 0.22).
TABLE 3. Factors Predictive of Survival After Hepatic Resection of CRM: Multivariate Analysis
DISCUSSION
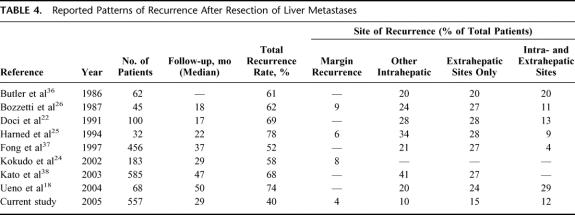
Although many surgeons strive to achieve surgical margins as wide as safely possible during hepatic resection for CRM, there has been no definitive evidence regarding the negative margin width necessary during hepatectomy to optimize long-term survival and to minimize surgical margin recurrence. Ekberg et al9 reported that a 1-cm margin was necessary to optimize long-term survival. Others10,12,13 subsequently endorsed this approach and extended its application to radiofrequency and cryoablation.14,15 These studies, however, were limited by small sample sizes, failure to stratify margin widths below 1 cm, and lack of multivariate analyses. Few studies1,22,23 have provided a separate multivariate analysis of patient survival based on the R0 resection rate. Furthermore, prior to the current study, only 3 single-institution studies24–26 reported on the effect of surgical margin status on local recurrence (Table 4). In fact, Kokudo et al24 was the only study to analyze local recurrence rates according to surgical margin width less than 1 cm. The current study represents the first multicenter report to examine the effect of surgical margin status after resection of hepatic CRM on both margin recurrence and survival.
TABLE 4. Reported Patterns of Recurrence After Resection of Liver Metastases
The effect of surgical margin status on the treatment of various malignancies has been extensively investigated. With breast and rectal cancer, a positive surgical margin has been reported to increase the incidence of local recurrence. Singletary27 noted that the rate of local recurrence for breast cancer was 0% to 10% with negative surgical margins compared with 4% to 31% with positive surgical margins. Similarly, Hall et al28 reported that the incidence of local recurrence was higher in patients with positive surgical margins (50%) compared with negative margins (24%) after resection of primary rectal carcinoma. Very few investigators, however, have directly evaluated patterns of recurrence after hepatic resection for CRM based on surgical margin status.1,22,23 Perhaps one reason for this is that the accurate assessment of the surgical margin in hepatic surgery can be difficult. Current techniques of gross evaluation followed by frozen section analysis of the surgical margin may overestimate the true positive margin rate because the ultrasonic dissectors aspirate a portion of the liver parenchyma interposed between the specimen and the normal liver, and the friability of the liver itself, can cause the liver to crack, making assessment of the true margin difficult. In the current study, we defined a positive surgical margin as the presence of exposed tumor along the line of transection or the presence of tumor cells at the line of transection detected by histologic examination. Specifically, a positive margin was considered to be a margin less than 1 mm. Although the definition of a “positive” margin varies in the literature24,28, we adopted a 1-mm cutoff to define a positive margin in the current study because inked margin analysis was not always performed and we wanted to avoid the potential sampling error associated with determinations of margin positivity based on pathology slides alone.
Following hepatic resection a 1-cm margin was not achieved in almost one half of the patients (259 out of 557; 46.5%). This was comparable to other reported series that noted rates of 34%24 and 40%10 for obtaining a surgical margin of 1 cm or wider at the time of hepatic resection for CRM. Proposals to exclude patients from surgery or ablation therapy based on a 1-cm margin rule need to be evaluated in light of these findings. Using a 1-cm rule for resection could exclude a large number of patients from the only therapeutic interventions (surgery and ablation) known to affect long-term survival.
The current study identified preoperative predictors of positive margin status. Specifically, patients with more aggressive biologic factors such as tumor number greater than 3 and a preoperative CEA level greater than 200 ng/mL were significantly more likely to have a positive surgical margin. This also explains why only 4 out of 21 patients with positive margins had isolated margin recurrence, with all 4 being alive at last follow-up. These findings corroborate the notion that tumors with a more aggressive biologic phenotype are more difficult to extirpate with negative surgical margins. Preoperative systemic chemotherapy for resectable hepatic CRM has recently been shown to be associated with a decreased rate of positive margins.29 Based on this, it may be that patients with aggressive biologic factors such as multiple tumors and an elevated CEA should receive adjuvant therapy in an attempt to improve survival in this subset of patients at high risk for recurrence.
In this study, different techniques were used to treat positive margins at the time of resection. When additional surgical resection was not feasible, ablation with cautery or radiofrequency was used to treat the positive margin. At the University of Texas M. D. Anderson Cancer Center, we currently prefer to use saline-linked cautery in combination with inflow occlusion to treat positive margins. Animal data have suggested that saline-linked cautery can ablate up to 1 cm in combination with inflow occlusion in a porcine animal model.30 No conclusions can be derived from the current study, however, with regard to the treatment of positive margins, given the various techniques used and the small number of patients with positive margins who subsequently recurred at the surgical margin.
Bozzetti et al26 found recurrence in 62% of their patients after a median follow-up of 18 months. Harned et al25 reported recurrence in 78% of patients 22 months after resection. In the current study, we report a 40% incidence of overall recurrence at a median follow-up of 29 months. In addition, our reported rates of recurrence at the surgical margin, other intrahepatic locations, as well as distant sites were all almost one half the rates reported by either Bozzetti et al26 or Harned et al25 (Table 4). The reason for the lower incidence of recurrence at all sites reported in the current study is probably multifactorial. Recent improvement in whole-body and hepatic imaging has allowed for more accurate selection of patients with CRM. Preoperative imaging is now able to detect minimal burdens of metastatic disease that in the past would have been detected only after enlarging and thus would have been classified at a later time as a recurrence. Forty percent of patients in the current study also received adjuvant chemotherapy, and recent advances in the use of systemic chemotherapy have been shown to improve the survival of patients with CRM.31 Finally, the systematic use of hepatic intraoperative ultrasound may in part explain the low incidence of margin and other intrahepatic recurrence.
In the current study, surgical margin recurrence was rare (3.8%). Although a positive surgical margin was associated with an increased risk of margin recurrence (11%), the width of the margin was not significant. Patients with a margin of 1 mm to 4 mm (5%) did have a slightly increased rate of margin recurrence compared with patients who had wider margins (2%); this did not reach statistical significance (P = 0.25). These findings are consistent with those of Kokudo et al24 who reported an increased risk of margin site recurrence in patients with a positive surgical margin defined as less than 2 mm. Gayowski et al32 and Ohlsson et al33 also reported that patients with surgical margins 1 mm or wider but less than 1 cm had outcomes comparable to those with surgical margins wider than 1 cm. Based on the aggregate data, a surgical margin of at least 1 mm appears to be the minimal requirement to reduce margin-related recurrences. This concept of successful limited complete resection is supported by the fact that CRMs are histopathologically well circumscribed,34 only 16% have satellitosis,1 Glisson sheath extension is uncommon (14.5%) and limited (<5 mm),24 and micrometastases are rare (2%).24
Local (margin) or regional- (other intrahepatic site) only disease accounted for the minority of recurrences (26.6%) in the current series. The overwhelming majority (73.4%) of patients had disease at an extrahepatic site as a component of their recurrence pattern. Others22,26,35,36 have similarly shown that the majority of patients with recurrence after hepatectomy for liver metastases develop extrahepatic disease. Given the high risk for systemic failure, as well as the recent advances in systemic adjuvant therapy, we do not advocate the use of regional chemotherapy in combination with hepatic resection for CRM. Rather, high-risk patients should be offered systemic adjuvant therapy, which has the ability to address both micrometastatic regional as well as distant disease.
In conclusion, the 58% 5-year survival rate reported in the current multicenter study confirms the improvement in survival reported in recent single institutional studies.5,6 It supports the concept of limited negative margin resection in patients with hepatic CRM.23,24,34 Although associated with an increased risk of surgical-margin recurrence, a positive margin after resection of hepatic CRM is also associated with advanced metastatic disease (multiple tumors) and adverse biology (CEA >200 ng/mL). The intraoperative treatment of a positive margin appears reasonable but its benefit, if any, is likely minimal and remains unproven.
ACKNOWLEDGMENTS
The authors thank Ruth J. Haynes for secretarial assistance in the preparation of the manuscript.
Discussions
Dr. Michael A. Choti (Baltimore, Maryland): I would like to congratulate Dr. Vauthey and his group for this interesting paper addressing the question of both positive and close surgical margins, as well as the issue of the pattern of recurrence. I have three quick questions for you, Dr. Vauthey.
The first relates to your definition of the surgical margin. You mention that it was either gross margin visible at the resection edge or histologically tumor cells at the resection margin, and yet some of your data showed less than 1 mm. As we know, margins in metastatic resection of the liver are difficult because of the techniques we use to go through the parenchyma cusha [sic], and tissue link devices sometimes create oblation, there is fracturing on the edge, so that the positive ink margin sometimes can make the definition of a positive margin quite subjective. How do you define it and how do we compare different margin positive reports in various studies? I think it is important to address that may not be possible.
The second question relates to the biology. If you could speculate why positive margins and surgical margin positivity in this study correlated in fact with adverse biologic parameters, CEA level, number of metastases, but didn't correlate, at least that I saw, on what would expect to be technical factors of margin positives such as, for example, tumor size.
And third, a related question: Did you look at other technical parameters that may be associated, or one would speculate would be associated, with margin positivity; for example, the location of the tumor within the liver, the type of resection, anatomic versus wedge resection, for example, breast-invected [sic] of parenchymal resection, the technique used, intraoperative ultrasonography, and whether the tumors were isoechoic, for example, which may make planning or a positive margin more likely, and perhaps speculate or comment on whether there was a difference in margin positivity by surgeon or by institution.
Dr. Reid B. Adams (Charlottesville, Virginia): A couple of quick questions regarding the data. This may be in the paper.
The first is, you have a fairly small subset of the patients you studied and you have an even smaller positive effect in those 45 patients. My question is, did you do a power calculation to determine whether or not you can detect a difference in the small subset of patients that you were studying, that is, the outcome in that group that had positive margins?
The second is, I didn't hear anything about postoperative adjuvant therapy. Was there any effect of postoperative adjuvant therapy on the group of patients that had positive or close margins?
Dr. John S. Bolton (New Orleans, Louisiana): Close margins are a fact of life for hepatic surgeons, as evidenced in this study where almost half the patients had margins less than a centimeter, and in quite a few other reports the percentage is actually well over 50% who have margins narrower than 1 cm. I think we are indebted to Dr. Vauthey and his coauthors for bringing the significance or lack thereafter of margin width into better focus. And to summarize, I think, his findings, only a positive margin is associated with marginal recurrence, and margin status is not an independent predictor for long-term outcome.
I do think we need to be careful about what conclusions we draw. My read is that positive margins are an indicator, not a governor, of prognosis. That doesn't mean that obtaining negative margins is not important but only that when we make a good-faith effort and fail to obtain negative margins, other biologic factors, particularly multiple lesions, are present and govern the outcome, which is less good than in patients with negative margins.
I have 3 questions, Dr. Vauthey. First, you classify the operative extent as either less than a hemihepatectomy or an extended resection. How many of the less than hemihepatectomies had nonanatomic wedge resections? The Memorial group in a nice study several years ago of about 250 patients showed wedge resections to be associated with worst outcome, and that was mediated by margin involvement in their study.
2. Why don't all or most patients with positive margins experience local recurrence? Could you just speculate on that? It was only 11% in your study, and that just seems surprisingly low.
3. Could you also speculate based on your findings in this study should the 1-cm margin which is recommended as the goal of ablation techniques, is that still valid? Or was it ever valid, I guess, for that matter? But would you comment on that?
Dr. Alan W. Hemming (Gainesville, Florida): Survival as reported in the paper appears to be overall survival rather than disease-free survival. Was the disease-free survival substantially different, and are some of the improved results that we are all currently seeing with resection due to improved patient selection and operative technique, or is it simply better chemotherapy that is delaying the recurrence?
The second question is: How were patients with recurrence only in the liver dealt with statistically, if they underwent repeat hepatic resection, or did any of the patients undergo repeat hepatic resection?
A little bit of a comment. I think you have to be a little careful about saying that positive margins are a marker of biologic aggressiveness of the tumor. That may be true if you are talking about positive margins in the hands of experienced liver surgeons that have done everything possible to obtain negative margins. It is quite another thing if someone has inadequately wedged out a tumor with positive margins. If you currently see a patient treated elsewhere referred with just that scenario, in other words, a wedged-out tumor with positive margins, are you going to reresect the patient or are you going to cite biologic aggressiveness and just treat with chemotherapy?
Dr. Joseph B. Cofer (Chattanooga, Tennessee): Your own data showed that the mean number of resections in your series were 2, and then you showed that a number of metastases greater than 3 was an independent predictor of poor survival. Other data, as you well know, have shown an inverse relationship of survival and recurrence to the number of lesions resected. Do you have a number beyond which you won't attempt to resect, even though you know you might be able to technically? Will you go after 5, 6? Is it fruitless at some point?
Dr. W. Roy Smythe (Temple, Texas): In thoracic surgery, we used to do anatomic lobectomies for metastatic disease and then moved to wedge resections, and actually more recently as we noted the same thing that you noted from metastatic disease in the liver and, as was pioneered by Bob Ginsberg of Memorial Sloan-Kettering, we actually core some of these lesions out of the lung now as an immediately adjacent margin appears to have no impact on recurrence. Will these data have any influence on the method that you used to resect metastatic lesions in the liver?
Dr. Bryan M. Clary (Durham, North Carolina): This is a very impressive series from Dr. Vauthey. I know that he has an interest in neoadjuvant chemotherapy, and as it is usually fairly evident preoperatively when you are going to have a close margin, I was wondering if the lack of significance for the closer margin, such as 1 to 4 mm, is a reflection of him selecting patients by placing them on neoadjuvant and allowing a period of time prior to performing resections?
Dr. Jean-Nicolas Vauthey (Houston, Texas): Thank you for the insightful questions and comments.
Dr. Choti, we had to define positive-resection margins. Because this is a retrospective study without routine frozen-section analysis of the inked margins, we believe the combination of exposed tumor and tumor cells at the margin of resection is the most appropriate definition. It may overestimate the number of patients who had true positive margins because of the vaporization or destruction of tissue associated with the use of the ultrasonic dissector or the crushing clamp. The current study suggests that the positive margins called later by the pathologist on final report are equally relevant.
Positive margin did not correlate with tumor size but correlated with tumor number. It seems we are back full circle to the initial papers that were published on resection for colorectal metastases. The papers by Hughes, Ekberg, and Cady pointed to the margins but also pointed to the number of tumors. So the question really is: How do we address these patients with adverse biology?
Dr. Adams, the study clearly determined a significantly worse survival and higher local recurrence rate with positive margins. In answer to your question regarding the risk of a type II error, this is a large multicenter study, and only patients with complete follow-up imaging were included. There are about 100 patients in the various subsets with negative margins so that if there is a statistical difference between the groups, it is likely small and negligible in terms of clinical significance.
To answer the question of Dr. Bolton, we have looked at patients who had less than a hemihepatectomy who had anatomic- or segment-oriented resection versus wedge resection, and in fact we have recently put together an abstract based on this data, and in contrast to the paper by Dr. DeMatteo from Memorial Sloan-Kettering, we did not find a difference in survival and in the rate of positive margins. So that may be a difference in approach. But it supports the limited resection advocated by the Japanese authors and confirms the pathological studies indicating that satellitosis, Glisson sheath extension, or micrometastases are uncommon in hepatic colorectal metastases.
Dr. Hemming asked about chemotherapy. Well, this series reflects in part the era predating effective chemotherapy for colorectal metastases as the series started in 1990. So I think we should look at a combined effect of better surgery, better imaging evaluation of the patient before surgery, maybe with PET scan, better evaluation intraoperatively with ultrasound, the use of 2-stage liver resection and reresection, the use of portal-vein embolization, and perhaps chemotherapy, which all combined led to this improvement in survival. And this is also reflected in the improvement in the recurrence rate.
Dr. Cofer, in answer to your question, we do not have a cutoff number for the resection of metastases. If we can completely resect, we do resect, especially in combination with multimodality therapy including systemic chemotherapy. We use the preoperative chemotherapy in patients with hepatic colorectal metastases at M. D. Anderson Cancer Center. The 45 patients with positive margins in our study had adverse biology, and these are the patients who benefit most from preoperative chemotherapy because of the association with multiple metastases. In fact, in the paper this month in Annals of Surgery (Ann Surg. 2004;240:1052–1061), patients with more than 3 metastases who do not respond to preoperative chemotherapy have a very poor prognosis.
Dr. Smythe, we are not ready to core hepatic colorectal metastases, while we do occasionally enucleate neuroendocrine metastases. Our results indicate that coring is not appropriate.
Dr. Clary asked a final question regarding the use of preoperative chemotherapy. We have published a series reporting the M. D. Anderson experience on this particular topic. We have shown, in fact, a very low positive margin rate (3%) after resection performed following preoperative systemic chemotherapy in spite of the fact that these patients had multiple tumors (J Gastrointest Surg. 2003;7:1082–1088). So I think there is a place now for considering systemic chemotherapy before surgery especially in high-risk patients rather than reresecting or using radiofrequency ablation intraoperatively to treat positive margins post hoc. By reversing the approach in these patients ie considering preoperative treatment (3–4 cycles preoperatively), we also obtain information as to whether the same chemotherapy is effective postoperatively based on the preoperative response rate.
As to radiofrequency ablation, the local recurrence rate is higher than the 2% reported here last year (Ann Surg. 2004;239:722–730), or the 4% for the current series. I think radiofrequency ablation is in many ways a blind procedure, and the 1-cm margin recommendation is based on an extrapolation derived from the experience with resection, but it does not stand in terms of pathology and anatomy.
Footnotes
Reprints: Jean-Nicolas Vauthey, MD, Department of Surgical Oncology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 444, Houston, TX 77030. E-mail: jvauthey@mdanderson.org.
REFERENCES
- 1.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 2.Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647–651. [DOI] [PubMed] [Google Scholar]
- 3.Hughes KS, Rosenstein RB, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of long-term survivors. Dis Colon Rectum. 1988;31:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–353. [DOI] [PubMed] [Google Scholar]
- 5.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients: Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 8.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekberg H, Tranberg KG, Andersson R, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. [DOI] [PubMed] [Google Scholar]
- 10.Cady B, Jenkins RL, Steele GD Jr, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirabe K, Takenaka K, Gion T, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077–1080. [PubMed] [Google Scholar]
- 12.Fuhrman GM, Curley SA, Hohn DC, et al. Improved survival after resection of colorectal liver metastases. Ann Surg Oncol. 1995;2:537–541. [DOI] [PubMed] [Google Scholar]
- 13.Steele G Jr, Bleday R, Mayer RJ, et al. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol. 1991;9:1105–1112. [DOI] [PubMed] [Google Scholar]
- 14.Scaife CL, Curley SA. Complication, local recurrence, and survival rates after radiofrequency ablation for hepatic malignancies. Surg Oncol Clin North Am. 2003;12:243–255. [DOI] [PubMed] [Google Scholar]
- 15.Ruers TJ, Joosten J, Jager GJ, et al. Long-term results of treating hepatic colorectal metastases with cryosurgery. Br J Surg. 2001;88:844–849. [DOI] [PubMed] [Google Scholar]
- 16.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin North Am. 2003;12:165–192, xi. [DOI] [PubMed] [Google Scholar]
- 17.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias D, Cavalcanti A, Sabourin JC, et al. Resection of liver metastases from colorectal cancer: the real impact of the surgical margin. Eur J Surg Oncol. 1998;24:174–179. [DOI] [PubMed] [Google Scholar]
- 19.Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clavien PA, Emond J, Vauthey JN, et al. Protection of the liver during hepatic surgery. J Gastrointest Surg. 2004;8:313–327. [DOI] [PubMed] [Google Scholar]
- 21.Strasberg SM. for the International Hepato-Pancreato-Biliary Association Terminology Committee Survey: the Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB. 2000;2:333–339. [Google Scholar]
- 22.Doci R, Gennari L, Bignami P, et al. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg. 1991;78:797–801. [DOI] [PubMed] [Google Scholar]
- 23.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokudo N, Miki Y, Sugai S, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137:833–840. [DOI] [PubMed] [Google Scholar]
- 25.Harned RK 2nd, Chezmar JL, Nelson RC. Recurrent tumor after resection of hepatic metastases from colorectal carcinoma: location and time of discovery as determined by CT. AJR Am J Roentgenol. 1994;163:93–97. [DOI] [PubMed] [Google Scholar]
- 26.Bozzetti F, Doci R, Bignami P, et al. Patterns of failure following surgical resection of colorectal cancer liver metastases: rationale for a multimodal approach. Ann Surg. 1987;205:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–393. [DOI] [PubMed] [Google Scholar]
- 28.Hall NR, Finan PJ, al-Jaberi T, et al. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent: predictor of survival but not local recurrence? Dis Colon Rectum. 1998;41:979–983. [DOI] [PubMed] [Google Scholar]
- 29.Parikh AA, Gentner B, Wu TT, et al. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. [DOI] [PubMed] [Google Scholar]
- 30.Topp SA, McClurken M, Lipson D, et al. Saline-linked surface radiofrequency ablation: factors affecting steam popping and depth of injury in the pig liver. Ann Surg. 2004;239:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis NL, Meropol NJ. Development of new agents for the treatment of advanced colorectal cancer. Clin Colorectal Cancer. 2003;3:154–164. [DOI] [PubMed] [Google Scholar]
- 32.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–710. [PMC free article] [PubMed] [Google Scholar]
- 33.Ohlsson B, Stenram U, Tranberg KG. Resection of colorectal liver metastases: 25-year experience. World J Surg. 1998;22:268–276. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto J, Sugihara K, Kosuge T, et al. Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann Surg. 1995;221:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordlinger B, Quilichini MA, Parc R, et al. Hepatic resection for colorectal liver metastases: influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg. 1987;205:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler J, Attiyeh FF, Daly JM. Hepatic resection for metastases of the colon and rectum. Surg Gynecol Obstet. 1986;162:109–113. [PubMed] [Google Scholar]
- 37.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. [DOI] [PubMed] [Google Scholar]
- 38.Kato T, Yasui K, Hirai T, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46:S22–31. [DOI] [PubMed] [Google Scholar]