Abstract
Objective:
To report outcome after laparoscopic Heller myotomy in a large number of patients.
Summary Background Data:
Laparoscopic Heller myotomy has been undertaken for over a decade, but most studies involve small numbers of patients with limited follow-up.
Methods:
Since 1992, 262 patients have undergone laparoscopic Heller myotomy and been prospectively followed. Concomitant fundoplication was undertaken for a patulous hiatus or large hiatal hernia or to buttress the repair of an esophagotomy until recently when it became routinely applied. With mean follow-up at 32months, symptoms were scored by patients on a Likert scale (frequency: 0 = Never to 10 = Every time I eat/always; severity: 0 = Not bothersome to 10 = Very bothersome).
Results:
Before myotomy, 79% received Botox or bag dilation: 52% had Botox, 59% underwent dilation, and 36% had both. Inadvertent esophagotomy occurred in 5%. Concomitant diverticulectomy was undertaken in 4%, and fundoplication was undertaken in 30%. Complications were infrequent. Median length of stay was 1 day. After myotomy, the frequency and severity of symptoms of achalasia and reflux significantly decreased. Eighty-eight percent of patients felt their symptoms were greatly improved or resolved, and 90% felt their outcome was satisfying or better. Ninety-three percent felt they would undergo myotomy again, if necessary.
Conclusions:
Laparoscopic Heller myotomy can safely and durably relieve symptoms of dysphagia while also reducing symptoms of reflux. Length of stay is short and patient satisfaction is very high with extended follow-up. Laparoscopic Heller myotomy is strongly encouraged for patients with symptomatic achalasia and is efficacious even after failures of dilation and/or Botox therapy.
Laparoscopic Heller myotomy can safely and durably relieve symptoms of achalasia. Patient satisfaction is very high with extended follow-up, even when Heller myotomy is undertaken after Botox or balloon dilation. Laparoscopic Heller myotomy is encouraged for patients with symptomatic achalasia and is efficacious even after failures of dilation and/or Botox.
Initial care for achalasia is often provided by nonsurgeons and, thereby, many patients have been initially treated with botulinum toxin (Botox) or have undergone balloon dilation prior to considerations for myotomy. Over the last decade, however, laparoscopic approaches to achalasia have become widely available and experience has grown, as documented by favorable results from many centers.1–8 Laparoscopic Heller myotomy is evolving into a “first-line” therapy for patients with achalasia, as well as a definitive therapy for salvage of patients failing endoscopic therapy for achalasia. Laparoscopic myotomy has now been undertaken at enough centers and for long enough that the full picture of the experience of laparoscopic Heller myotomy should be coming into focus.
We undertook our first laparoscopic Heller myotomy in 1992. We have prospectively followed patients since then, building upon our earlier experience with myotomy via celiotomy or thoracotomy. While our initial videoscopic myotomies were undertaken through thoracoscopy, after our initial experience of less than 20 patients, we converted our approach to involve laparoscopy because of perceived simplicity and superior outcomes. Our initial results with thoracoscopy have been documented,9 and we believed they were inadequate to justify continuing that approach.
Many questions over the past decade have arisen regarding outcomes after laparoscopic Heller myotomy, including the frequency and severity of gastroesophageal reflux, the need for an antireflux fundoplication, the need for intraoperative endoscopy, the extent of myotomy, the need for robotics, and long-term relief of dysphagia. As well, rates of success and failure have been sought, as well as the need for interventions and revisions after myotomy.
This report documents our experience with videoscopic Heller myotomy since we began undertaking laparoscopic Heller myotomies. This report encompasses our journey with laparoscopic Heller myotomy over the last decade, documents our results, and delineates our current approach. In undertaking this review of our experience, we have sought to address several key issues, specifically, rates of complications associated with laparoscopic Heller myotomy, rates of success as determined by patient judgment, and rates of reinterventions. We hypothesized prior to reviewing our cumulative experience that success following laparoscopic Heller myotomy (as judged by patients) would be very high, complications would be relatively infrequent, and interventions after laparoscopic Heller myotomy would be infrequent, though late recurrence of symptoms of achalasia and consequences of reflux would occur.
MATERIALS AND METHODS
Preoperative Assessment
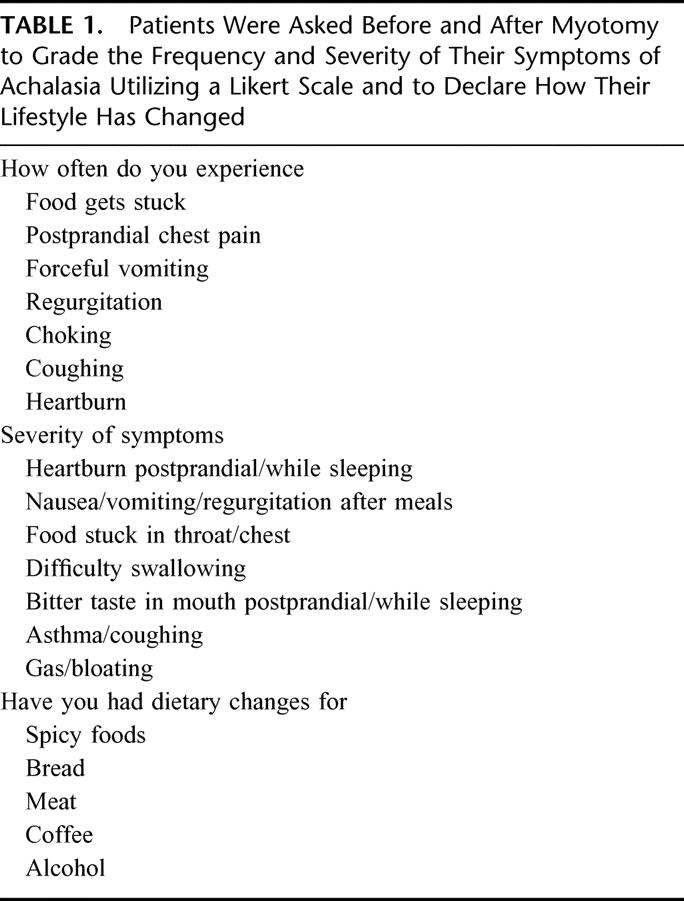
The diagnosis of achalasia was secure in all patients. All patients underwent esophageal manometry, which documented incomplete relaxation of the lower esophageal sphincter mechanism and aperistalsis of the esophageal body, and/or barium esophagram, which showed a distal narrowing or “bird's beak” of the distal esophagus. Prior to myotomy, patients were asked to grade the frequency of symptoms of achalasia, including dysphagia and heartburn symptoms, utilizing a Likert scale (0 = Never; 10 = Always/every time I eat) (Table 1). Prior to myotomy, symptom severity was also graded on a Likert scale (0 = Not bothersome; 10 = Very bothersome) (Table 1). Modifications in dietary habits and sleeping habits, current medications, and previous procedures related to achalasia were also recorded (Table 1).
TABLE 1. Patients Were Asked Before and After Myotomy to Grade the Frequency and Severity of Their Symptoms of Achalasia Utilizing a Likert Scale and to Declare How Their Lifestyle Has Changed
Laparoscopic Heller Myotomy
Patients were operated upon using a 5-port technique (using 4 10-mm trocars and 1 5-mm trocar). Concomitant endoscopy was used to guide the extent of the myotomy in all patients to avoid inadequate myotomy in either the cephalad or caudad direction on the esophagus and to avoid an unnecessarily long myotomy on the gastric cardia.4 Once the obstruction due to achalasia is relieved, it has long been our belief that further myotomy is superfluous, and thereby risks of extensive myotomy are not justified.
Our specific technique for laparoscopic Heller myotomy with intraoperative endoscopy has been previously described.10 Toupet or anterior fundoplication was initially applied in patients that had a large hiatal hernia, that had a patulous esophageal hiatus, or to buttress the repair of an intraoperative esophagotomy. More recently, concomitant anterior fundoplication has been unitized in all patients undergoing laparoscopic Heller myotomy in response to a randomized clinical trial supporting its routine application.11 Anterior fundoplications were constructed to bring the anterior fundus of the stomach up and over the anterior esophagus, generally covering most, but not all, of the myotomized segment. The fundus was generally secured with 4 sutures to esophageal muscle, 2 on the left side and 2 on the right side of the myotomy. Then the esophageal hiatus was sufficiently closed and the fundoplication was secured to the right crus to relieve tension on the fundoplication or twisting of the distal esophagus.
Myotomy was considered to be adequate once 4 criteria were met: bright translumination of the myotomized segment crossing the Z-line as seen through both the laparoscope and through the endoscope, opening of the gastroesophageal junction with gentle insufflation through the endoscope, easy passage of the endoscope into the stomach, and absence of transmural burn or perforation by either direct (visualization with the endoscope or laparoscope) or indirect (presence of bubbles during insufflation through the endoscope while the myotomized segment is under saline irrigant) examination.4
Postoperative Management
Upon leaving the recovery room, patients routinely underwent esophagram to evaluate esophageal emptying and to document subclinical esophagotomy. In the absence of leak, if the esophagus emptied swiftly, a liquid diet was initiated and patients were discharged home on the morning following surgery. They were instructed to resume a more regular diet over the next 2 to 3 weeks but to initially consume liquids and very soft foods such as soups, applesauce, and mashed potatoes. If esophageal emptying was slow following myotomy, postoperative edema was considered to be the problem and liquids were started only after emptying was clinically adequate or repeat contrast studies showed improved emptying. At that point, the usual postoperative diet was initiated and advanced as previously described. If emptying did not improve after myotomy, inadequate myotomy was considered.
Patients were seen in the clinic within the first 1–3weeks following discharge. They were then seen in clinic or contacted by mail or by telephone in the later postoperative period and followed annually thereafter. Follow-up is now available in some patients for more than 9 years.
At the time of each contact, patients were asked to grade the frequency and severity of their symptoms of dysphagia and heartburn (Table 1). Current antiacid medications and any postoperative interventions were noted. Finally, patients were asked to grade their overall outcomes as excellent (complete or near complete resolution of symptoms), good (greatly improved symptoms), fair (slightly improved symptoms), or poor (no improvement or worsening of symptoms) relative to before myotomy. They were also asked to grade their experience from very unsatisfying to very satisfying and to declare if they would again have the operation knowing what they know now. Patients who ultimately underwent esophagectomy for continued or recurrent severe symptoms were considered to have poor outcomes and were then censored from analysis of incidence and severity of symptoms.
Data Management and Statistical Analysis
Data were maintained on an Excel (Microsoft, Redmond, WA) spreadsheet and analyzed by χ2 analysis, Fisher exact test, Student t test, or Mann-Whitney U test, when appropriate using True Epistat (Services, Richardson, TX). Significance was accepted with 95% probability. Where appropriate, data are presented as mean ± SD.
RESULTS
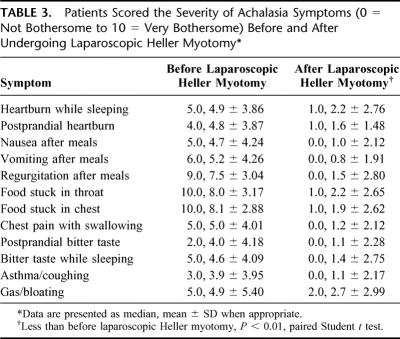
Laparoscopic Heller myotomy has been undertaken in 262 patients, 54% male and 46% female, of average age 49years ± 17.7. The duration of symptoms prior to myotomy was notable in most patients; duration of symptoms averaged 7 years ± 7.4, with a median duration of 5 years. The frequency and severity of preoperative symptoms are denoted in Table 2 and Table 3; 100% of patients complained of dysphagia. A significant majority of patients modified their eating habits and sleeping habits prior to myotomy to palliate their symptoms of achalasia (Fig. 1). They commonly avoided spicy foods, bulky foods (eg, bread, meat, fresh fruit), and coffee (Fig. 2). They commonly slept upright and often in a recliner.
TABLE 2. Patients Scored the Frequency of Symptoms of Achalasia (0 = Never to 10 = Every Time I Eat/Always) Before and After Laparoscopic Heller Myotomy
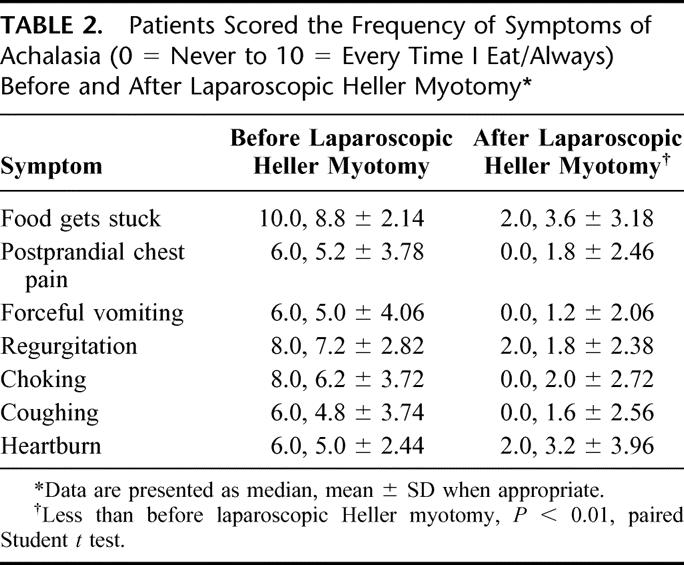
TABLE 3. Patients Scored the Severity of Achalasia Symptoms (0 = Not Bothersome to 10 = Very Bothersome) Before and After Undergoing Laparoscopic Heller Myotomy
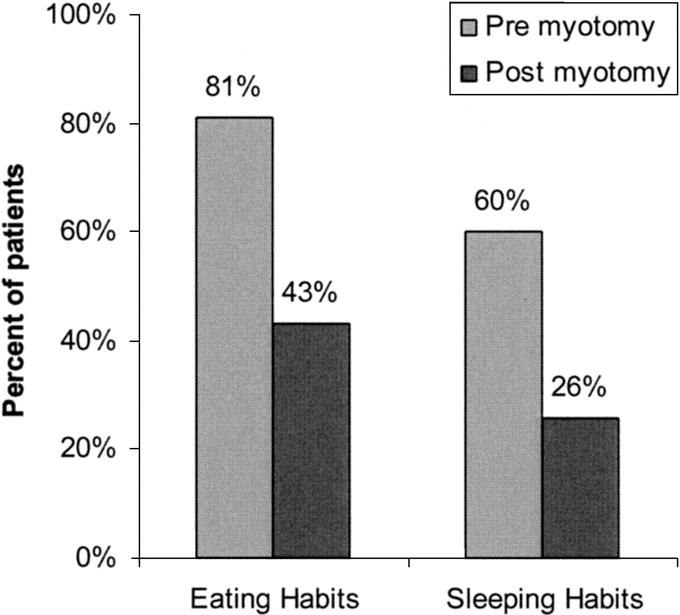
FIGURE 1. Patients reported modifying their eating and sleeping habits before and after laparoscopic Heller myotomy. These modifications were less frequent (P < 0.01, χ2 test) after myotomy.
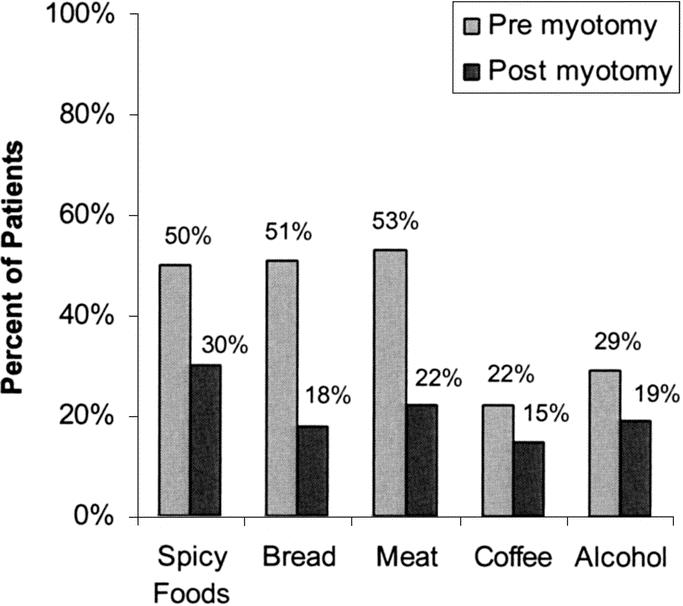
FIGURE 2. Patients reported avoiding these foods before and after laparoscopic Heller myotomy. This occurred were less frequently (P < 0.05, χ2 test) after myotomy.
Prior to myotomy, 59% of patients had undergone pneumatic (ie, balloon) dilation, 52% of patients had undergone Botox injections, 36% had undergone both, and 79% had undergone either (Fig. 3). As well, antiacid therapy, given as proton-pump inhibitors or H-2 receptor blockade, was frequently administered, generally empirically without documentation of excess reflux by pH testing. Eight patients had undergone previous myotomies, and the myotomy noted in this manuscript was a revisional or reoperative myotomy.
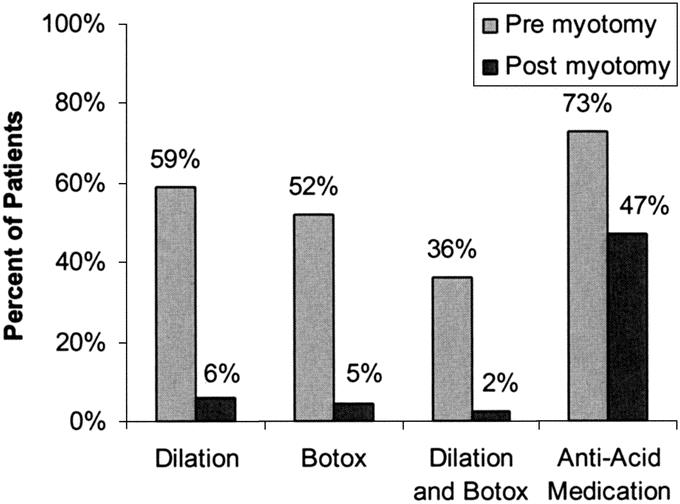
FIGURE 3. Nonoperative therapies undertaken in patients before and after undergoing laparoscopic Heller myotomy.
At the time of laparoscopic myotomy, concomitant diverticulectomy was undertaken in 11 patients. Three patients had gastrostomy tubes removed and their gastrotomies closed. Fundoplication was undertaken because of specific indications in 56 (9 Toupet fundoplications and 47 anterior fundoplications) patients and, later, routinely in 23 consecutive patients (all anterior fundoplications). Other procedures concomitantly undertaken included cholecystectomy in 3patients and parathyroidectomy in 1 patient. The median length of hospital stay was 1 day. Length of stay ranged from 1 to 60days and averaged 3 days ± 4.6.
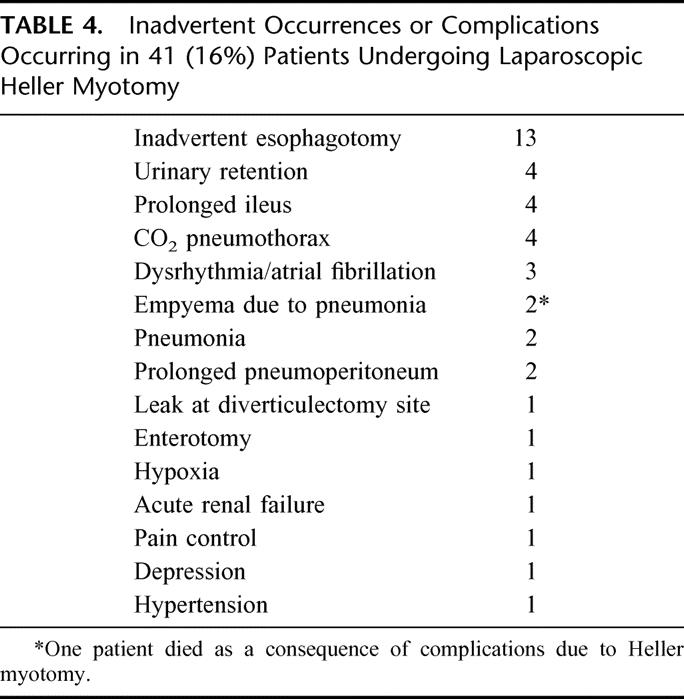
Postoperative complications or inadvertent intraoperative events occurred in 41 (16%) patients. Specifically, inadvertent esophagotomy in occurred in 13 patients and enterotomy occurred in 1 patient (Table 4). Inadvertent esophagotomy did not impact outcome.12 One patient experiencing a postoperative leak did so after an exemplary operation and postoperative esophagogram. Twelve hours after myotomy, he experienced retching and forceful vomiting and, as a consequence, subsequent esophagram documented a leak at his myotomy. He was returned to the operating room, and his esophagotomy was closed and an anterior fundoplication was constructed. His recovery thereafter was uneventful. CO2 pneumothorax occurred in 4 patients and was uneventful. A limited leak from a diverticulectomy site was noted with delayed esophagogram and healed with TPN and nonoperative care. One patient also experienced transient renal failure without explanation. Two patients developed uncomplicated pneumonias. Empyema developed as a consequence of pneumonia in 2 patients. One of these 2 patients died due to sepsis, being the only death in this series due to complications of myotomy.
TABLE 4. Inadvertent Occurrences or Complications Occurring in 41 (16%) Patients Undergoing Laparoscopic Heller Myotomy
Following myotomy, intervention was uncommonly undertaken. Balloon dilation was undertaken in 15 (5.7%) patients, and 12 (4.6%) patients underwent Botox injections, while 6 (2.3%) patients underwent both (Fig. 3) and 8% underwent either. Balloon dilations and Botox injections were generally undertaken without specific indications but rather because of ill-defined symptoms. Revisional myotomies were subsequently undertaken in 4 patients because of persistent symptoms of dysphagia and studies confirming a nonrelaxing lower esophageal sphincter without stricture. These were undertaken at 28 and 66 ± 93.8 days following initial myotomy.
Esophagectomy was ultimately undertaken in 4 patients, all during late-follow-up. Two patients never experienced improvement in dysphagia following laparoscopic Heller myotomy. Following esophagectomy, these 2 patients continued to complain of dysphagia. Two other patients undergoing esophagectomy stated they had excellent to good outcome in early follow-up but developed recurrent dysphagia and underwent esophagectomy for relief of symptoms. For all patients undergoing esophagectomy, profound dysmotility of the esophagus appeared to be the cause of persistent dysphagia. All patients had adequate myotomies by timed barium study and esophagoscopy.
Reflux symptoms were noted in 238 (91%) patients before myotomy. Antacid medication use (proton pump inhibitors or H-2 blocker therapy) was noted before myotomy in 191 (73%) patients. Following myotomy, significantly fewer patients reported use of these medications (Fig. 3). Utilization of these medications following myotomy was generally empiric and not based on endoscopic findings or specific gastroesophageal reflux testing (eg, ambulatory pH testing).
Current follow-up is established in 216 patients. An additional 20 patients have died due to congestive heart failure (1), cancer (1), myocardial infarction (2), or unknown causes (16). It is believed that achalasia and/or laparoscopic Heller myotomy played no roles in their deaths. Current contact is lost with 30 patients, though follow-up beyond short term was obtained for each. Median follow-up is 25.3months, and mean follow-up is 31.8 ± 28.71 months. Follow-up is limited by the more recent undertaking of most of the myotomies (Fig. 4). All symptoms associated with achalasia improved dramatically in frequency and severity (Tables 2 and 3). This is particularly true for symptoms associated with dysphagia.
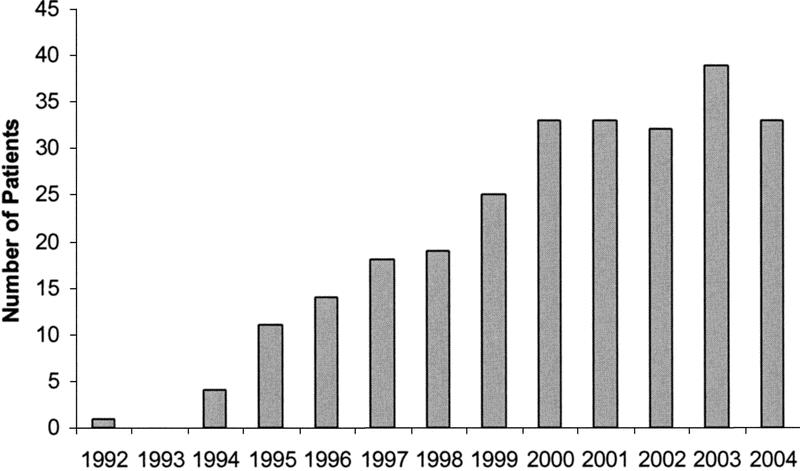
FIGURE 4. The number of laparoscopic Heller myotomies undertaken annually through October 2004.
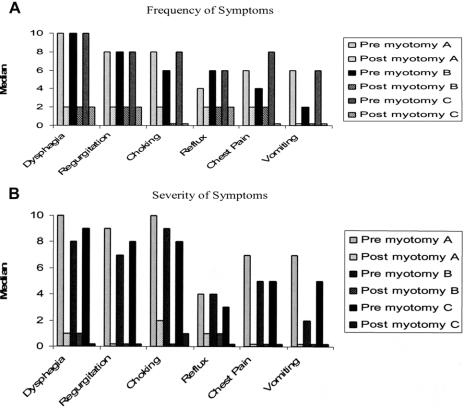
For comparison purposes, patients were stratified into groups of patients that underwent Heller myotomy without fundoplication, Heller myotomy with fundoplication for specific indications, and Heller myotomy with routine application of anterior fundoplication. The frequency and severity of preoperative and postoperative symptoms of these patients are compared in Figure 5. While all groups did well, patients undergoing concomitant fundoplication experienced a bit less (P = 0.09) choking and chest pain after myotomy. There were no notable differences in the frequency or severity of reflux among these groups of patients, and, as well, no notable differences in symptoms associated with dysphagia (Fig. 5).
FIGURE 5. Achalasia symptoms before and after laparoscopic Heller myotomy. For comparison purposes, patients were stratified into groups: A (Heller myotomy without fundoplication), B (Heller myotomy with fundoplication for specific indications), and C (Heller myotomy with routine application of fundoplication). Frequency of symptoms was graded 0 (Never) to 10 (Always/every time I eat). Severity of symptoms was graded 0 (Not bothersome) to 10 (Very bothersome). Frequency and severity of symptoms decreased significantly (P < 0.01, paired Student t test) after myotomy, whether or not a fundoplication was applied.
At most recent follow-up, 88% of patients rated their relief of symptoms as good or excellent. Poor or fair outcomes were noted by 12% of patients with most recent follow-up (Fig. 6). Of patents reporting fair or poor outcomes, myotomy was assessed to be adequate by esophagogram and/or by endoscopy in all. Fair or poor outcomes appeared to be due to dysmotility proximal to the myotomy rather than due to inadequate relief from lower esophageal sphincter function. Following laparoscopic Heller myotomy, 90% of patients thought that their experience was either very satisfying or satisfying (Fig. 7). As well, following laparoscopic Heller myotomy, 93% of the patients said that they would “still have the operation” if they knew before the operation what they know now.
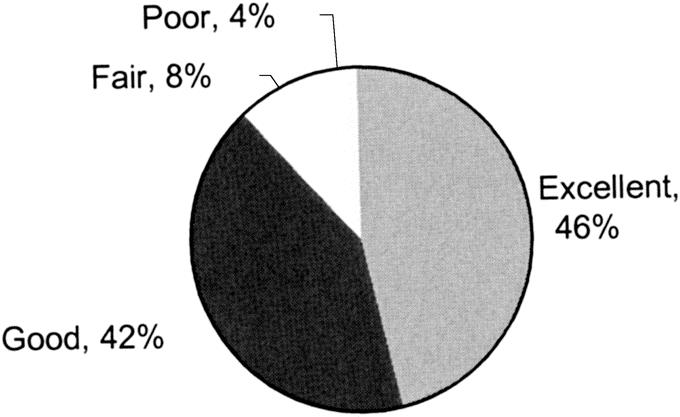
FIGURE 6. Symptom relief following laparoscopic Heller myotomy as graded by patients at latest follow-up. Excellent, nearly/completely resolved symptoms; good, greatly improved symptoms; fair, slightly improved symptoms; poor, no improvement/worsened symptoms.
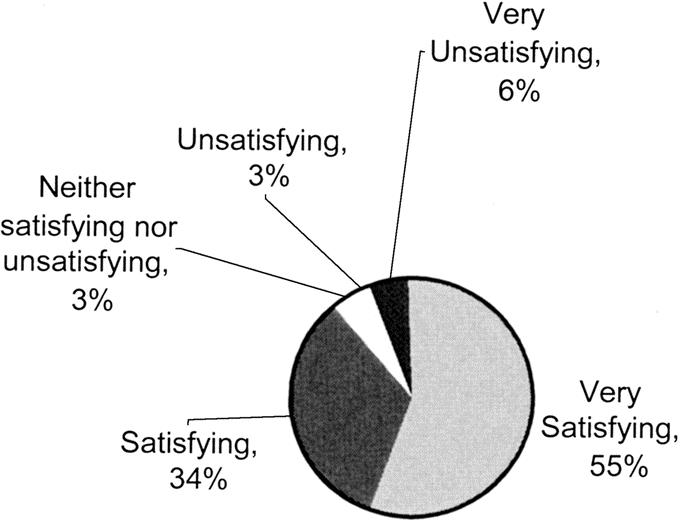
FIGURE 7. Overall experience of patients that underwent laparoscopic Heller myotomy reported at latest follow-up. Very satisfying; Satisfying; Neither satisfying nor unsatisfying; Unsatisfying; Very unsatisfying.
DISCUSSION
Experience with laparoscopic Heller myotomy for symptomatic achalasia continues to build. Over the past decade, many issues about laparoscopic Heller myotomy have been considered, and while we now know that laparoscopic Heller myotomy can be undertaken with generally strong results, many questions remain. This report about laparoscopic Heller myotomy for symptomatic achalasia is the largest series yet reported. This report helps answer some questions about outcomes and complications after laparoscopic Heller myotomy, begins to answer some others, and forms a foundation for discussions about specific questions and issues about future applications of laparoscopic Heller myotomy.
This series is a consecutive series of “all comers.” Thus, the age range is broad, though most patients were of middle age. The gender distribution was nearly equal. The duration of symptoms prior to myotomy was notable, averaging many years. Preoperative symptoms of achalasia were frequent and significant and imparted tremendous morbidity, especially obstructive symptoms exacerbated by eating.
Before myotomy, most patients had changed their lifestyles and eating habits to accommodate their obstructive process and had learned to live with and accept altered lifestyles for years. Changes in eating habits were particularly impressive.
With laparoscopic Heller myotomy, many concomitant procedures were undertaken, although in a minority of patients. Concomitant esophageal diverticulectomy was undertaken in a small but notable number of patients. Diverticulectomy was undertaken without meaningful mishap, though 1 patient experienced extravasation of contrast at a delayed esophagogram and received TPN while refraining from oral intake until a subsequent esophagogram demonstrated no leak. This patient has done very well, as have the patients undergoing diverticulectomy in general.13 Cholecystectomies and repairs of gastrostomies were infrequently undertaken and were safely undertaken without sequela. Most commonly, anterior fundoplication was undertaken. Very early in our experience, Toupet fundoplications were applied, but they subjectively seemed to provide excessive resistance to esophageal emptying, and their application was quickly ended. Building on our early experience with antireflux surgery, we became comfortable with anterior fundoplications, which we selectively applied. Anterior fundoplications ideally provided protection after esophagotomy, and outcomes following anterior fundoplications subjectively seemed better than after posterior (Toupet) fundoplications. With the report of a randomized trial supporting routine application,11 we now routinely apply anterior fundoplication, though our results clinically seem no better than when it was selectively applied.
Complications occurred in a minority of patients after laparoscopic Heller myotomy. Most, like CO2 pneumothorax, were uneventful or nearly so. Others, such as transient cardiac dysrhythmias, imparted no morbidity and go along with operating on older patients. Some, such as pneumonia, seemed due to exacerbations of preoperative conditions, such as aspiration due to esophageal obstruction. Unfortunately, pneumonia was complicated by empyema in 2 patients and 1died. This death, due to sepsis in an elderly lady, was the only death in this series.
Inadvertent esophagotomy occurred in a small but significant number of patients in this series. Some might presume that inadvertent esophagotomy occurs more often after or as a consequence of preoperative endoscopic therapy. Our data to date suggest this14 but do not prove this.15 Inadvertent esophagotomy occurring with laparoscopic Heller myotomy occurred for technical reasons, specifically, when the muscle was so stuck to the submucosa that no plane between the 2 could be developed and the submucosa and mucosa were violated with the myotomy. Repair was undertaken with polypropylene sutures, and an anterior fundoplication was applied to buttress the repair. Esophagotomy and repair, including fundoplication, do not seem to impact long-term outcome, even when it occurs postoperatively, as it did in 1patient.12 Nonetheless, esophagotomy with laparoscopic Heller myotomy is very disconcerting to both surgeon and patient. Its occurrence certainly cannot be encouraged, but its occurrence does not need to impact long-term outcome, and it certainly denotes that the myotomy is deep enough.
After laparoscopic Heller myotomy, length of stay was short, and long stays in only a few patients increased the mean length of stay over 1 day. The enterotomy with laparoscopic Heller myotomy was not apparent for days following the myotomy and was detected late after myotomy or progressed to enterotomy over the days following myotomy. Nonetheless, a few long hospital stays can greatly increase the mean length of stay when the median is so short (ie, 1.0day). Presumably, more discharges could have occurred the day of surgery, but to date there seems no urgency to accomplish this, and discharge the morning after myotomy allows for further coaching on diet and expectations.
Endoscopic or operative interventions were uncommonly undertaken after myotomy, especially the latter. A very small number of patients required reoperations within a month of myotomy (2), or late after myotomy (2). Early failures were a consequence of the myotomy not being carried adequately caudad. Extending the myotomy onto the stomach relieved the obstructive process and relieved symptoms. Late operations included revisional myotomies and esophagectomies. Esophagectomy was applied because dysmotility along the esophagus was felt to be the primary problem. The myotomy in each seemed adequate by timed barium studies, and, for each, barium tablets passed very slowly until the myotomized segment was reached. Patients that have had revisional, or reoperative, laparoscopic Heller myotomies continue to do well. Our results after esophagectomy are mixed, though others report better long-term results,16 though with great morbidity.
Relative to reoperative procedures, pneumatic dilations and/or Botox are much more frequently applied after myotomy, though their application is infrequent. Generally, their application is brought about by well-intended gastroenterologists without data specifically pointing to an inadequate myotomy or specific distal esophageal emptying restrictions (eg, strictures). Rather, their application is empiric for patients with ongoing complaints after myotomy. Often, the complaints are nonspecific, such as “chest pain” or “chest discomfort,” and not notably exacerbated by eating. Patients with these complaints before myotomy generally continue to have them after myotomy, and they are presumably due to esophageal spasm or dysmotility. Dilation or Botox would not be expected to help, and they generally do not.
Recurrent obstructive symptoms of achalasia should always raise concerns for a reflux induced stricture. Gastroesophageal reflux has been noted in some patients, consistent with other reports,1,3,5 but reflux-induced strictures have not been encountered. Pneumatic or brusk dilation will help patients with reflux-induced strictures, denoting the importance of investigating recurrent obstructive symptoms after myotomy with timed barium studies and esophagoscopy. These studies should document all anatomic correlates to obstructive symptoms at or above the gastroesophageal junction. With recurrent obstructive symptoms, always first consider and look for a reflux-induced stricture. In the absence of that, look for an “again functioning” LES and, failing that, extremely poor esophageal emptying as a consequence of dysmotility.
Following myotomy, patients should expect reductions in the frequency and severity of symptoms. Though some people will have persistent or recurrent symptoms after myotomy, most patients will have profound and persistent relief from troubling symptoms of esophageal obstruction. Furthermore, they will have relief from symptoms not directly associated with achalasia, like coughing. As well, patients will have dramatic reductions in the frequency and severity of heartburn, whether or not concomitant fundoplication is undertaken. Symptoms of gas and bloating do not increase after relief of the outlet obstruction with or without concomitant fundoplication; rather, they notably decrease. This is similar to our findings after fundoplication for GERD.17
Routine application of an antireflux procedure concomitant with myotomy seems to be the current standard. This approach is supported with strong clinical data.11 However, fundoplication may be superfluous1,14 as our data with or without fundoplication is equally strong, for whatever reason the fundoplication was applied. Furthermore, reflux in the control group of the randomized trial11 seemed excessive and was greater than the authors had previously noted after myotomy without fundoplication.5 Nonetheless, we have not studied many of our patients with ambulatory pH studies. With increased availability of the Bravo pH system, maybe we will be able to study more of our patients and further delineate who needs a concomitant antireflux procedure.
Antisecretory medication use was commonly applied prior to myotomy. Almost always, this is without ambulatory pH testing, but rather in response to symptoms. While a tight lower esophageal sphincter should limit gastroesophageal reflux, a tight lower esophageal sphincter and esophageal dysmotility should also limit esophageal clearance. Given the lack of short-term complications of antisecretory medications, empiric therapy treating symptoms is not a monumental issue, but open-ended antisecretory therapy cannot be encouraged. After myotomy, antisecretory therapy is again almost always empiric. If given beyond short term, ambulatory pH testing should be undertaken to learn about reflux after myotomy and to better direct long-term care, including the need for surveillance endoscopy.
Follow-up beyond 5 years is available for a relatively large number of patients, though more than half of the patients in this series underwent myotomy less than 5 years ago (Fig. 4). As well, follow-up is limited by being able to stay in touch with patients. Some patients are lost to follow-up for a variety of reasons. Relocation is, by far and away, the biggest problem for follow-up. No patients refuse to see us or communicate with us. We continue to make great efforts with follow-up and will continue to do so. Early outcome after myotomy does not uniformly predict long-term outcome, especially for patients with persistent symptoms of esophageal obstruction, but outcome does not change in many patients after early follow-up.18,19
Overall, patients have been pleased with their outcomes after laparoscopic Heller myotomy, with or without fundoplication, consistent with other reports. Nearly 9 of 10 feel their symptoms of achalasia are greatly improved or resolved. Patients reporting poor outcomes have had adequate myotomies but are plagued by symptoms due to profound esophageal dysmotility. As well, 9 of 10 patients feel their experience has been at least satisfying, and more than 9 of 10 would undergo the operation again if necessary, thereby promoting the continued application of laparoscopic Heller myotomy.
Discussions
Dr. John G. Hunter (Portland, Oregon): This paper by Rosemurgy and colleagues from south Florida represents an important contribution to our understanding of the minimally invasive surgical management of achalasia.
In the 1980s, despite much surgical literature demonstrating that Heller myotomy better frees the patient with achalasia from troublesome dysphagia than did either balloon dilation or Botox, patients 10 to 1 chose balloon dilation to thoracotomy when offered both options. Laparoscopic surgery largely changed that.
In the early 1990s, GI doctors admitted at the outcomes of thoracoscopic or laparoscopic Heller myotomy were of sufficient quality that it might be a decent option if dilation or Botox failed. In 1998, in a landmark state-of-the-art lecture at the International Society for Diseases of the Esophagus, John Dent, a GI physician from Adelaide, stated that all available evidence strongly indicated that, for achalasia, the primary therapy should be laparoscopic Heller myotomy and partial fundoplication. What an abrupt reversal!
A formerly rare (I admit I never cared for an achalasia patient during my residency) operation for a rare disease became relatively commonplace, especially in centers where expertise in esophagoscopy and minimally invasive surgery cluster. Such was clearly the case in south Florida.
After reading the very clear manuscript of Dr. Rosemurgy, I have only 3 questions: I notice that most of your patients had Botox or balloon therapy before myotomy. Have you seen this number decrease with time, more patients referred primarily for surgery, or is my good friend Worth Boyd still using Botox or balloon dilation first on most of your patients?
Following on the first question, what percentage of the entire achalasia population coming to south Florida does this series represent? Do the 262 patients in this study represent a minority or a majority of patients receiving therapy for achalasia in your esophageal center? How successful are the nonoperative therapies?
Last, what do we do with the myotomy failures? We have found that residual resting pressure in the LES best defines the likelihood a patient will respond to remyotomy. While we may still offer redo myotomy to patients with dysphagia, poor esophageal emptying, and a lower esophageal sphincter pressure less than 10, our experience has been that most of these patients require esophagectomy. On the other hand, patients with resting LESP pressures greater than 15 have done reasonably well with redo myotomy. How do you decide whether reoperation is warranted, and how do you determine what operation to advise?
Dr. Stephen B. Vogel (Gainesville, Florida): I would like to congratulate Dr. Rosemurgy and the other authors on this outstanding paper documenting excellent relief of the symptoms of achalasia with minimum complications, extremely shortened length of stay, and, perhaps most importantly, minimal postoperative symptoms of esophageal reflux.
My first question concerns the issue of postoperative gastroesophageal reflux. During the past decades and prior to the laparoscopic era, the standard approach to achalasia was a limited myotomy through the left chest, preserving the integrity of the phrenoesophageal ligaments, thereby leading to minimal incidence of postoperative reflux. Since the laparoscopic transabdominal approach to the myotomy mobilizes the EG junction and carries the myotomy onto the stomach, the potential for postoperative reflux is fairly high. In this series, the postoperative incidence of “heartburn” or symptoms of reflux are quite low, with the overall group reporting extremely satisfactory results. It is also interesting that preoperatively, significant percentages of patients report symptoms of “heartburn.” Is it possible that these patients with known achalasia and a high LES pressure have esophageal-esophageal reflux as the etiology of their symptoms, and following surgery, the myotomy leads to better esophageal emptying and a decrease in these preoperative symptoms? We have often used nuclear medicine esophageal liquid emptying studies to document that esophageal-esophageal reflux occurs frequently and leads to symptoms mimicking gastroesophageal reflux in patients with achalasia. Is this the explanation of the significant improvement in symptoms during the postoperative period?
My second question refers to the fairly high incidence of prior Botox injections in an attempt to palliate the achalasia nonoperatively. It has been our experience that Botox injections lead to a significant thickening of the esophageal wall, with essentially a “fusing” of the mucosa and the submucosa. Did you find evidence of this during surgery, and could these findings have caused in any way the small number of complications that occurred? Finally, and as the manuscript considers the issue of postoperative reflux, I would like to hear your comments on whether the anterior fundoplication actually is effective in terms of preventing reflux or, as suggested, it may be irrelevant. Certainly your findings suggest an extremely low incidence of this postoperative condition.
Dr. Kenneth W. Sharp (Nashville, Tennessee): We have a series now of 207 laparoscopic or thoracoscopic Heller myotomies we have recorded at Vanderbilt University, and I note that our striking similarity and results as far as esophagotomies, morbidity, mortality, and outcomes are extremely similar to yours. So that immediately biases me to agree with many of your conclusions. I have 2 brief comments.
One is the number of patients who underwent preoperative balloon dilatation at 59%. That is 154 patients who underwent a procedure that carries a 3% to 5% risk of perforation and a 1% chance of mortality. At Vanderbilt, we have educated our gastroenterologists, and there has not been a pneumatic dilatation for achalasia done since 1994. I would encourage you to make the same effort with your gastroenterologists.
The second comment. I appreciate the manuscript's recognition of our work on a randomized prospective trial comparing an anterior hemifundoplication added to a Heller myotomy and its results in preventing reflux. You make the point that there is no correlation in the symptoms with whether or not the patient has had an antireflux procedure or not.
What I would caution you is that one of the most important lessons I have learned over the years in taking care of achalasia is that there is essentially no correlation between symptoms of reflux and heartburn and true abnormal acid exposure when measured by 24-hour pH studies.
The paper we presented here 3 years ago showed a zero correlation between abnormal acid reflux and symptoms of heartburn. There is a very striking disconnect there. These patients who report chest pain postoperatively cannot tell the difference between esophageal spasm and heartburn.
There was a short abstract presented at DDW a few years ago in which balloons were inflated in the esophagus of patients with achalasia; they could not tell the degree of balloon dilatation as normal patients can. They also instilled hydrochloric acid or saline, and achalasia patients could not tell the difference like normals could. These folks do not have normal sensation.
I have 2 or 3 quick questions. In the manuscript, you mention you used the Toupet fundoplication early on in your experience and then abandoned it because of poor outcomes. Can you tell me a little bit more about that and what you did about it?
I would also like to know how you handled the megaesophagus or the patient with the true sigmoid esophagus, the patient with a 15-cm esophagus. I have found those folks subjectively in small numbers not to do quite as well with a Heller myotomy.
Finally, Dr. Hunter made the cogent question about: What do you do with your failures? How do you investigate them? Do you do routine manometry studies on them to determine what the residual LES pressure is? And we, as Dr.Hunter mentioned, we are tending not to redo myotomies inpatients who have low LES pressures after this procedure. We haven't found it really made much more difference for them. I would like to hear your comments on how you investigate and manage your failures.
Dr. Alexander S. Rosemurgy, II (Tampa, Florida): We have not noticed a decrease in the administration of Botox or the use of bag dilations. Probably more Botox is used now than ever. Maybe there is a decrease in the use of bag dilation at our facility, but Botox is looked at as being safe and efficacious. I think that there is some sense that Botox moves the patient down some algorithm. Botox doesn't “cure” the patient; nonetheless, it continues to be used. A lot of patients get sent in from cities outside Tampa, and bag dilation there seems to be more frequently applied than in our immediate environment. We currently are embarking on a randomized trial of myotomy versus bag dilation to identify their respective roles.
The failure of myotomy to palliate symptoms is a perplexing issue. The first thing I always consider is a reflux-induced stricture. If there is not a reflux-induced stricture, then I would consider remyotomy if there is an emptying problem at the esophagus gastric junction, regardless of what the LES pressures are. I then do myotomy in a different location across the LES and then an anterior fundoplication.
We stopped doing Toupet fundoplications because we perceived that there was excessive postoperative dysphagia relative to patients receiving no antireflux procedure. With our growing experience in antireflux surgery, we began to utilize anterior fundoplications more often. We are much happier with anterior fundoplications than with Toupet fundoplications.
Going back to Botox and dilation, the only use for Botox at this point for us is as a therapeutic trial for patients with an uncertain diagnosis of achalasia. Sometimes the diagnosis isn't as clear cut as it should be, and Botox is a worthwhile therapeutic trial.
I do think Botox and dilation make myotomy more difficult. Over the years, I have recorded how difficult each myotomy is so I can go back and look at how premyotomy therapy impacts myotomy. There probably is an inherent bias in reporting this relationship. I do think Botox and dilation make the myotomy more difficult. Do preoperative interventions impact outcome? Our data are a bit conflicting, depending on the patients studied.
Persistant dysmotility after myotomy is a real issue. I agree with what Dr. Vogel was saying. There is such a thing as esophageal rumination. With UGI contrast studies and barium tablets, the barium tablet may descend in the esophagus and go back and forth for a while before it ultimately gets down to the lower esophagus, and after the myotomy it goes quickly through the gastroesophageal junction. If patients vociferously complain of dysphagia, as four patients did in this trial, and it leads to esophagectomy, I believe it has to be undertaken with some concern, because it is a relatively morbid operation for these patients and the outcome is not as good as we would like it to be.
Last, but not least, the pH studies are really important. We have not done that only because patients won't do it. The Bravo system hopefully changes that. But I rise to agree with Dr. Sharp that there may be a striking disconnect between the symptoms of reflux and the incidence of reflux based on pH studies.
As far as the megaesophagus and the S-shaped esophagus are concerned, I believe the most important factor in determining outcome is to establish adequate emptying. I will mobilize the esophagus up into the mediastinum and attempt to straighten it out, whatever that is worth, and make sure that I have a good length, about 8 cm, of intraabdominal esophagus, reconstruct the hiatus, and construct an anterior fundoplication, making sure it is not too tight to prevent reflux and dysphagia. Patients with time will have improvement in the appearance of their esophagus by x-ray study, for whatever that is worth, but symptoms are well palliated as long as there is no redundant area in the esophagus in the chest where food or liquids will pool. Thank you.
Footnotes
Reprints: Alexander S. Rosemurgy, MD, Professor of Surgery, Professor of Medicine, University of South Florida, C/O Tampa General Hospital, PO Box 1289, Room F-145, Tampa, FL 33601. E-mail: arosemur@hsc.usf.edu.
REFERENCES
- 1.Lyass D, Thoman J, Steiner P, et al. Current status of an antireflux procedure in laparoscopic Heller myotomy. Surg Endosc. 2003;17:554–558. [DOI] [PubMed] [Google Scholar]
- 2.Ramacciato G, Mercantini P, Amodio PM, et al. Minimally invasive surgical treatment of esophageal achalasia. JSLS. 2003;7:219–225. [PMC free article] [PubMed] [Google Scholar]
- 3.Ponce M, Oritz V, Juan M, et al. Gastroesophageal reflux, quality of life, and satisfaction in patients with achalasia treated by open cardiomyotomy and partial fundoplication. Am J Surg. 2003;185:560–564. [DOI] [PubMed] [Google Scholar]
- 4.Bloomston M, Brady P, Rosemurgy A. Videoscopic Heller myotomy with intraoperative endoscopy promotes optimal outcomes. JSLS. 2002;6:133–138. [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp K, Khaitan L, Schotz S, et al. 100 Consecutive minimally invasive Heller myotomies: lessons learned. Ann Surg. 2002;235:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finley R, Clifton J, Stewart K, et al. Laparoscopic Heller myotomy improves esophageal emptying and the symptoms of achalasia. Arch Surg. 2001;136:892–896. [DOI] [PubMed] [Google Scholar]
- 7.Bloomston M, Serafini F, Rosemurgy A. Videoscopic Heller myotomy as first-line therapy for severe achalasia. Am Surg. 2001;67:1105–1109. [PubMed] [Google Scholar]
- 8.Zaninotto G, Costantini M, Molena D, et al. Minimally invasive surgery for esophageal achalasia. J Laparoendosc Adv Surg Tech. 2001;11:351–359. [DOI] [PubMed] [Google Scholar]
- 9.Bloomston M, Serafini F, Boyce HW, et al. The “learning curve” in videoscopic Heller myotomy. JSLS. 2002;6:41–47. [PMC free article] [PubMed] [Google Scholar]
- 10.Bloomston M, Boyce W, Mamel J, et al. Videoscopic Heller myotomy for achalasia: results beyond short term follow-up. J Surg Res. 2000;92:150–156. [DOI] [PubMed] [Google Scholar]
- 11.Richards W, Torquati A, Holzman M, et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia. Ann Surg. 2004;240:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakita S, Bloomston M, Giarelli N, et al. Esophagotomy during laparoscopic Heller myotomy can not be predicted by application of preoperative therapy and does not influence long-term outcome. J Gastrointest Surg. In press. [DOI] [PubMed]
- 13.Fraiji E, Bloomston M, Carey L, et al. Laparoscopic management of symptomatic achalasia associated with epiphrenic diverticulum. Surg Endosc. 2003;17:1600–1603. [DOI] [PubMed] [Google Scholar]
- 14.Bloomston M, Rosemurgy A. Selective application of fundoplication during laparoscopic Heller myotomy. Surg Laparosc Endo Percutan Tech. 2002;12:309–315. [DOI] [PubMed] [Google Scholar]
- 15.Bloomston M, Fraiji E, Boyce HW, et al. Preoperative Intervention does not affect esophageal muscle histology or patient outcomes in patients undergoing laparoscopic Heller myotomy. J Gastrointest Surg. 2003;7:181–188. [DOI] [PubMed] [Google Scholar]
- 16.Miller D, Allen M, Trastek V, et al. Esophageal resection for recurrent achalasia. Ann Thorac Surg. 1995;60:922–925. [DOI] [PubMed] [Google Scholar]
- 17.Bloomston M, Zervos E, Gonzalez R, et al. Quality of life and antireflux medication use following laparoscopic Nissen fundoplication. Am Surg. 1998;64:509–513. [PubMed] [Google Scholar]
- 18.Bloomston M, Durkin A, Boyce HW, et al. Early results of laparoscopic Heller myotomy do not necessarily predict long-term outcome. Am J Surg. 2994;187:403–407. [DOI] [PubMed] [Google Scholar]
- 19.DiSimone M, Felice V, D'Errico A, et al. Onset timing of delayed complications and criteria of follow-up after operation for esophageal achalasia. Ann Thorac Surg. 1996;61:1106–1111. [DOI] [PubMed] [Google Scholar]