Abstract
Objective:
Complications of anastomotic healing are a common source of morbidity and mortality after esophagogastrostomy. The delay phenomenon is seen when a skin flap is partially devascularized in a staged procedure prior to its definitive placement, resulting in increased blood flow at the time of grafting. This effect may be applied to esophagogastrectomy, potentially reducing anastomotic complications.
Summary Background Data:
The purpose of this investigation was to apply the delay principle to the gastrointestinal tract, investigate mechanisms by which it occurs and examine the effects of delay on anastomotic healing.
Methods:
Thirty-seven opossums were assigned to Sham (n = 5), Immediate (n = 14), and Delay (n = 18) groups. Each underwent laparotomy and measurement of baseline gastric fundus blood flow. The Delay and Immediate animals underwent ligation of the left, right, and short gastric vessels and subsequent measurement of gastric fundus blood flow. The Delay group underwent repeat measurement of blood flow, esophagogastrectomy, gastric tubularization, and esophagogastrostomy 28 days after vessel ligation. The Immediate group completed the procedure immediately after vessel ligation. The anastomoses in both groups were harvested 32 days after esophagogastrostomy. The Sham group underwent blood flow measurement on initial laparotomy, followed by harvesting of esophagogastric junction 60 days later. Sections taken through the anastomoses were examined with trichrome-staining and immunohistochemistry (IHC) for actin. Collagen content of the gastric submucosa 5 mm below the anastomosis was quantified, and preservation of the muscularis propria and muscularis mucosa was determined histologically. Capillary content of the esophagogastric junction was quantified using IHC for vascular endothelium in the Delay and Sham groups.
Results:
Blood flow decreased by 73% following vessel ligation in Delay and Immediate groups. The Delay group had over 3 times the gastric blood flow of the Immediate group at the time of anastomosis at 16 (interquartile range [IQR] 11–17) versus 5, (IQR 5–6) mL/min/100 g (P = 0.000003). Two Immediate animals developed anastomotic leak and died; the Delay group had no complications. Submucosal collagen content in Sham, Delay, and Immediate groups were 57% (IQR 52–62), 65% (IQR 57–72), and 71% (IQR 60–82), respectively (P = 0.0004). The median distance of full-thickness atrophy of the muscularis propria was 0.10 mm (IQR 0–0.60 mm) in the Delay group and 0.53 mm (IQR 0.03–0.80 mm) in the Immediate group (P = 0.346). Five percent of the Delay group had atrophy of the muscularis mucosa, whereas 19% of Immediate animals had atrophy of this layer (P = 0.023). Compared with the Sham group, all Delay animals developed dilation of the right gastroepiploic artery and vein. A median of 27 (IQR 23–33) capillaries per 20× field was observed in the Sham fundus and 38 (IQR 31–46) in the Delay fundus (P = 0.037).
Conclusions:
The delay effect is associated with both vasodilation and angiogenesis and results in increased blood flow to the gastric fundus prior to esophagogastric anastomosis. Animals undergoing delayed operations have less anastomotic collagen deposition and ischemic injury than those undergoing immediate resection. Clinical application of the delay effect in patients undergoing esophagogastrectomy may lead to a decreased incidence of leak and stricture formation.
Leakage and stricture are common causes of morbidity and mortality after esophagectomy. The delay effect as observed in the stomach results in increased blood flow to the gastric fundus prior to esophagogastric anastomosis. Animals undergoing delayed operations have less anastomotic collagen deposition and ischemic injury than those undergoing immediate resection. Clinical application of the delay effect in patients undergoing esophagogastrectomy may lead to a decreased incidence of leak and stricture formation.
The gastric tube is a well-established conduit for esophageal replacement in the treatment of esophageal malignancy, Barrett esophagus with high-grade dysplasia, and end-stage benign disorders.1 Unfortunately, anastomotic leakage and/or stricture are common complications after esophagectomy. Anastomotic leaks may lead to sepsis and carry a high mortality rate.2,3 Strictures create long-term dysphagia, which can greatly diminish quality of life. In the setting of advanced regional cancer when long-term survival is unlikely, the failure to alleviate dysphagia definitively is unacceptable.
Ischemia has been cited as the primary mechanism responsible for anastomotic dehiscence and stricture formation.3 Because a significant amount of gastric devascularization is required to facilitate pull-up of the gastric conduit after esophageal resection, the neoesophagus is dependent upon collateral blood flow that is based upon a single vascular pedicle.4 This procedure results in acute ischemia of the gastric fundus and thus poor arterial inflow and/or venous drainage at the level of the esophagogastrostomy, which is created at a point most distal from the pedicle.4,5
Prior to the introduction of tissue expanders and free tissue transfer, plastic surgeons often used the “delay” phenomenon in the preparation of large skin flaps for transfer. In this process, the definitive transfer of a pedicled tissue flap is delayed several weeks after it has been partially devascularized, thereby allowing the development of increased blood flow to the distal aspect of a pedicle.6 Although the mechanism of action is unknown, studies have suggested that the compensatory increase in blood flow occurs as a result of arterial dilation.6,7 In the gastrointestinal equivalent of the delay procedure, the gastric pull-up and anastomosis are performed several weeks after the stomach has been partially devascularized. This procedure may enhance the perfusion of the distal gastric tube at the time of anastomosis and thus decrease anastomotic complications after esophagogastrectomy. The purpose of this investigation was to apply the delay principle to the gastrointestinal tract, explore the mechanisms by which it occurs, and examine the effects on anastomotic healing.
METHODS
Animal Model and Study Design
The North American opossum (Didelphis virginiana) was chosen as the animal model for this study because it possesses physiologic and anatomic similarities to the human foregut.8 Thirty-seven animals, managed under the regulations of the Animal Care and Use Committee of the Portland Veterans Administration Medical Center, were assigned to Sham (n = 5), Delay (n = 18), and Immediate (n = 14) groups. The Delay and Immediate animals underwent laparotomy and vascular ligation, with measurement of gastric fundus blood flow before and after ligation. In the Immediate group, esophagogastrectomy, gastric tubularization, and esophagogastrostomy were completed during the initial laparotomy. In Delay animals, a second laparotomy was performed 28 days after vessel ligation, during which the group underwent blood flow measurements, esophagogastrectomy, gastric tubularization, and esophagogastrostomy. The anastomoses in both groups were harvested 32 days after esophagogastrostomy. The Sham group underwent an initial laparotomy in which blood flow was measured. On postoperative day 60, the esophagogastric junction was harvested (Fig. 1).
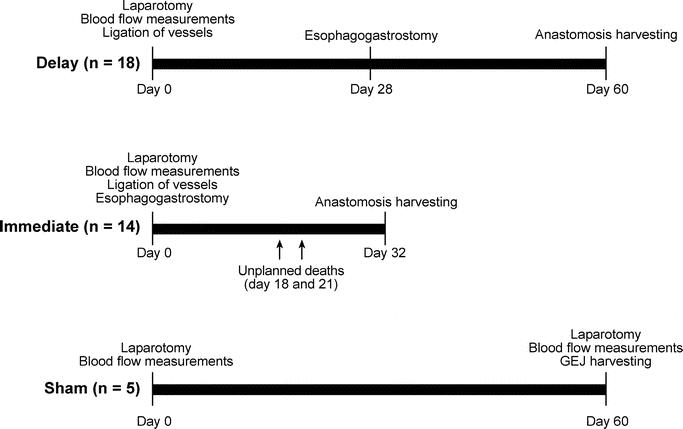
FIGURE 1. Timeline of interventions for the Delay, Immediate, and Sham groups.
The endpoints assessed were the delay effect on gastric blood flow (laser Doppler flowmetry), anastomotic healing (stricture and leak rate, collagen density, preservation of the muscularis propria and muscularis mucosa), and mechanism of delay (subjective assessment of vessel diameter and quantity of capillaries at the level of the anastomosis).
Surgical Technique
All animals underwent a 2-week acclimation period and were fasted for 12 hours prior to operation. Prior to initiating the study, visceral angiography was performed in a separate group of animals to define gastric vascular anatomy and confirm that the right gastroepiploic vessels were the only blood supply to the stomach after ligation of the left, right, and short gastric vessels (Fig. 2).
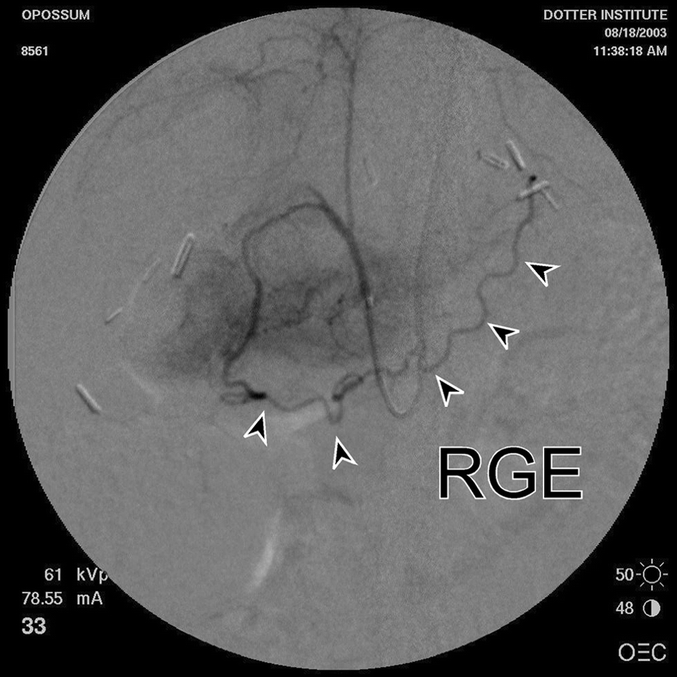
FIGURE 2. Postligation visceral angiography in a Delay animal. Arrowheads identify the right gastroepiploic artery (RGE), the single remaining artery supplying the gastric pedicle after ligation of the left, right, and short gastric vessels.
Induction anesthesia was accomplished with 5% isoflurane and endotracheal anesthesia maintained with 2.5% isoflurane and oxygen. Oxygen saturation was maintained above 95% throughout the procedure. Preinterventional weight, hematocrit, and blood pressure were measured and recorded. The abdomen was accessed through a midline laparotomy, and the stomach was marked with a suture 5 mm distal to the tip of the gastric fundus. All blood flow measurements were recorded in situ at the level of the marking suture. The stomach was bathed with warm saline throughout the procedure to prevent vasoconstriction. Peritoneal temperature was measured using a digital thermometer (Mabis Healthcare Inc, Lake Forest, IL) and recorded immediately before each blood-flow measurement. Intraperitoneal temperature was maintained at the appropriate core temperature of the North American opossum (93°F/34°C). Blood flow was measured by laser Doppler flowmetry, a technique that has been extensively validated.9–11 The Laserflo BPM2 laser Doppler flowmeter (Vasamedics, Eden Prairie, MN) was calibrated. Baseline blood flow was measured 3 consecutive times and recorded. All subsequent measurements were performed in an identical manner. The left, right, and short gastric vessels were ligated. The right gastroepiploic artery and vein were left intact. The diameter of the right gastroepiploic vessels was noted before and after ligation of the specified vessels. Postligation blood flow was measured. In the Delay group, the abdomen was closed, whereas the remainder of the procedure was completed in the Immediate group. The esophagogastric junction was resected and placed in formalin. This was followed by gastric tubularization using a 2-layer running 5–0 monofilament suture. The esophagogastrostomy was completed with a single layer of interrupted 5–0 monofilament suture (Fig. 3).
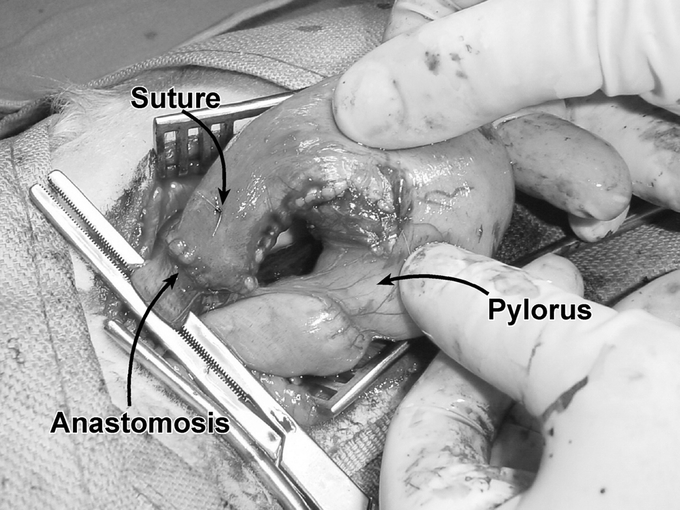
FIGURE 3. Esophagogastrostomy. A hand-sewn anastomosis was performed at the distal end of the gastric pedicle. A marking suture was placed 5 mm distal to the tip of the gastric fundus (prior to resection) and remained in place throughout the entire study period. The pyloris indicates the distal end of the gastric tube.
The Delay group underwent a second laparotomy after 28 days of vessel ligation. Blood flow was recorded, and the diameter of the right gastroepiploic vessels was noted. The animals then underwent esophagogastrectomy, gastric tubularization, and esophagogastrostomy. The anastomoses in the Delay and Immediate groups were harvested 32 days after esophagogastrostomy and preserved in formalin, and the animals were euthanized.
Five Sham animals underwent an initial laparotomy, during which the right gastroepiploic vessel caliber was noted and baseline blood flow was measured. Sixty days later, the Sham animals underwent a second laparotomy where blood flow was measured and right gastroepiploic vessel caliber was assessed. The esophagogastric junction was resected and preserved in formalin, and the animals were euthanized.
Tissue Preparation
Longitudinal sections were taken through the anterior aspect of the esophagogastric anastomosis in Delay and Immediate groups, and through the esophagogastric junction in the Sham animals. Each was embedded in paraffin and trichrome-stained (Masson trichrome; Sigma-Aldrich Corp, St. Louis, MO).12 Immunohistochemistry (IHC) for actin was performed (Smooth Muscle Actin-M0851; Dako Cytomation, Carpenteria, CA).13
Anastomotic Collagen Deposition
To determine collagen content, the trichrome-stained specimens were analyzed using a 3-chip CCD camera mounted on a conventional light microscope. Images were digitally acquired at a fixed resolution and analyzed using a 10× objective and Leica IM50 (Leica Microsystems, Cambridge, UK) and Image Pro Plus system (Media Cybernetics, San Diego, CA). In each specimen, 3 representative areas of interest were taken within the submucosa of the gastric tube 5 mm distal to the anastomosis. The submucosa was defined as the region between the margins of the muscularis mucosa and the muscularis propria. For each region of interest, an examiner selected the optimal color range which highlighted the blue pixels that contained collagen. The ratio of blue-stained collagen pixels to the total number of pixels within the area of interest was taken as the fraction of subanastomotic collagen content. The examiner was blinded to the identity and group of each specimen.
For collagen subtype analysis, 3 longitudinally oriented samples of tissue were taken from the anastomosis of each group. IHC for collagen types I, III, IV, and V was performed using the protocol described by Schulze et al14 The tissue was examined by a pathologist to assess differences in the density of each subtype and pattern of distribution among the 3 groups.
Gastric Muscularis Propria Preservation
Longitudinal sections through the anastomoses were prepared with IHC for actin and evaluated under light microscopy. Measurements were taken at 4× magnification using an eyepiece ocular micrometer by a single examiner blinded to group identity. The muscularis propria was considered preserved if it continued uninterrupted from the esophageal to the gastric suture marks. If any discontinuity was present, atrophy was considered present and quantified as the length of the gap in the muscularis. The gastric muscularis propria below the anastomosis was also examined for evidence of atrophy using the same technique.
Gastric Muscularis Mucosa Preservation
Longitudinal sections of the anastomosis, stained with trichrome, were used to quantify the extent of atrophy in the muscularis mucosa. The entire glass-mounted section was digitally scanned at 4000 dpi using an optical scanner (CoolScan; Nikon Tokyo, Japan). Images were imported into the desktop graphics application Adobe Illustrator (Adobe Inc, San Jose, CA). A Bezier curve was drawn to trace the gastric muscularis mucosa from the anastomosis to the end of the specimen. In regions where the muscularis mucosa was atrophic or interrupted, the path of the curve was interpolated. The total length of this curve was measured using the Pathlength plug-in (Telegraphics Software, http://www.telegraphics.com.au). Areas where the muscularis mucosa appeared discontinuous or indistinct from the surrounding tissue were identified. The number and length of gaps due to atrophy of the muscularis mucosa were recorded. Gaps associated with artifacts from specimen processing were not counted or measured. The sum of the length of gaps divided by the total length of muscularis was calculated as the percentage of atrophy of the muscularis mucosa. The examiner was blinded to the group and identity of each specimen.
Capillary Quantification after Delay
The esophagogastric junctions removed during the second laparotomy in Sham and Delay groups was used for capillary counts. A transverse section of stomach taken 5 mm beneath the squamocolumnar junction was fixed in formalin and embedded in paraffin. Capillary vascular endothelium was identified using IHC for Factor VIII (von Willebrand Factor-A0062; Dako) via a standardized immunoperoxidase technique.15 Specimens were viewed at 5× magnification to facilitate the identification of 3 representative areas of the submucosa with capillary endothelium (staining brown for factor VIII). A single examiner, blinded to group and identity, evaluated the IHC-prepared slides under 20× magnification using light microscopy. Capillaries were identified and counted using methods adapted from Weidner et al15 Each distinct endothelial-cell cluster was counted as a single capillary. The presence of a visible vessel lumen was not required for a structure to be defined as a capillary.
Statistical Analysis
In all cases, a histogram of the data and the normal curve based on sample mean and variance were compared visually to determine whether a parametric or nonparametric statistical test was appropriate.16 Comparisons were considered statistically different at an α of 0.05.
Blood flow in mL/min/100 g tissue was recorded for each time point. The Kruskal-Wallis test for equality of medians was used to compare baseline blood flow between Delay, Sham, and Immediate groups and blood flow at time of anastomosis between the Delay and Immediate groups. The Wilcoxon matched-pairs signed-ranks test was used to compare changes in blood flow between time points for both groups. Pearson correlation coefficient was used to evaluate the relationship between weight, blood pressure, temperature, hematocrit, and gastric fundus blood flow. Fisher exact test was used to compare survival between the Delay and Immediate groups. ANOVA with Tukey HSD post hoc test was used to evaluate collagen deposition between the 3 groups. Muscularis preservation was compared between the 3 groups using a χ2 test. Mann-Whitney U was used to assess relative muscularis atrophy at the anastomosis between the Delay and Immediate groups.
The average number of counted capillaries in 3 20× fields was recorded per animal. The Mann-Whitney U test for post hoc comparisons was used to compare capillary counts between the groups. Interquartile ranges of measurements are presented in parentheses.
RESULTS
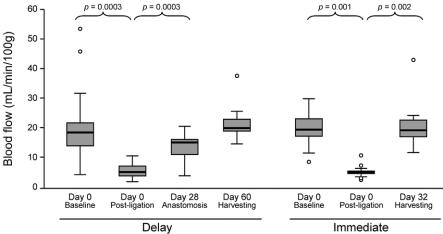
The median baseline blood flow was similar among the 3 groups, with 20 (15–23) mL/min/100 g tissue in the Sham group, 19 (15–26) mL/min/100 g tissue in the Delay group, and 20 (17–24) mL/min/100 g tissue in the Immediate group (P = 0.880). There was no significant correlation between baseline blood flow and weight, blood pressure, temperature, or hematocrit. Following vessel ligation, blood flow decreased from 19 (15–26) to 6 (4–8) mL/min/100 g tissue in the Delay group and from 20 (17–24) to 5 (5–6) mL/min/100 g in the Immediate group (P < 0.001 for each group compared with baseline) (Fig. 4). At the time of esophagogastric anastomosis, the Delay group had over 3 times the blood flow of the Immediate group (16 [11–17] versus 5 [5–6] mL/min/100 g; P = 0.000003) (Fig. 5). Final blood flow measurements in the Delay and Immediate animals returned to baseline values. The baseline and final blood flow in the Sham group did not differ (P = 0.893).
FIGURE 4. Time courses of gastric fundus blood flow in the Delay and Immediate groups. Blood flow was measured by laser Doppler flowmetry. Solid line indicates median blood flow. Shaded boxes indicate interquartile range. Comparisons between baseline and postligation blood flow and between the postligation and next scheduled measurement of blood flow were statistically significant.
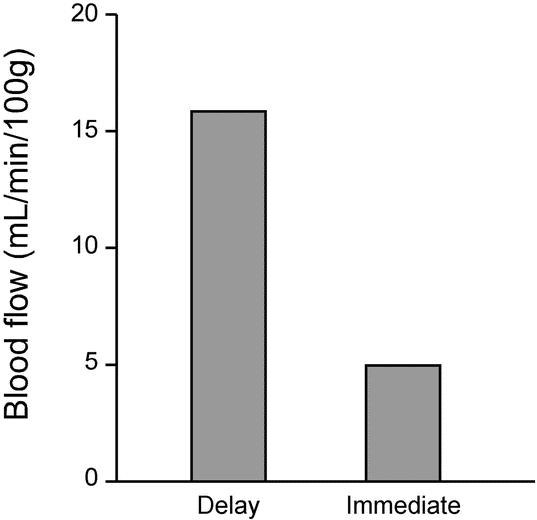
FIGURE 5. Median blood flow at time of esophagogastrostomy in the Delay and Immediate groups.
No clinically significant strictures developed in either group. Two animals in the Immediate group died secondary to anastomotic leak (Fig. 6), while no complications occurred in the Delay group (P = 0.183). There was no weight loss observed in any of the groups.
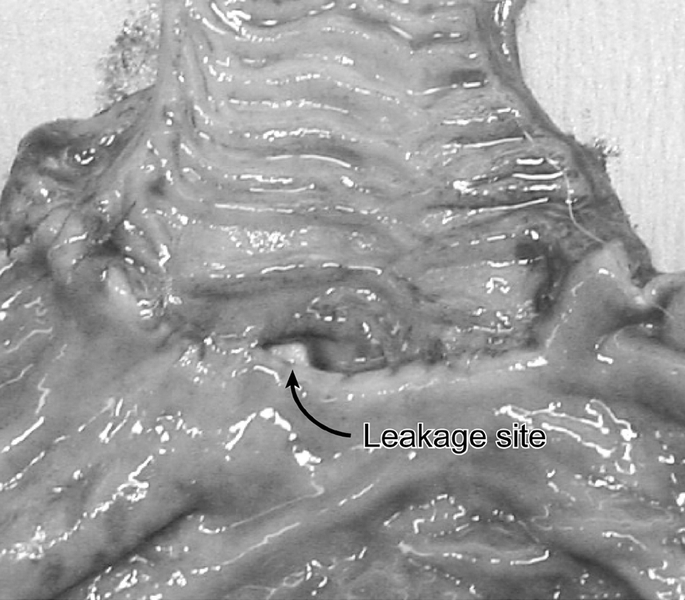
FIGURE 6. Anastomotic leakage at the esophagogastrostomy. This animal, which had been assigned to the Immediate group, developed sepsis and expired. The anastomosis was harvested, and the luminal side of the longitudinally opened specimen is displayed. The site of leakage is indicated between the esophageal (top) and gastric (bottom) conduit.
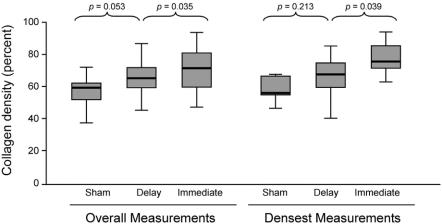
The overall collagen content in the submucosa was determined to be 57% (52–62) in the Sham group, 65% (57–72) in the Delay group, and 71% (60–82) in the Immediate group (P = 0.0004). Post hoc analysis demonstrated that the Immediate and Delay groups were statistically different (P = 0.035), but the Sham and Delay groups were not (P = 0.053). When the densest region of collagen deposition was taken into consideration in each specimen, a similar and more pronounced relationship was seen among the groups, with the Sham group having a mean of 64% (62–72) collagen content, Delay 72% (66–77), and Immediate 80% (75–85) (P = 0.004) (Fig. 7). Again, the Immediate and Delay groups were statistically different (P = 0.039), but the Sham and Delay groups were not (P = 0.213). Immunohistochemistry for collagen types I, III, IV, and V did not reveal any differences in distribution or density of each collagen type among the groups.
FIGURE 7. Collagen density in the submucosa of the gastric fundus. Using digital colorimetric quantification, measurements of collagen density were taken in 3 representative regions of interest 5 mm distal to the anastomosis. Overall measurements using every measured value in each group are given. When only the highest measurement in each animal was considered, the same trends persisted. A significant difference was noted between the Delay and Immediate groups, and a trend toward significance was noted between the Sham and Delay groups.
Complete preservation of the muscularis propria across the anastomosis was evident in 56% (10 of 18) of the Delay animals and 36% (5 of 14) of the Immediate animals (P = 0.265). Measurable atrophy of the gastric muscularis propria was only appreciated directly adjacent to the anastomosis. The mean length of atrophy in this layer was 0.10 mm (0–0.60 mm) in the Delay group and 0.53 mm (0.03–0.80 mm) in the Immediate group (P = 0.346). There was complete preservation of the esophageal muscularis in all specimens.
Examination of the anastomoses revealed regions of atrophy in the muscularis mucosa of the gastric tube. In these regions, the borders of the muscularis mucosa became indistinct and were manifest as gaps between runs of intact muscularis. In the Delay group, 5.1% of this tissue layer was atrophic, with a mean of 0.5 gaps per specimen. The Immediate group had 19.0% atrophy of this tissue layer, with a mean of 1.5 gaps per specimen. No atrophy of the muscularis mucosa was evident in the Sham group (P = 0.01, comparison between groups). There was a significant difference between the Immediate and Delay groups (P = 0.023).
Gross dilation of the right gastroepiploic artery and vein diameter was evident in all Delay animals at 28 days after vessel ligation. In contrast, this was not seen in any of the Sham animals (Fig. 8). Counts in 3 representative fields per animal showed a median of 27 (23–33) capillaries per 20× field in the Sham group, compared with 38 (31–46) in the Delay group (P = 0.037) (Fig. 9).
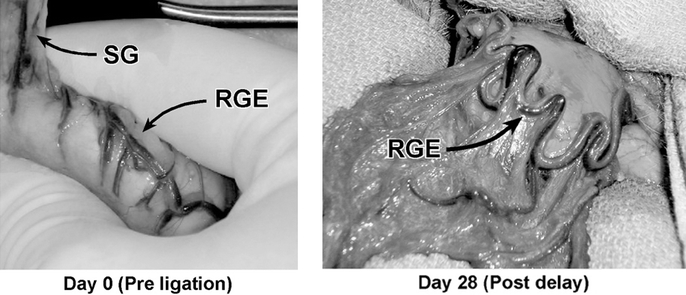
FIGURE 8. Right gastroepiploic (RGE) artery and vein, examined at baseline before ligation and after a delay period of 28 days. Qualitatively, the artery appears consistently more distended after the delay period compared with baseline. SG indicates short gastric vessels.
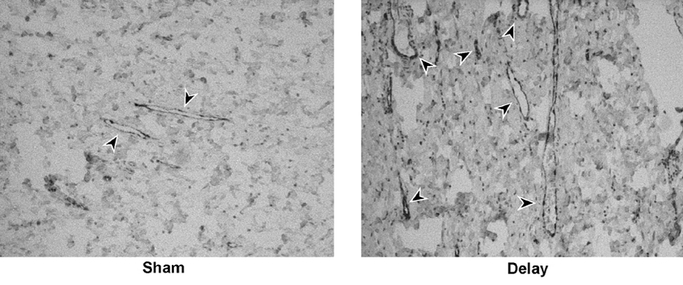
FIGURE 9. Capillary identification with immunohistochemistry for vascular endothelium. Representative transverse sections of gastric fundus from the Sham and Delay groups are shown. Arrowheads indicate structures identified as capillaries. In the Sham group, esophagogastric junctions were harvested 60 days after initial the laparotomy. In the Delay group, esophagogastric junctions were harvested after a 28-day period of delay.
Comment
Following esophagectomy, a gastric tube is created from the greater curvature of the stomach to serve as the neoesophagus. This procedure requires substantial gastric devascularization and results in acute ischemia of the fundus. Corrosion cast studies have established that the cranial 20% of the neoesophagus is supplied solely by a microscopic network of capillaries and arterioles which are dependent upon right gastroepiploic artery inflow.4 It is this milieu that accounts, in part, for the high incidence of anastomotic complications and subsequent mortality associated with esophagectomy.2,3,17,18 The impetus for the present study and others was founded in a desire to improve survival, reduce complications, and improve the quality of life in patients who have undergone esophageal resection.7,17–24 In this study, we established that the delayed, right gastroepiploic-based opossum stomach resulted in a significant increase in blood flow to the distal aspect of the pedicle at the time of esophagogastrostomy when compared with animals that underwent immediate resection and anastomosis. In addition, the results suggest that more blood flow at the time of esophagogastrostomy results in improved healing characteristics as evidenced by a reduction in both collagen deposition and ischemic changes at the level of the anastomosis.
In selecting a model, we wished to perform a gastric devascularization analogous to that performed in humans during creation of the neoesophagus. Opossums were chosen because of their anatomic similarities to the human foregut and because they have similar gastric vascular anatomy.8,25 These animals possess a generous length of intraabdominal esophagus, which facilitates distal esophagogastrectomy, gastric tubularization, and anastomosis without requiring entry into the thoracic cavity. Because the neoesophagus remains within the abdominal cavity, it is not subjected to the theoretical compression and resultant venous congestion that occurs when it resides within the posterior mediastinum. This may explain the lower anastomotic complication rate and absence of global conduit ischemia observed in our study compared with what would be expected in humans undergoing this procedure. Our technique for esophagogastrostomy was based on well-established methods.26 Kovacs and colleagues27 revealed similar postoperative collagen deposition in canine esophagogastric anastomoses in a comparison of various techniques.
Although incompletely understood, the proposed mechanism(s) by which the delay phenomenon occurs include conditioning of tissues in response to hypoxia,28 vasodilation of existing vessels,11–13,29 and neovascularization.30,31 It has also been suggested that closure of arteriovenous shunts may contribute to this effect.32 To date, the thrust of research surrounding delay has focused primarily on skin flaps, and little is known regarding mechanisms of delay in the gastrointestinal tract.6,33 In addition, while most skin flap studies allow only 7 days for the delay process to occur, the present study afforded 1 month.
We hypothesized that the delay effect in the stomach occurs both via vasodilation of existing vessels and angiogenesis. After 1 month of delay, the right gastroepiploic artery and vein were dramatically dilated on subjective assessment in the Delay group when compared with Shams. Investigations have demonstrated that tissue hypoxia results in the production of nitric oxide, which in turn leads to arterial dilation and neovascularization.34 Vascular endothelium growth factor (VEGF), which has been shown to stimulate nitric oxide synthase in endothelial cells,35 is up-regulated in the face of acute ischemia.36 It is feasible that the ischemia caused by delay could serve to trigger the production of nitric oxide, resulting in the observed vasodilation of the gastroepiploic vessels.
Using established methods for detecting angiogenesis,15 the Delay group was demonstrated to have a significantly higher number of capillaries within the fundus submucosa when compared with Shams. Tissue hypoxia results in a feedback loop which stimulates the production of hypoxia-induced factor α and potent angiogenesis factors such as VEGF and platelet derived growth factor.37 In the rat transverse rectus abdominis myocutaneous flap model, delay resulted in increased VEGF expression, angiogenesis, and increased survival of the flap.29 This same group established that the improved flap survival associated with the delay effect can be achieved pharmacologically with preoperative injections of VEGF.38 It is important to note that, should angiogenesis prove to be the primary mechanism by which delay occurs, the effects of this technique on esophageal cancer biology must be considered prior to clinical implementation.
It is generally accepted that ischemia is the root cause of both early (leak) and late (stricture) complications of esophagogastrostomy.3 Strictures which occur more than 1 year following anastomosis are thought to be primarily reflux related.3 We examined several endpoints in an attempt to determine whether gastrointestinal delay can be exploited to impact anastomotic healing favorably after esophagogastrectomy. There were 2 anastomotic leaks leading to death in the Immediate group and none in the animals that underwent delay. Although this comparison did not reach statistical significance, there was a trend towards a protective effect of delay in preventing leak. The lack of anastomotic stricture in all animals 1 month after esophagogastrostomy is likely secondary to protective mechanisms inherent in the animal model. Unlike the opossum, patients present not only the potential for ischemia but also additional risk factors for anastomotic complications, such as obesity, history of neoadjuvant therapy, diabetes, chronic obstructive pulmonary disease, and cardiac disease.39–41
Collagen is essential for the appropriate healing of anastomotic tissue. Insufficient collagen deposition may occur in the face of acute and severe perioperative ischemia, which can lead to anastomotic necrosis and dehiscence in the early postoperative period. Conversely, large amounts of collagen deposition over several weeks may occur in response to the healing of an early dehiscence or a lesser ischemic insult with or without subclinical leak. It is apparent from our study that the increased blood flow at the time of anastomosis is associated with the deposition of a more “physiologic” amount of collagen when compared with the Immediate group. Urschel18,21 and Urschel et al42 first suggested that the delay effect could be applied to the stomach to improve anastomotic healing after esophagectomy. This group evaluated gastric hydroxyproline concentration, gastrotomy dehiscence rates, and bursting strength following left gastric artery ligation and 2-week delay in a rat model. After 5 days of anastomotic healing, there was significantly less dehiscence in the delay group; however, there was no difference in hydroxyproline concentration and bursting strength between delay and immediate animals.43 Akiyama and colleagues7 attempted delay in 24 patients using percutaneous embolization of the left gastric and splenic arteries 3 weeks prior to esophagectomy for cancer. Intraoperative laser flowmetry demonstrated a 23% reduction in blood flow after creation of the gastric tube versus a 65% reduction in patients that did not undergo preoperative embolization. There was no late follow-up, and the sample size was insufficient to enable a determination of delay effect on anastomotic healing. Whether a delay-induced increase in blood flow at the time of esophagectomy translates into improved anastomotic healing in humans requires a clinical trial.
Because collagen synthesis and cross-linking are highly dependent upon systemic factors such as tissue oxygenation and nutritional status, we theorized that there may be a predominance of a specific collagen subtype associated with the “protective effect” of delay.44 More specifically, we hypothesized that collagen type III, which is predominant during the repair process in the early phases of wound healing, would be less abundant than type I (final matrix) in Delay animals compared with Immediates. Although there was less total collagen in the Delay group, immunohistochemistry for types I, III, IV, and V did not reveal any differences in the distribution or density of each type of collagen among the groups. It is possible that the significant early effects of delay on the regulation of collagen subtypes were obscured by the subsequent remodeling of collagen that occurs several weeks after tissue injury.
We evaluated the preservation of the muscularis propria and muscularis mucosa in the region of the anastomosis in each group. Studies of small-bowel ischemia have demonstrated that low grades of ischemia are manifest by a loss of the muscularis mucosa, and higher grades of ischemia are associated with involvement of the inner layer of muscularis propria.45 In our analysis, the muscularis mucosa was completely preserved in the Sham animals, arguing against the possibility that the loss of the muscularis was the result of the laparotomy itself or the anesthetic agents. Similar to others, our investigation demonstrated complete preservation of the esophageal muscularis.46 This was not surprising, given that the esophageal portion of the anastomosis maintained its native vascular inflow. The percentage of muscularis mucosa atrophy was significantly greater in the Immediate group than the Delay group, suggesting that the Delay group had benefited from improved perfusion. Our analysis was conducted throughout the entire length of the gastric tissue that was available for histologic analysis. We noted that the extent of atrophy in the muscularis mucosa was not solely limited to the region potentially constricted by the suture. Thus, the observed atrophy was likely a result of underlying ischemia involving the distal gastric tube rather than from suture artifact (Fig. 10).
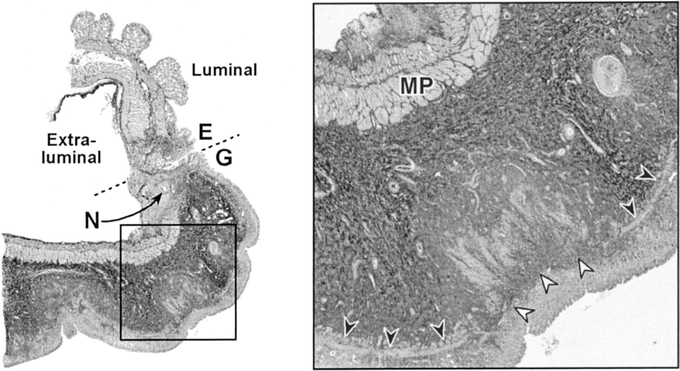
FIGURE 10. Representative trichrome-stained section of the anastomosis in an Immediate animal. Broken line represents anastomosis between the esophagus (E) and the gastric tube (G). N denotes a needle hole. The inset depicts a higher magnification to show structures of interest: muscularis propria (MP), intact muscularis mucosa (black arrowheads), and areas of atrophy in the muscularis mucosa (white arrowheads).
Although there was a trend favoring preservation of the gastric muscularis propria in the Delay animals, this did not reach statistical significance. We also noted that, in all groups, the entire muscularis propria was intact except at the point immediately adjacent to the anastomosis; because the atrophy of the muscularis propria was more closely limited to the region of the anastomosis, it is possible that variations in length of atrophy may reflect suture artifact or highly localized ischemia resulting from suture compression. This analysis, however, excluded the 2 Immediate animals which developed anastomotic leaks, and these 2 animals probably developed the most pronounced effects of ischemia. Multiple areas of atrophy in the muscularis propria may have developed in these animals, but histologic analysis was not feasible due to the effects of the massive inflammatory reaction associated with the leakage.
Prior to the clinical application of delay, it will be essential to demonstrate that the increase in blood flow achieved with this technique will translate into a decrease in anastomotic dehiscence and stricture formation. There are promising ways in which the delay phenomenon may be exploited. The technical benefit of this particular approach is primarily its simplicity. Time provides the necessary element for the delay phenomenon to take place, and as a result, no advanced training or additional operative equipment is necessary. As the concept of delay moves into the clinical arena, a combination of laparoscopic staging, regional devascularization, and vasoactive agent delivery several weeks prior to esophageal resection may be feasible. This technique may be applicable to other gastrointestinal anastomoses that are at risk for ischemic injury.
Discussions
Dr. William C. Lineaweaver (Jackson, Mississippi): Gastrointestinal wound healing offers some extraordinarily rich areas of study. As in the general study of wound healing, gastrointestinal wounds offer opportunities to study scarring, regeneration, and long-term consequences such as neoplasia, as well as the inflammatory and biochemical processes that mediate these phenomena. In addition, gastrointestinal wounds can include studies of function, including secretory, absorptive, and endocrine processes, motility, changes in flora, lumen patency, and valve competencies.
These authors introduce a number of these concepts into this study. They have identified a significant wound healing problem in clinical gastroesophageal reconstruction and designed a delay procedure addressing this problem by attempting to modify wound healing at the surgical site.
Their study shows that the surgical delay increased blood flow and capillary counts in the region of the reconstructive procedure. The delay group had decreased collagen at the surgical site and did have better preservation of the tissue architecture. There were no significant differences in stricture formation or leaks. These findings represent substantial effects but raise at least 2 questions in my mind.
First, are there other models or modifications of this model that have measurable rates of leak or stricture? Studies of such a model would provide data more suggestive of clinical application.
Second, findings of neovascularization and decreased collagen content could arguably be interpreted as evidence of impaired wound healing. Biomechanical wound healing studies such as bursting strength, breaking strength, and tensile strength could help clarify whether this delay procedure results in a weaker wound or stronger healing of the surgical site. Have the authors undertaken such studies? I look forward to further studies from this group.
Last year, I had the privilege of presenting a paper to the Southern Surgical Association on the delay phenomena and associated cytokine activity in a rat flap model. The discussants of that paper were Drs. M. J. Jurkiewicz and Leonard Furlow. Dr. Jurkiewicz noted that prior to my paper, over 80 years had passed since surgical delay was discussed at this association, and Dr. Furlow remarked that most surgeons today probably think that the term delay phenomena refers to the handling of surgeons’ fees by insurance companies.
I congratulate today's authors for expanding the experimental investigation of this basic wound healing concept and note that today's paper represents a temporal acceleration of 8000% in the rate of appearance of delay phenomenon papers at the Southern Surgical Association. If continued, this trend could result in next year's program being devoted exclusively to papers dealing with the delay phenomenon.
Dr. Bruce D. Schirmer (Charlottesville, Virginia): I wish to congratulate Drs. Reavis, Hunter, and Jobe for performing a very complete and elegant study. The manuscript they furnished me is well written, scholarly, and the data are clearly presented.
The authors suggest the potential in the manuscript for using the delay concept in the clinical arena through a combination of laparoscopic staging, regional devascularization, and vasoactive agent deliveries several weeks prior to esophageal resection. I have several questions for the authors regarding such potential application.
First, what is the basis for the length of 28 days delay in animals in the delay group? Most delay studies for flaps involve a period of about 7 days, and at 32 days for the immediate surgery group blood flow in the gastric fundus was returned to baseline level. Should one consider a shorter delay period?
Second, as the authors have correctly pointed out in the manuscript, the differences in leak rate between the groups was not significant. Yet this animal model does have advantages for healing over the human situation since the animals don't have any comorbid medical problems. Therefore, I ask the authors to speculate on which esophageal tumors and preoperative conditions in humans would warrant initial trials of the delay phenomenon. Also, would they be concerned about injecting angiogenesis factors in the fundus if the tumor were involving or adjacent to that area?
Finally, this model focuses on eliminating the ischemia of the gastric fundus in esophagogastric anastomosis. Should we look at the contributions of esophageal ischemia as important to the morbidity in esophagogastric anastomosis, and how would the authors propose doing that?
Dr. Adrian Barbul (Baltimore, Maryland): I enjoyed the paper very much as it addresses a very important issue that has been bedeviling surgeons for many, many years. As a comment, I would start by saying that the majority of the incidence of both dehiscence and stricture occurs in the esophagus and in the rectum, which lacks serosal lining. I am wondering if that factor has been taken into account in the present model.
The question that I have relates to the opposite direction that circulation seems to have on collagen deposition in this model. It is one of the basic tenets in wound healing that ischemic tissue will heal with less collagen deposition, if at all. I would like the authors to comment on this seemingly contradictory finding.
Dr. Walter G. Wolfe (Durham, North Carolina): I enjoyed the paper. I rise only to mention that Ivor Lewis, who presented his work at the Hunterian Lecture in '46 on his procedure for resection of carcinoma of the esophagus, used the “delay phenomenon.” He mobilized the stomach and then closed the abdomen, hydrated the patient with a gastrostomy, then returned several days later to do a right thoracotomy and complete the esophagectomy and esophagogastrostomy. So, in some ways, there is nothing new under the sun as it appears he might have been the first to use the “delay phenomenon.”
Dr. Luis O. Vasconez (Birmingham, Alabama): I enjoyed very much the paper and I am glad of the authors’ interest in the phenomenon of “delay.” I should caution to indicate that, although the data may be appropriate to those animals, it has to be very carefully translated to the humans, if at all. I think that the observed increase in flow is temporary or variable, depending on the animal's blood pressure and other factors. I think we all know that if we devascularize an organ or structure such as was done on the test animal, we are going to have some changes in the mucosa and so forth.
It is actually a source of disappointment to me as a plastic surgeon that the voluminous written plastic surgical literature on the delay phenomenon has been effectively translated to clinical applications in an arguable few instances.
Dr. Kevin M. Reavis (Portland, Oregon): Our study established that delay is associated with both vasodilation and angiogenesis and results in increased blood flow to the gastric fundus prior to esophagogastric anastomosis. Animals undergoing delayed operations had less anastomotic collagen deposition and ischemic injury than those undergoing immediate resection. In fact, we noted that out of the 18 delay animals, none suffered anastomotic complications; however, 2 of the 14 immediate animals suffered leaks and died. With this in mind, Dr. Lineaweaver asked several questions.
The first question pertained to whether there are other models which are used to investigate the delay phenomenon. There have been clinical studies out of Japan by Akiyama and Dr. Nagawa. Dr. Akiyama percutaneously embolized the splenic artery and left gastric artery in hopes of achieving delay and improved anastomotic healing in esophageal cancer patients. One patient suffered a leak in the experimental group and there were none in the control group. This study focused primarily on perioperative outcome, and there was no long-term follow-up with respect to the incidence of stricture formation. Dr. Nagawa investigated the concept of “supercharging” in which the transverse cervical vessels were connected to the short gastric vessels in hopes of augmenting the blood flow to the distal aspect of the pedicle, closer to the anastomosis. In several studies, Dr. Urschel and colleagues described the use of the rat stomach with ligation of the left gastric artery as a gastrointestinal model for delay. This group established a return to baseline gastric blood flow several days after ligation of the left gastric artery.
With respect to other methods by which to evaluate the quality of anastomotic healing, we did not examine bursting strength in this study. I think it is a potentially useful tool in this area of research given that it provides easy to appreciate clinical data. That being said, I wonder if after 1 month of healing, the esophagus or stomach on either side of the anastomosis would tear before there was anastomotic disruption.
Further areas of study will center on characterizing the optimal delay period prior to resection and anastomosis and examining the effects of radiotherapy on anastomotic healing within the context of the delay model described here today.
Dr. Schirmer asked about the selection of the 28-day delay period. Data support shorter time periods regarding the use of skin and myocutaneous flaps. According to Dhar and colleagues, the most dramatic increase in blood flow occurred at days 3 to 5. In discussing this aspect of the study during the design phase, we thought about what would be an appropriate time course. We hypothesized that after vessel ligation, blood flow may continue to increase for up to 3 weeks. If the use of delay was to be applied to a patient with cancer in future clinical trials, we felt that no more than 4 weeks should pass between initial diagnosis of malignancy, intervention, and final resection. Therefore, 28 days was selected as the delay period. However, the complete return of blood flow may occur prior to this interval. With this in mind, we have recently designed a protocol which will investigate the rate of blood flow return at day 2, day 5, day 7, and day 14, as well as determine the level of up-regulation of VEGF and angiogenesis during the shorter time periods.
Dr. Schirmer asked about comorbidities and which patients would most greatly benefit from delay. Patients who we theorize would be most appropriate for this approach would be those considered highest risk for complications. At least 4 patient types meet this criteria. First are those patients who have benign disease but are at risk for compromised anastomotic healing. This includes patients who have poorly controlled diabetes mellitus, steroid dependence, or patients who are otherwise immunocompromised, such as solid-organ-transplant recipients. Second are patients who have connective tissue disorders; for example, patients with scleroderma. Third are patients who are nutritionally bankrupt either secondary to cachexia from malignancy or secondary to dysphagia following caustic ingestion and inability to maintain adequate nutrition with tube feeds and/or total parental nutrition. Fourth are patients who have undergone radiochemotherapy with resultant compromised tissue healing at the level of the anastomosis.
Dr. Schirmer asked about the appropriateness of angiogenic agents in the presence of malignancy. Initially, pharmacologic delay would be appropriate on protocol in patients who are undergoing resection for benign disease. Prior to the regional delivery of angiogenic factors in the area at risk for ischemia in patients with cancer, we would need to place a vasoinhibitory factor such as squalamine that may be placed directly on the tumor; this would theoretically prevent tumor angiogenesis while stimulating angiogenesis to the future anastomotic site. One would need to be certain that there was little or no systemic distribution of angiogenic agents in patients with cancer.
Dr. Schirmer asked if we should evaluate the esophageal side of the anastomosis for ischemia. In this model it was not necessary because devascularization of the esophagus prior to resection and anastomosis was not required, given the generous intraabdominal esophagus of the opossum. This was evidenced by the fact that the muscularis propria and mucosa in the esophageal portion was preserved right up to the level of the anastomosis in all of the immediate and delay animals. Also, in clinical practice, an esophagojejunostomy rarely develops anastomotic stenosis compared to the incidence observed in patients with an esophagogastrostomy.
Dr. Barbul queried as to the significance of increasing gastric fundus blood flow and decreasing collagen deposition observed at the level of the anastomosis. There was actually an increase in the total amount of collagen in animals that underwent delay when compared to the sham group. I think Dr. Lineaweaver phrased it perfectly in terms of the architectural matrix of the healing tissue. It seems that with increasing blood flow there is a greater amount of physiologic healing such that the tissue more closely resembles that of the sham animals than the immediate animals.
Finally, Dr. Vasconez made an astute observation that before we take this from the experimental realm to the clinical realm, careful studies need to be undertaken. I agree with him and feel that initially delay should be incorporated on protocol in the care of patients undergoing resection for benign disease. Eventually, we would like to incorporate delay into a 2-stage operation, that being laparoscopic staging combined with gastric devascularization followed by definitive resection and anastomosis.
We are currently developing a randomized trial which will evaluate effect of delay on anastomotic healing.
Footnotes
This work was supported in part by National Institutes of Health grants K23 DK066165-01 and RO3 CA105959-01, the Oregon Health & Science University Gerlinger Foundation, and the Society of American Gastrointestinal and Endoscopic Surgeons.
Reprints: Blair A. Jobe, MD, Portland VA Medical Center, Surgical Service–P3GS, PO Box 1034, Portland, OR 97207. E-mail: jobeb@ohsu.edu.
REFERENCES
- 1.Watson TJ, Peters JH, DeMeester TR. Esophageal replacement for end-stage benign esophageal disease. Surg Clin North Am. 1997;77:1099–1113. [DOI] [PubMed] [Google Scholar]
- 2.Casson AG, Porter GA, Veugelers PJ. Evolution and critical appraisal of anastomotic technique following resection of esophageal adenocarcinoma. Dis Esophagus. 2002;15:296–302. [DOI] [PubMed] [Google Scholar]
- 3.Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg. 2004;198:536–541. [DOI] [PubMed] [Google Scholar]
- 4.Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg. 1992;54:1110–1115. [DOI] [PubMed] [Google Scholar]
- 5.Pierie JP, de Graaf PW, van Vroonhoven TJ, et al. The vascularization of a gastric tube as a substitute for the esophagus is affected by its diameter. Dis Esophagus. 1998;11:231–235. [DOI] [PubMed] [Google Scholar]
- 6.Dhar SC, Taylor GI. The delay phenomenon: the story unfolds. Plast Reconstr Surg. 1999;104:2079–2091. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama S, Ito S, Sekiguchi H, et al. Preoperative embolization of gastric arteries for esophageal cancer. Surgery. 1996;120:542–546. [DOI] [PubMed] [Google Scholar]
- 8.Forse RA, MacDonald PH, Mercer CD. Anastomotic and regional blood flow following esophagogastrectomy in an opossum model. J Invest Surg. 1999;12:45–52. [DOI] [PubMed] [Google Scholar]
- 9.Shandall A, Lowndes R, Young HL. Colonic anastomotic healing and oxygen tension. Br J Surg. 1985;72:606–609. [DOI] [PubMed] [Google Scholar]
- 10.Boyle NH, Pearce A, Owen WJ, et al. Validation of scanning laser Doppler flowmetry against single point laser Doppler flowmetry in the measurement of human gastric serosal/muscularis perfusion. Int J Surg Invest. 2000;2:203–211. [PubMed] [Google Scholar]
- 11.Nishida H, Giostra E, Spahr L, et al. Validation of color Doppler EUS for azygos blood flow measurement in patients with cirrhosis: application to the acute hemodynamic effects of somatostatin, octreotide, or placebo. Gastrointest Endosc. 2001;54:24–30. [DOI] [PubMed] [Google Scholar]
- 12.Carson FL. Histotechnology: A Self-Instructional Text. Chicago: ASCP Press; 1990. [Google Scholar]
- 13.Sorensen B. Corporate Training Manager. DakoCytomation Denmark A/S.
- 14.Schulze K, Ellerbroek S, Martin J. Matrix composition in opossum esophagus. Dig Dis Sci. 2001;46:968–975. [DOI] [PubMed] [Google Scholar]
- 15.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 16.Sheskin D. Handbook of Parametric and Nonparametric Statistical Procedures. 3rd ed. Boca Raton: Chapman & Hall/CRC; 2004. [Google Scholar]
- 17.Nagawa H, Seto Y, Nakatsuka T, et al. Microvascular anastomosis for additional blood flow in reconstruction after intrathoracic esophageal carcinoma surgery. Am J Surg. 1997;173:131–133. [DOI] [PubMed] [Google Scholar]
- 18.Urschel JD. Ischemic conditioning of the stomach may reduce the incidence of esophagogastric anastomotic leaks complicating esophagectomy: a hypothesis. Dis Esophagus. 1997;10:217–219. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M, Sugiyama A, Ikegami T, et al. Revascularization using the short gastric vessels of the gastric tube after subtotal esophagectomy for intrathoracic esophageal carcinoma. J Am Coll Surg. 2000;190:71–77. [DOI] [PubMed] [Google Scholar]
- 20.Randelovic T, Knezevic J, Pesko P, et al. [Complications in esophagojejunal anastomosis]. Acta Chir Iugosl. 1994;41(2 suppl 2):225–228. [PubMed] [Google Scholar]
- 21.Urschel JD. Ischemic conditioning of the rat stomach: implications for esophageal replacement with stomach. J Cardiovasc Surg (Torino). 1995;36:191–193. [PubMed] [Google Scholar]
- 22.Sekido M, Yamamoto Y, Minakawa H, et al. Use of the “supercharge” technique in esophageal and pharyngeal reconstruction to augment microvascular blood flow. Surgery. 2003;134:420–424. [DOI] [PubMed] [Google Scholar]
- 23.Canty TG Sr, LoSasso BE. One-stage esophagectomy and in situ colon interposition for esophageal replacement in children. J Pediatr Surg. 1997;32:334–336. [DOI] [PubMed] [Google Scholar]
- 24.DeMeester SR. Colon interposition following esophagectomy. Dis Esophagus. 2001;14:169–172. [DOI] [PubMed] [Google Scholar]
- 25.Schroder W, Beckurts KT, Stahler D, et al. Microcirculatory changes associated with gastric tube formation in the pig. Eur Surg Res. 2002;34:411–417. [DOI] [PubMed] [Google Scholar]
- 26.Law S, Fok M, Chu KM, et al. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg. 1997;226:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacs T, Koves I, Pandi E, et al. Bursting strength and collagen content changes in esophageal anastomoses: comparative experimental study in dogs. Ann Chir Gynaecol. 2001;90:266–270. [PubMed] [Google Scholar]
- 28.McFarlane RM, Heagy FC, Radin S, et al. A study of the delay phenomenon in experimental pedicle flaps. Plast Reconstr Surg. 1965;35:245–262. [DOI] [PubMed] [Google Scholar]
- 29.Lineaweaver WC, Lei MP, Mustain W, et al. Vascular endothelium growth factor, surgical delay, and skin flap survival. Ann Surg. 2004;239:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barthe Garcia P, Suarez Nieto C, Rojo Ortega JM. Morphological changes in the vascularisation of delayed flaps in rabbits. Br J Plast Surg. 1991;44:285–290. [DOI] [PubMed] [Google Scholar]
- 31.Arranz Lopez JL, Suarez Nieto C, Barthe Garcia P, et al. Evaluation of angiogenesis in delayed skin flaps using a monoclonal antibody for the vascular endothelium. Br J Plast Surg. 1995;48:479–486. [DOI] [PubMed] [Google Scholar]
- 32.Reinisch JF. The pathophysiology of skin flap circulation: the delay phenomenon. Plast Reconstr Surg. 1974;54:585–598. [DOI] [PubMed] [Google Scholar]
- 33.Carroll SM, Carroll CM, Stremel RW, et al. Vascular delay and administration of basic fibroblast growth factor augment latissimus dorsi muscle flap perfusion and function. Plast Reconstr Surg. 2000;105:964–971. [DOI] [PubMed] [Google Scholar]
- 34.Smith RS Jr, Lin KF, Agata J, et al. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2002;22:1279–1285. [DOI] [PubMed] [Google Scholar]
- 35.Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996;27:838–844. [DOI] [PubMed] [Google Scholar]
- 36.Shima DT, Deutsch U, D'Amore PA. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995;370:203–208. [DOI] [PubMed] [Google Scholar]
- 37.Gleadle JM, Ebert BL, Firth JD, et al. Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents. Am J Physiol. 1995;268(6 pt 1):C1362–1368. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F, Fischer K, Komorowska-Timek E, et al. Improvement of skin paddle survival by application of vascular endothelial growth factor in a rat TRAM flap model. Ann Plast Surg. 2001;46:314–319. [DOI] [PubMed] [Google Scholar]
- 39.Honkoop P, Siersema PD, Tilanus HW, et al. Benign anastomotic strictures after transhiatal esophagectomy and cervical esophagogastrostomy: risk factors and management. J Thorac Cardiovasc Surg. 1996;111:1141–1146. [DOI] [PubMed] [Google Scholar]
- 40.Pierie JP, de Graaf PW, Poen H, et al. Incidence and management of benign anastomotic stricture after cervical oesophagogastrostomy. Br J Surg. 1993;80:471–474. [DOI] [PubMed] [Google Scholar]
- 41.Dewar L, Gelfand G, Finley RJ, et al. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg. 1992;163:484–489. [DOI] [PubMed] [Google Scholar]
- 42.Urschel JD, Antkowiak JG, Delacure MD, et al. Ischemic conditioning (delay phenomenon) improves esophagogastric anastomotic wound healing in the rat. J Surg Oncol. 1997;66:254–256. [DOI] [PubMed] [Google Scholar]
- 43.Urschel JD, Takita H, Antkowiak JG. The effect of ischemic conditioning on gastric wound healing in the rat: implications for esophageal replacement with stomach. J Cardiovasc Surg (Torino). 1997;38:535–538. [PubMed] [Google Scholar]
- 44.Cotran RS, Kumar V, Collins T, et al. Robbins Pathologic Basis Of Disease. 6th ed. Philadelphia: Saunders; 1999. [Google Scholar]
- 45.Hegde SS, Seidel SA, Ladipo JK, et al. Effects of mesenteric ischemia and reperfusion on small bowel electrical activity. J Surg Res. 1998;74:86–95. [DOI] [PubMed] [Google Scholar]
- 46.Oesch I, Helikson MA, Shermeta DW, et al. Esophageal reconstruction with free jejunal grafts: an experimental study. J Pediatr Surg. 1980;15:433–436. [DOI] [PubMed] [Google Scholar]