Abstract
Objective:
Protein degradation, negative nitrogen balance and compromised structure of essential organs have been associated with resistance and decreased production of anabolic hormones. In turn, increased levels of anabolic hormones are associated with improved survival. The aims of the present study were to determine the pattern of anabolic hormones, resting energy expenditure and cytokines in severely thermally injured pediatric patients and to compare these parameters in female and male patients.
Methods:
Sixty-five children (1 to 16 years of age) sustaining a severe thermal injury (≥40% TBSA) were included into the study. Patients were further divided into females (n = 22) and males (n = 43). Patient demographics, nutritional support, incidence of sepsis, inhalation injury, and mortality were noted. Resting energy expenditure was measured during hospital course by indirect calorimetry. Blood was drawn 0, 10, 20, and 40 days postburn and serum insulin-like growth factor-I (IGF-I), insulin-like growth factor binding protein-1 and -3 (IGFBP-1, and -3), growth hormone, insulin, and cytokines were measured.
Results:
There were no significant differences between females and males for demographics, nutritional intake, or concomitant injuries. In both groups, endogenous anabolic agents were drastically decreased by 3- to 5-fold up to 40 days posttrauma. Females had significantly higher levels of IGF-I, IGFBP-3, growth hormone, and insulin when compared with males, P < 0.05. Increased levels of anabolic hormones were associated with decreased stay on the ICU (females 36 ± 22 days versus males 53± 39 days), decreased serum IL-1β and TNF-α as well as resting energy expenditure, P < 0.05.
Conclusion:
Data indicate that despite adequate nutritional support, severe thermal injury leads to decreased anabolic hormones over a prolonged period of time. Female patients had significantly increased levels of anabolic hormones, which are associated with decreased proinflammatory mediators and hypermetabolism, leading to a significant shorter ICU stay compared with male patients.
We determined the pattern of anabolic hormones, resting energy expenditure and cytokines in severely thermally injured pediatric patients and compared these parameters in female and male patients. Despite adequate nutritional support, severe thermal injury leads to increased cytokine and resting energy expenditure, as well as decreased anabolic agents, over a prolonged period of time. Female patients had significantly increased levels of anabolic hormones, which are associated with decreased pro-inflammatory mediators and hypermetabolism leading to a shorter ICU stay.
The stress response to burn injury is similar to any critical illness or severe trauma only differing by its severity and duration. The hypermetabolic response after major burn is characterized by a hyperdynamic response with increased body temperature, oxygen and glucose consumption, CO2 production, glycogenolysis, proteolysis, lipolysis, and futile substrate cycling.1 This response begins on the fifth day postinjury and continues up to 9 months postburn, causing erosion of lean body mass, muscle weakness, immunodepression, and poor wound healing.2 In no other disease or trauma is the hypermetabolic response as severe as it is following a thermal injury. While patients with peritonitis may have metabolic rates elevated from 5% to 25% and severely multiple traumatized patients 30% to 75% above normal, severely burned patients with a body surface area burned greater than 40% may have metabolic requirements twice normal.3 The increased metabolic requirements in patients with major burns can cause major tissue breakdown leading to nitrogen loss and a potentially lethal depletion of essential protein stores.4 The energy requirements are met by the mobilization of proteins and amino acids. Increased protein turnover, degradation, and negative nitrogen balance are all characteristic of this severe critical illness.4,5 As a consequence, the structure and function of essential organs, such as liver, skeletal muscle, skin, immune system, and cellular membrane transport functions, are compromised.6 An increased and prolonged proinflammatory acute-phase response enhances protein degradation, and catabolism is associated with increased incidence of multiorgan system failure and ongoing sepsis.7
Protein degradation and negative nitrogen balance have been associated with resistance and decreased production of anabolic hormones.8 Several studies administering anabolic hormones showed positive effects on wound healing, muscle protein synthesis, and the immune system.9,10 On the other hand, Gianotti et al11 found no significant decrease of growth hormone (GH) and insulin-like growth factor-I (IGF-I) in burned patients. In addition, there are no clinical studies with larger patient series looking at anabolic agents after a severe injury. Therefore, one aim of the present study was to determine the pattern of anabolic hormones, cytokines, and hypermetabolism in severely thermally injured pediatric patients. Over the last years, many experimental and clinical studies looked at differences in the outcome of female and male patients.12,13 While some studies found improved survival for female patients, others did not find any differences in outcome.14,15 In light of the hypothesis that increased levels of anabolic agents are associated with improved survival, the second aim of the present study was to determine the concentration of endogenous anabolic hormones and associated cytokines and resting energy expenditure (REE) in female and male patients.
PATIENTS AND METHODS
Thermally injured children with the following inclusion criteria were enrolled in a prospective study: 1 to 16 years of age, admitted within 3 days after injury to our institute, and burns covering more than 40% TBSA with a third-degree component of >10%, which required a minimum harvesting of 1 donor site for skin grafting. Patient demographics (age, date of burn and admission, sex, burn size, and depth of burn) and concomitant injuries, such as inhalation injury, sepsis, morbidity, and mortality, were recorded. Infections were defined as a blood culture identifying the pathogen during hospitalization or at autopsy, in combination with leucocytosis or leucopenia, hyperthermia or hypothermia, and tachycardia. Infections were divided into mild infections, such as wound infections and severe life-threatening infections (sepsis), according to the guideline of the Society of Critical Care Medicine. Wound healing was evaluated with respect to the number of surgeries required and time between operations for the cohorts. For further evaluation, patients were divided according to their gender and demographics, and concomitant injuries were determined in both groups.
Patients were resuscitated according to the Galveston formula with 5000 mL/m2 TBSA burned + 2000 mL/m2 TBSA lactated Ringer solution given in increments over the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision and the wounds covered with available autograft skin with allograft used to cover any remaining open areas. After the first operative procedure, 5 to 10 days were required for the donor site to heal, and patients were then taken back to the operation theater. This procedure was repeated until all open wound areas were covered with autologous skin material. Blood was drawn at the time of admit, then 10, 20, and 40 days after burn. Almost every patient data was available for measurement at each time point.
All patients underwent the same nutritional treatment to a standardized protocol. The initial assessment for nutritional need is calculated by the Curreri formula, which is an accepted standard for estimating basal energy expenditure. This formula calls for 25 kcal/kg/d plus 40 kcal/% TBSA burned/d. It provides the needs, plus the additional caloric needs of the burn wounds. In children, formulas based on body surface area are more appropriate because of greater body surface per kilogram. For children, we used the Galveston formulas, Galveston Infant, Galveston Revised, Galveston Adolescent. The formula change with age based on the body-surface alterations that occur with growth. Roughly, the intake is calculated as 1500 kcal/m2 body surface + 1500kcal/m2 area burn. The composition of the nutritional supplement is also important. The optimal dietary composition contains 1 to 2 g/kg/d of protein, which provides a calorie-to-nitrogen ratio at around 100:1 with the suggested caloric intakes. Nonprotein calories can be given either as carbohydrate or as fat, with clinical advantages for the carbohydrates. The diet was delivered by enteral nutrition when possible to all our patients. Total parenteral nutrition would have been only used as a supplemental form of nutrition when calculated intake could have been not be achieved, which was never the case.
Serum IGF-I, IGF binding protein-1 (IGFBP-1), IGFBP-3, GH, and insulin were determined by RIA (Nichols Inst. Diagnostics, San Juan Capistrano, CA) or a human ELISA (Endogen, Woburn, MA, or Biosource Int., Camarillo, CA) on days 0 (admit), 10, 20, and 40 days after burn.
Serum IL-1, IL-6, IL-10, and TNF were determined by ELISA (Biosource Int., Camarillo, CA) following the guidelines of each kit.
Indirect Calorimetry
REE was measured using a Sensor-Medics 2900 metabolic cart (Yorba Linda, CA). All indirect calorimetry measurements were made at 30°C, which is the standard environmental setting for all patient rooms in both our adult and pediatric acute burn ICUs. Composition of inspired and expired gases was sampled and analyzed at 60-second intervals. Values of VCO2, VO2, and REE were accepted when they were at a steady state for 5 minutes. The average REE was calculated from these steady-state measurements. For statistical comparison, energy expenditure was expressed both as absolute REE and as the percentage of the basal metabolic rate predicted by the Harris-Benedict equation.
Ethics and Statistics
The study was reviewed and approved by the institutional review board of the University Texas Medical Branch, Galveston, Texas. Prior to the study, each subject, parent, or child's legal guardian had to sign a written informed consent form. Analysis of variance with post hoc Bonferroni's correction, paired and unpaired Student t test, χ2 analysis, and Mann-Whitney tests were used where appropriate. Data are expressed as means ± SD or SEM, where appropriate. Significance was accepted at P < 0.05.
RESULTS
Demographics
Sixty-five severely burned children were included in the present study. The average time from burn to hospital admission was 2 days. Patients’ demographics are shown in Table 1. Patients remained with a mean of 53 ± 39 days on the ICU. The overall mortality was 5% (3/65 patients). The deaths were late deaths, around the 50th day postburn. Patients were divided by gender; 42 of the burned patients were males and 23 were females. There was no significant difference in age, TBSA, and third-degree burn, incidence of inhalation injury, and incidence of sepsis between males and females. There were no differences in caloric intake or distribution between groups. It is important to notice that caloric intake was 92% from the calculated need (Table 1). There was a significant difference in length of ICU stay. While males stayed 65 days, female patients stayed only 36 days, P < 0.05 (Table 1). The total incidence of infections was 32%, with 17% minor infections and 15% life-threatening major infections (Table 1). We found that females had more minor infections, 22%, then major infections, 8%, whereas males had 14% minor infections and 19% major infections (Table 1).
TABLE 1. Patient Demographics, Nutritional Intake and Albumin Substitution Requirements of Severely Burned Pediatric Patients (n = 65)
All the patients required approximately 6 surgeries, with 7 to 8 days between operations. Both gender required similar amounts of surgeries, but the time between surgeries was shorter in females by 1.8 days compared with males (Table 1). The shortened period indicates an accelerated and improved wound healing in female patients. Despite that there was no statistical difference between males and females, we suggest that the difference is too small for the cohort size, and by increasing the patient number a statistical difference between the 2 patient cohorts would be detected.
Anabolic Hormone Concentration Postburn
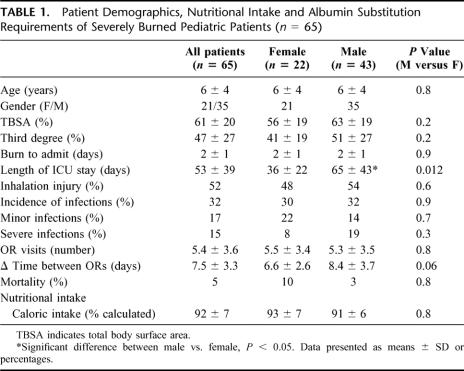
Despite adequate nutrition, serum IGF-I drastically decreased 3- to 4-fold below normal levels immediately after burn in all patients and remained low 40 days after thermal injury, P < 0.05 (Fig. 1A). Serum IGFBP-3 decreased by 3-fold immediately after the insult and remained low 10, 20, and 40 days after the insult (Fig. 1B). IGFBP-1 was found increased at days 0 and 10 after thermal injury and then steadily decreased over time and approached normal levels at 40 days postburn (Fig. 1C). Similarly to IGF-I, serum GH decreased immediately after burn and continued to decrease over the study period. Serum GH was found 5-fold decreased 40 days after burn (Fig. 1D). Serum insulin was decreased immediately after burn but increased over time (Fig. 1E).
FIGURE 1. A, Serum IGF-I levels dropped 3- to 4-fold immediately after burn. Forty days after trauma, IGF-I levels were still significantly decreased compared with normal values. Normal values for unburned, normal age-matched children: IGF-I: 365 ± 15 μg/mL. B, Serum IGFBP-3 was decreased 4-fold compared with normal. Forty days postburn, levels did not reach normal concentrations. Normal levels: IGFBP-3: 2.8 ± 0.9 μg/mL. C, Serum IGFBP-1 was increased by 2-fold during the first 10 days postburn. At 20 days after trauma, levels decreased and reached normal values. Normal levels: IGFBP-1 115 ± 15 μg/mL. D, Serum insulin was decreased immediately after burn. Insulin increased almost 2-fold over the study period. Normal levels: 20 ± 5 IU/mL. E, Serum GH was 4- to 5-fold decreased after the burn trauma. Serum GH demonstrated no recovery over the 40-day study period and remained decreased. Normal levels: GH 8 ± 1 μg/mL.
Predicted REE
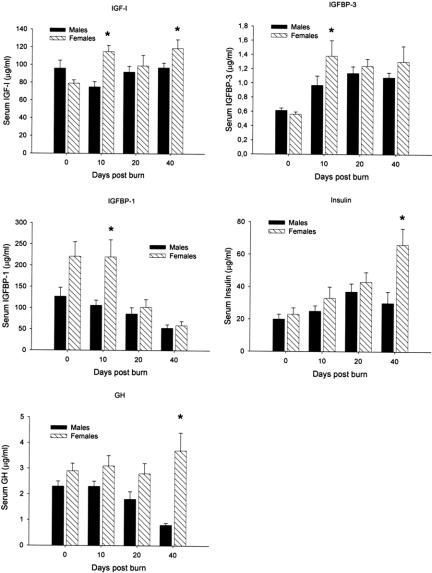
Predicted energy expenditure was increased during acute hospitalization and at discharge by 130%. Predicted REE decreased towards normal levels 6 months after burn but was still elevated at 120% predicted from normal (Fig. 2).
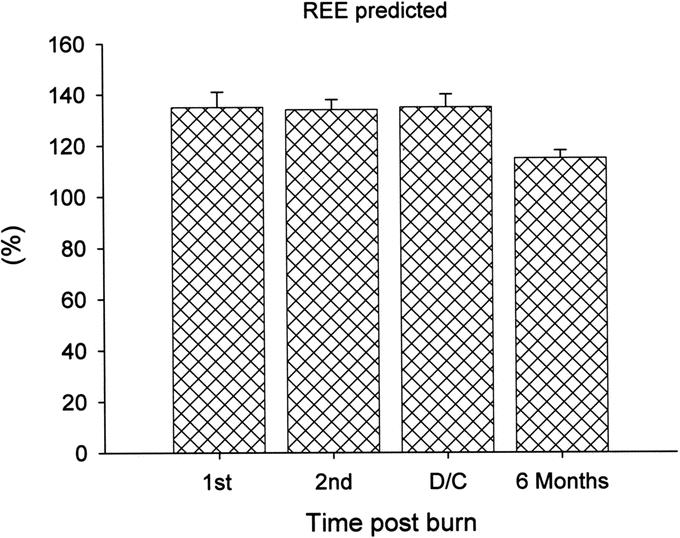
FIGURE 2. Predicted energy expenditure was increased during acute hospitalization and at discharge by 130%. Predicted REE decreased towards normal levels 6 months after burn but was still elevated, at 120% predicted from normal.
Serum Cytokines
Serum IL-1β was significantly increased compared with normal levels over the 40-day study period (Fig. 3A). Serum IL-6 was also increased but decreased towards normal levels at 40 days postburn (Fig. 3B). Similarly, serum TNF-α was increased immediately after burn but decreased to approach normal levels 40 days after injury (Fig. 3C).
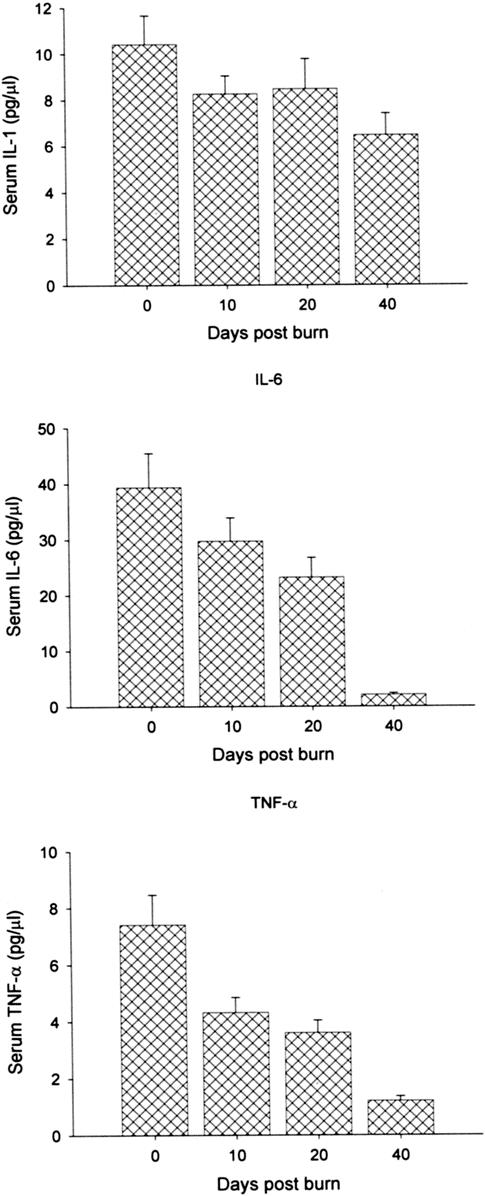
FIGURE 3. A–C, Serum IL-1β (A) was significantly increased compared with normal levels over the 40-day study period. Normal serum IL-1β: 0.1–0.8 pg/mL. B, Serum Il-6 was also increased but decreased towards normal levels at 40 days postburn. Normal IL-6: 0–5 pg/mL. C, Similarly, serum TNF-α was increased immediately after burn but decreased to approach normal levels 40 days after injury. Normal serum TNF-α: 0–2 pg/mL.
Anabolic Hormones, Males Versus Females
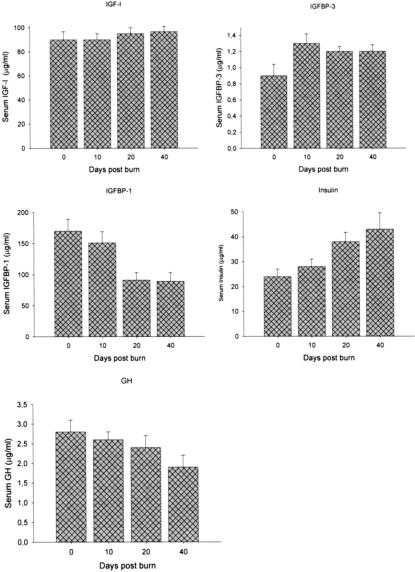
Males and females had decreased serum IGF-I levels after the burn. Females had significant increased serum IGF-I levels at 10 and 40 days after burn when compared with males, P < 0.05 (Fig. 4A). In association with increased IGF-I levels, females had increased IGFBP-3 at 10 days postinsult when compared with controls (Fig. 4B). Serum IGFBP-1 was found increased in females at 10 days postburn (Fig. 4C). Serum insulin was slightly increased in the female group at all time points, with a significant difference at 40 days after burn when compared with males, P < 0.05 (Fig. 4D). For serum GH we found that females had higher concentrations of GH at 10, 20, and 40 days after trauma. A significant increase of GH was found 40 days postburn, P < 0.05 (Fig. 4E).
FIGURE 4. A, In females and males, serum IGF-I levels dropped immediately after burn. Ten and 40 days after trauma, IGF-I levels were significantly increased in female patients when compared with males. *Significant differences between females versus males, P < 0.05. Normal values for unburned, normal, age-matched children: IGF-I: 365 ± 15 μg/mL. B, Serum IGFBP-3 decreased immediately in both groups. In accordance with IGF-I levels, IGFBP-3 levels were significantly increased in female patients compared with males. *Significant differences between females versus males, P < 0.05. Normal levels: IGFBP-3: 2.8 ± 0.9 μg/mL. C, Serum IGFBP-1 was found significantly increased 10 days postburn in female patients compared with males. *Significant differences between females versus males, P < 0.05. Normal levels: IGFBP-1 115 ± 15 μg/mL. D, Serum insulin was in the normal range in both groups in the early phase postburn, but then increased. Female patients had significantly higher insulin levels compared with males 40 days postburn. *Significant differences between females versus males, P < 0.05. Normal levels: 20 ± 5 IU/mL. E, Serum GH decreased in male patients over the 40-day study period. GH was increased in female patients and significantly greater 40 days postburn. *Significant differences between females versus males, P < 0.05. Normal levels: GH 8 ± 1 μg/mL.
Predicted REE, Males Versus Females
Predicted REE was significantly increased in males during acute hospital stay, at discharge, and at 6 months after burn when compared with females, P < 0.05 (Fig. 5). Interestingly, females returned to baseline at 6 months after burn, whereas males remained elevated at 120% predicted REE.
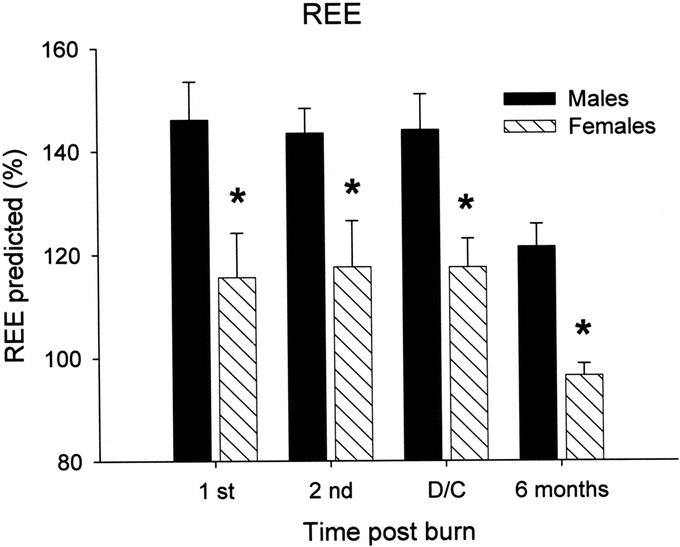
FIGURE 5. Predicted REE was significantly increased in males during acute hospital stay, at discharge, and at 6 months after burn when compared with females, P < 0.05 (Fig. 5). Females returned to baseline at 6 months after burn, whereas males remained elevated, at 120% predicted REE. *Significant differences between females versus males, P < 0.05.
Serum Cytokines, Males Versus Females
Serum TNF-α was significantly decreased female patients 40 days after injury compared with males, P < 0.05 (Fig. 6A). Interleukin-1β was decreased in females on days 10 and 20 after burn when compared with males, P < 0.05 (Fig. 6B). There were no differences between males and females in IL-6 levels.
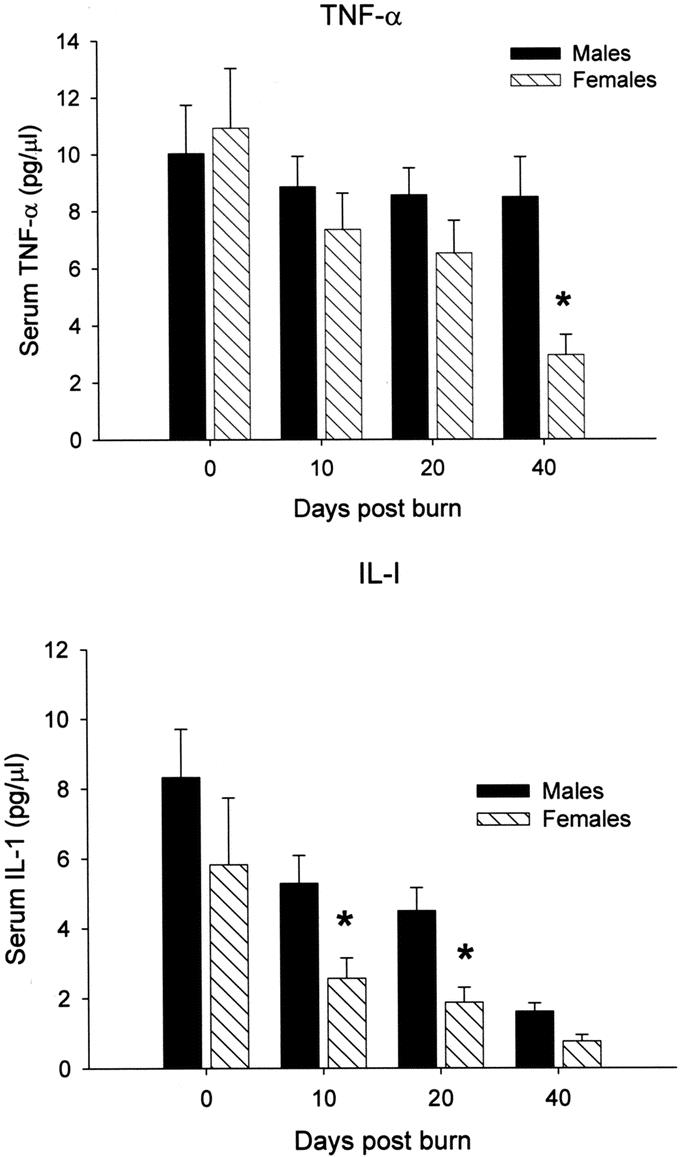
FIGURE 6. A, Serum TNF-α was significantly decreased female patients 40 days after injury compared with males, P < 0.05. Normal serum TNF-α: 0–2 pg/mL. B, Interleukin-1β was decreased in females on days 10 and 20 after burn when compared with males, P < 0.05. Normal serum IL-1β: 0.1–0.8pg/mL. *Significant differences between females versus males, P< 0.05.
DISCUSSION
The metabolic rate in burns is extremely high, and energy requirements are immense and met by the mobilization of proteins and amino acids.2 Increased protein turnover, degradation, and negative nitrogen balance are characteristics of this severe critical illness.4 As a consequence, the structure and function of essential organs are compromised, such as skeletal muscle, skin, immune system, and cellular membrane transport functions.2,6 This compromise can lead to multiorgan dysfunction or even death.2 Catecholamines involved in the hypermetabolic response to burn injury are released from sympathetic nerve ends and the adrenal medulla. Norepinephrine levels are raised 2- to 10-fold in proportion to burn size. A close correlation exists between the increase in plasma catecholamines and metabolic rate. Catecholamines block and decrease via cellular mediators endogenous anabolic hormones.16 We showed in the present study that the GH-IGF-I axis is drastically decreased during the aftermath of a burn trauma. The reason for the drop in GH and IGF-I is unclear because cytokines are also thought to mediate the catabolic states induced by infection and trauma. Interleukin-1β directly inhibits the anabolic IGF-I-GH axis.17 Several studies showed that proinflammatory cytokines are significantly increased over a period of 40 days after massive trauma.18 Thus, it can be surmised that GH and IGF-I are affected by multiple factors, such as cytokines or catecholamines or that the overwhelming hypermetabolic response leads to a lack of hormone synthesis. However, the pathophysiologic and clinical relevance of decreased GH and IGF-I levels is a deficit in transmembrane amino acid transport, protein catabolism, and compromised immune system.19–21
Glucose kinetics in severely burned patients is almost always abnormal.22 In burned patients, glucose is almost entirely used through inefficient anaerobic mechanisms, as characterized by increased lactate production, which accounts for increased glucose consumption.22,23 Glucose production, particularly from alanine, is elevated in almost all patients with severe burn.4 The increased gluconeogenesis renders these amino acids unavailable for reincorporation into body protein. Nitrogen is excreted primarily in urea, thus contributing to the progressive depletion of body protein stores. Plasma insulin levels usually remain normal or slightly elevated in burn patients.24 The fact that the basal rate of glucose production is elevated despite normal or elevated plasma insulin levels indicates hepatic insulin resistance, since under normal conditions elevated serum insulin would lower the rate of glucose production.25 Furthermore, the plasma glucose concentration is frequently increased, which would normally directly inhibit glucose production.25 In the present study, we confirmed previous studies that insulin levels were in the normal range. However, immediately posttrauma insulin was significantly decreased but increased over time.
Recent laboratory studies have demonstrated that immune responses differ between male and female rodents, and some clinical studies have suggested gender differences regarding incidence and mortality from sepsis.12 The differences appear because of both deleterious testosterone and beneficial estrogen effects; clinical trials of testosterone blockage and/or estrogen administration for male subjects have been suggested.14,26 However, Beery27 already showed that estrogens and testosterone are not the only factors determining the improved outcome in female patients, that another factors must be present. In the present study, we found a difference between male and female patients in anabolic hormone concentration. IGF-I and its binding proteins and GH were increased in female patients compared with male patients. Given the pathophysiology that protein catabolism leads to prolonged need for ventilation, delay in mobilization, compromised immune system, and delay in wound healing, higher concentration of anabolic hormones should have beneficial effects in terms of improving clinical outcome. In fact, we found that female patients remained only 36 days on the ICU, whereas male patients remained 53 days. As there was no difference in nutritional intake, burn size, and age between female and males, we suggest that the shortened ICU stay for females is probably due to the higher concentration of anabolic hormones leading to improved wound healing and less infections. A possible explanation why females have higher hormones compared with males could be cellular mediators such as cytokines. In a rodent model, Diodato et al28 showed that plasma levels of IL-6, TNF-α, and prostaglandin E(2) (PGE(2)) after CLP were significantly increased in males subjected to prior hemorrhage. In contrast, plasma levels of IL-6 and TNF-α in females did not increase under such conditions.28 PGE(2) levels were comparable in males and females following CLP; however, prior hemorrhage significantly reduced PGE(2) levels in females, whereas no change was observed in males. Proinflammatory cytokines are known to decrease anabolic hormones;17 thus, it appears likely that decreased proinflammatory cytokine expression is associated with higher hormone concentration.
To date, few studies have examined anabolic hormone differences between males and females in critically ill or septic patients. Based on our findings, we believe it would be worthwhile looking at differences for anabolic hormones in male and female critically ill patients, as endogenous anabolic hormones are drastically decreased in the aftermath of a severe trauma. However, female pediatric patients showed higher levels of endogenous anabolic hormones when compared with male pediatric patients. Hence catabolism, hypermetabolism, and compromise of the structure and function of essential organs are attenuated in severely burned female patients. This results in an improved clinical outcome, which was found in the present study in a significantly shorter ICU stay for females when compared with males. Possible explanations for a shorter ICU stay are decreased incidence of severe sepsis and improved wound healing. We showed that females had a lower incidence of severe infections and shorter time periods between OR visits, thus indicating faster wound healing. As we did not reach statistical significance, a larger cohort has to be examined to verify the present findings.
Discussions
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): Dr. Herndon has added this study to his extensive program in integrative clinical and laboratory research, the importance of which was cited by Dr. Townsend in his excellent presidential address.
These results expand our understanding of postinjury hypermetabolism and identify a possible therapeutic role for endogenous anabolic agents. To assess the author's interpretation of these data, we need additional information.
The early changes in circulating levels of the endogenous anabolic agents could be explained, at least in part, by change in the volume of distribution as a consequence of hemodilution and edema formation. Do you have data pertinent to that possibility, that is, intake and output records and weight change throughout the entire study period?
Since the increased levels of the endogenous anabolic hormones, that is, IGF-1 and its binding protein IGF binding protein 3, and growth hormone are still 50% or less of predicted normal and are increased only relative to those in males and then usually at 40 days postburn, what indices of anabolism were increased in the female patients? Was lean body mass less, was UUN less, and was protein synthetic rate increased?
In that same vein, were the increased insulin levels in the females at 40 days associated with higher blood glucose levels as a reflection of carbohydrate loading?
The incidence of sepsis as indicated in Table 1 appears to be generally comparable. But were the causative agents and forms of infection similar?
Gram-negative sepsis has a greater impact on carbohydrate metabolism (I think that is mediated by inhibition of phosphoenolpyruvate carboxykinase). Consequently, more such infections in the males could have influenced the outcome. The 2-fold longer ICU stay in the males suggests that there was a significant difference in infection severity. If not, how is that longer ICU stay explained?
Another concern relates to the menstrual status of these patients, who aged in range from 1 to 16 years. Hunt and Purdue have reported that the protective gender effect was confined to premenopausal females with burn injury. Consequently, we need to know the differences in endogenous anabolic agents were present in only patients post-menarche and whether they were related to the ovulatory status at the time of sampling.
Last, if the noted differences are advantageous as you claim, why was there a more than 3-fold greater mortality rate in the female patients?
Dr. William G. Cioffi, Jr. (Providence, Rhode Island): Dr. Herndon and his colleagues have reported on the effect of gender on the hypermetabolic response following thermal injury in pediatric burn patients, and they found that young females have higher circulating levels of IGF-1, growth hormone, insulin, etc, during the acute care phase compared to their male counterparts. Clinically relevant differences included shorter ICU stays and a decreased hypermetabolic response, as indexed by resting energy expenditure in the female cohort.
The effect of gender on the response to disease, injury, and infection is complex. Females have a higher prevalence of autoimmune diseases, suggesting a more proinflammatory phenotype. The late Roger Bone reported that females have a better outcome than males when he analyzed outcomes from a series of sepsis trials in the late 1990s. We reported to this Association in a well-controlled animal model that testosterone was detrimental following trauma hemorrhage in terms of the cardiovascular response in a rodent model. And Chaudry, Rue and Blad from Birmingham have further extended these observations, clearly demonstrating in animal models that sex hormones have strong immunomodulatory effects.
Unfortunately, the clinical data are much less clear and quite conflicting. Some suggest that female gender confers a survival advantage, but many do not. The current paper suggests that female gender may confer a survival advantage following thermal injury by modulating hypermetabolic response.
The most significant outcome variable affected was resting energy expenditure. The changes in circulating anabolic hormone levels were less clear, although IGF-1 and its principal binding protein, BP-3, were clearly affected by gender. IC length of stay, as mentioned by Dr.Pruitt, was significant but is clearly a more subjective measure.
Dr. Herndon and colleagues have been leaders in studying in a very detailed fashion the effect of burns on protein and glucose metabolism following thermal injury. Consequently, do you have any data using your stable isotope methodology lending support to your current findings? Do you have simple nitrogen balance data? Was wound healing improved? Did females suffer fewer wounds or pulmonary infections, accounting for the IC length of stays? Do you believe the differences in anabolic hormone levels, which were still quite low in the females compared to normals, were great enough to make a difference?
The central issue, however, is what is driving these observations. Your patients are young, as pointed out by Dr.Pruitt, and the females undoubtedly have not started menses. Do you have any data on sex hormone levels tying your gender-related observations to sex hormone levels?
This is an intriguing study further supporting the concept that gender and most likely sex hormones may modulate the response to injury. What do you propose as a next step given your current findings? Are you ready to modulate this effect by either administering estrogens or blocking anergin function?
Dr. David N. Herndon (Galveston, Texas): I thank Dr. Pruitt and Dr. Cioffi for their excellent comments.
Dr. Pruitt's comment about the distribution is quite pertinent. These patients have been followed by dual image x-ray absortiometries (DEXA) and daily weights looking at volume standards and distribution of volume through hospital course, and there were indeed no major differences between males and females in this particular effort. But a more detailed response in that regard has to be researched. However, we did demonstrate that caloric intake was similar between the 2 groups.
Both Dr. Cioffi and Dr. Pruitt asked the question about whether postmenarche individuals significantly weighed this study. They really do not. The mean age is 6 plus or minus 4. It is a younger population that is being studied here that does not have the major variations in hormone levels that teenagers and young women do with males.
We are currently measuring and analyzing hormone levels in our pediatric burn population. We hypothesize that there are no major differences in the production of hormones over time, at least none that would explain these particular differences. Perhaps the differences are genetically weighed.
There were questions about what steps to take next. This is a controlled population. We have studied the use of anabolic agents through hospital course to try to improve the course of these individuals and have shown decreases in time to wound healing. In fact, when we compared the women to the men in the controlled population, it appeared that donor-site healing times were improved, though not statistically so, in the females. There was no statistical differential in incidence of infection, but the severity and length of the infections were longer in males.
The types of infections, the causative organisms, were not statistically different, but the duration of response to infectious episodes was indeed different, perhaps indicating that there were more subtle changes in immune responses in the male population than the female population. And I believe that those differences need to be further dissected. Clearly, much larger studies need to be done to try to dissect whether hormonal differences should be modulated directly in the patient population.
We did do cross-leg analysis using stable isotope infusion studies and looked at fractional synthetic rate, as well as cross-leg nitrogen balance and total body nitrogen balance amongst males and females. Preliminary data show differences between males and females, with females having an attenuated muscle breakdown, but further analysis is necessary. The data will be included into future studies.
In a larger study requiring a larger n, we clearly indicated that these findings are substantiated by a negative nitrogen balance and an increased catabolism in the males relative to the females, perhaps related to the changes in inflammatory cytokines and the reciprocal changes in anabolic hormones. But this is only a suggested mechanism, and further mechanistic studies performed in the animal model, as well as in humans, will substantiate which pathways are affected.
Concerning Dr. Pruitt's final comment that there was a 2% mortality in males and 10% in females so there was more than triple mortality in the females than there was in the males: There is no statistical difference in mortality between the 2 groups, and a much larger series of patients would be required to see any mortality effects. The mortality in this particular group is quite small, but in much larger groups that we have examined we have another paper in press looking at mortality over time.
Footnotes
Study was supported by the Shriners Hospital for Children, North America, and by the Deutsche Forschungsgemeinschaft (DFG Je 233/6-1).
Reprints: Marc G. Jeschke, MD, PhD, Shriners Hospital for Children and Department of Surgery, UTMB 815 Market Street, Galveston, TX 77550. E-mail: majeschk@utmb.edu or mcjeschke@hotmail.com.
REFERENCES
- 1.Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127–132. [DOI] [PubMed] [Google Scholar]
- 2.Takkala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NT, Goldman CD, Ho HS, et al. Systemic stress response after laparoscopic and open gastric bypass. J Am Coll Surg. 2002;194:557–566. [DOI] [PubMed] [Google Scholar]
- 4.Rennie MJ. Muscle protein turnover and wasting due to injury and disease. Br Med Bull. 1985;41:257–264. [DOI] [PubMed] [Google Scholar]
- 5.Arnold J, Campbell IT, Samuels TA, et al. Increased whole body protein breakdown predominates over increased whole body protein synthesis in multiple organ failure. Clin Sci (Colch). 1993;84:655–661. [DOI] [PubMed] [Google Scholar]
- 6.Biolo GTG, Ciocchi B, Situlin R, et al. Metabolic response to injury and sepsis: changes in protein metabolism. Nutrition. 1997;13:52S–57S. [DOI] [PubMed] [Google Scholar]
- 7.Hart DW, Wolf SE, Chinkes DL, et al. Beta-blockade and growth hormone after burn. Ann Surg. 2002;236:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boonen S, Mohan S, Dequeker J, et al. Down-regulation of the serum stimulatory components of the insulin-like growth factor (IGF) system (IGF-I, IGF-II, IGF binding protein [BP]-3, and IGFBP-5) in age-related (type II) femoral neck osteoporosis. J Bone Miner Res. 1999;14:2150–2158. [DOI] [PubMed] [Google Scholar]
- 9.Herndon DN, Pierre EJ, Stokes KN, et al. Growth hormone treatment for burned children. Horm Res. 1996;45(suppl 1):29–31. [DOI] [PubMed] [Google Scholar]
- 10.Welbourne TC, Milford L, Carter P. The role of growth hormone insubstrate utilization. Baillieres Clin Endocrinol Metab. 1997;11:699–707. [DOI] [PubMed] [Google Scholar]
- 11.Gianotti L, Stella M, Bollero D, et al. Activity of GH/IGF-1 axis in burn patients: comparison with normal subjects and patients with GH deficiency. J Endocrinol Invest. 2002;25:116–124. [DOI] [PubMed] [Google Scholar]
- 12.Angele MK, Xu YX, Ayala A, et al. Gender dimorphism in trauma-hemorrhage-induced thymocyte apoptosis. Shock. 1999;12:316–322. [DOI] [PubMed] [Google Scholar]
- 13.Chaudry IH, Samy TS, Schwacha MG, et al. Endocrine targets in experimental shock. J Trauma. 2003;54(5 suppl):S118–125. [DOI] [PubMed] [Google Scholar]
- 14.Croce MA, Fabian TC, Malhotra AK, et al. Does gender difference influence outcome? J Trauma. 2002;53:889–894. [DOI] [PubMed] [Google Scholar]
- 15.Mostafa G, Huynh T, Sing RF, et al. Gender-related outcomes in trauma. J Trauma. 2002;53:430–434. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Li N, Li JS, et al. The role of endotoxin, TNF-alpha, and IL-6 in inducing the state of growth hormone insensitivity. World J Gastroenterol. 2002;8:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delhanty PJ. Interleukin-1 beta suppresses growth hormone-induced acid-labile subunit mRNA levels and secretion in primary hepatocytes. Biochem Biophys Res Commun. 1998;243:269–272. [DOI] [PubMed] [Google Scholar]
- 18.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med. 2000;28:1578–1584. [DOI] [PubMed] [Google Scholar]
- 19.Tessari P, Inchiostro S, Biolo G, et al. Differential effects of hyperinsulinemia and hyperaminoacidemia on leucin-carbon metabolism in-vivo. J Clin Invest. 1987;79:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe RR. Control of muscle protein breakdown: effects of activity and nutritional states. Int J Sport Nutr Exerc Metab. 2001;11(suppl):S164–169. [DOI] [PubMed] [Google Scholar]
- 21.Takagi K, Suzuki F, Barrow RE, et al. Recombinant human growth hormone modulates Th1 and Th2 cytokine response in burned mice. Ann Surg. 1998;228:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demling RH, Seigne P. Metabolic management of patients with severe burns. World J Surg. 2000;24:673–680. [DOI] [PubMed] [Google Scholar]
- 23.Lal SO, Wolf SE, Herndon DN. Growth hormone, burns and tissue healing. Growth Horm IGF Res. 2000;10(suppl B):S39–43. [DOI] [PubMed] [Google Scholar]
- 24.Gore DC, Wolf SE, Herndon DN, et al. Relative influence of glucose and insulin on peripheral amino acid metabolism in severely burned patients. JPEN J Parenter Enteral Nutr. 2002;26:271–277. [DOI] [PubMed] [Google Scholar]
- 25.Van den Berghe G. Insulin therapy for the critically ill patient. Clin Cornerstone. 2003;5:56–63. [DOI] [PubMed] [Google Scholar]
- 26.Jarrar D, Kuebler JF, Wang P, et al. DHEA: a novel adjunct for the treatment of male trauma patients. Trends Mol Med. 2001;7:81–85. [DOI] [PubMed] [Google Scholar]
- 27.Beery TA. Sex differences in infection and sepsis. Crit Care Nurs. Clin North Am. 2003;15:55–62. [DOI] [PubMed] [Google Scholar]
- 28.Diodato MD, Knoferl MW, Schwacha MG, et al. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14:162–169. [DOI] [PubMed] [Google Scholar]