Abstract
Objective:
Evaluate experience over 15 years with treatment of this lesion.
Summary Background Data:
Biliary cystadenoma, a benign hepatic tumor arising from Von Meyenberg complexes, usually present as septated intrahepatic cystic lesions.
Methods:
Data were collected concurrently and retrospectively on patients identified from hospital medical records reviewed for pertinent International Classification of Diseases, Ninth Revision, Clinical Modification and CPT codes, pathology logs, and from operative case logs. Pathology specimens were rereviewed to confirm the diagnosis of biliary cystadenoma or biliary cystadenocarcinoma by 2 GI pathologists.
Results:
From October 1989 to April 2004 at our institution, 19 (18F:1M) patients had pathologically confirmed biliary cystadenomas, including one with a biliary cystadenocarcinoma. The mean age was 48 ± 15 years at initial evaluation. Complaints included abdominal pain in 74%, abdominal distension in 26%, and nausea/vomiting in 11%. Only 1 patient presented with an incidental finding. Symptoms had been present for 3 ± 5 years, with 1 to 4 different surgeons and many other physicians involved in the diagnosis or treatment prior to definitive ablation. Eight patients had undergone 20 previous treatments, including multiple percutaneous aspirations in 4 and 11 operative procedures. CT or US was diagnostic in 95%, with internal septations present in the hepatic cysts. Definitive operative intervention consisted of hepatic resection in 12 patients, enucleation in 6 patients, and fenestration and complete fulguration in 1 patient. There were no perioperative deaths. No recurrences were observed after definitive therapy, with follow-up of 4 ± 4 years.
Conclusions:
Biliary cystadenoma must be recognized and treated differently than most hepatic cysts. There remains a need for education about the imaging findings for biliary cystadenoma to reduce the demonstrated delay in appropriate treatment. Traditional treatment of simple cysts such as aspiration, drainage, and marsupialization results in near universal recurrence and occasional malignant degeneration. This experience demonstrates effective options include total ablation by standard hepatic resection and cyst enucleation.
Biliary cystadenomas represent a small minority of hepatic cysts but are often unrecognized and mistreated, even though imaging modalities are usually diagnostic. Treatment requires total ablation by resection, enucleation, or possibly fenestration and complete fulguration to avoid recurrence and the risk of malignant degeneration.
Cystic lesions of the liver are common, with some authors reporting a prevalence of 18%1 in asymptomatic patients. The differential diagnoses of cystic lesions of the liver include simple cysts, degeneration of metastatic tumors, bilomas, hematomas, abscesses, parasitic disease, polycystic liver disease (PCLD), Caroli disease, and biliary cystadenoma in as high as 5% to 11% of patients with larger cysts presenting for treatment or in older autopsy series.2–6 We present our experience with 19 patients over the last 15 years with biliary cystadenomas and cystadenocarcinomas. This series highlights the common findings of biliary cystadenomas and treatment options for long-term success.
METHODS
This study was approved by our institutional review board. Hospital records were searched for patients with International Classification of Diseases, Ninth Revision, Clinical Modification diagnoses of biliary cystadenoma, cystadenoma, and cystadenocarcinoma, as well as other hepatic cysts. In addition, case logs were searched from surgeons at our institution who perform hepatobiliary operations. Once identified from hospital and physician records, the pathologic specimens from operative resection were reviewed by 2 specialists in gastrointestinal pathology for verification of diagnosis (DW, MKW). Patient records were examined for details of demographic characteristics, symptoms, previous treatments, and patient outcomes. Patients were contacted in the spring of 2004 for follow-up outcome data. Results are shown as mean ± SD.
RESULTS
Between October 1989 and May 2004, 18 patients were identified by physicians at our institution with the diagnosis of biliary cystadenoma and 1 with biliary cystadenocarcinoma. Patients were 48.3 ± 14.6 years at the time of presentation (range, 19 to 68 years). Eighteen patients (95%) were female and 1 was male. In the 19 patients, symptoms had been present for 3.1 ± 4.8 years and included abdominal pain in 74%, abdominal fullness or bloating in 26%, and nausea/vomiting in 11%. Other less common symptoms included weight loss, mass effect, and in one, symptoms of cholangitis. One patient presented with incidental findings of a hepatic cystic mass. Patients had been evaluated by between 1 and 4 other surgeons and many more physicians, including internists and gastroenterologists prior to presentation in our clinics. Patients were in generally good health, with the most common comorbidities including hypertension in 3, non–insulin-dependent diabetes mellitus in 2, and hepatitis C virus in 1. American Society of Anesthesiology scores were 2.4 ± 0.6, with a median of 2.
Evaluation often included numerous abdominal imaging studies prior to treatment. Transabdominal ultrasound in 13 and computed tomography (CT) in 15 were the most commonly used imaging modalities, but others included magnetic resonance imaging in 1, nuclear medicine uptake studies in 1, and positron emission tomography for 1 patient with suspected malignancy. Ultrasound studies revealed anechoic, cystic structures with numerous septations, and similarly, CT findings revealed multicystic, multiseptated structures except in 1, which appeared as a solitary cyst. In addition, 3 patients underwent endoscopic retrograde cholangiopancreatography (ERCP), 1 of which had abnormal liver function studies consistent with obstructive jaundice. Laboratory studies, including complete blood count, serum chemistries, and tumor markers, were otherwise unremarkable in 18 of 19 patients.
Eleven patients received no treatment prior to definitive ablation procedures. Eight patients had 20 procedures prior to definitive ablation. Two of these were at our institution, both misdiagnosed as simple cysts. In total, prior treatments included attempts at percutaneous aspiration in 4 patients, open or laparoscopic drainage in 5, open or laparoscopic fenestration of the cyst in 5, and 1 attempt for drainage using a Roux-en-Y limb.
For definitive ablative treatment, a cyst enucleation was performed in 6 patients, a left lobectomy was performed in 7 patients, a right lobectomy in 2 patients, a bisegmentectomy in 2 patients, a nonanatomic resection in 1 patient, and a laparoscopic fenestration and complete fulguration in 1 patient. Some patients underwent multiple procedures. Operative duration was 277.0 ± 100.0 minutes. Cyst size was 10.9 ± 4.4 cm. Twelve patients required no transfusion during the operative procedure or subsequent hospital stay. Of the 7 patients that required a transfusion, the mean requirement was 5.0 ± 3.0 units of packed red blood cells. Length of hospital stay was 7.0 ± 4.6 days.
There have been no recurrences after definitive ablation, with a follow-up of 3.5 ± 4.2 years. Ten complications occurred in 7 of the 19 patients (37%). A biloma treated by percutaneous drainage and a biliary leak treated by ERCP and stent placement occurred in 1 patient each. Infectious complications included intraabdominal abscess treated by percutaneous drainage in 2 patients and 1 line infection. Atrial fibrillation and respiratory failure requiring prolonged ventilatory support occurred in 1 patient. Acute lung injury due to a systemic inflammatory response occurred in 1 patient that required prolonged mechanical ventilation. A pleural effusion occurred in 1 patient that required no additional treatment. One patient developed an incisional hernia several years after resection that required operative repair.
DISCUSSION
Biliary cystadenoma most often presents as an intrahepatic lesion. It may also manifest as an extension of the extrahepatic biliary tree in less than 10%.7 The origin of these lesions is postulated to be proliferation of ectopic embryonic tissues that otherwise aid in development of the adult gallbladder.8 The gross and microscopic characteristics of biliary cystadenoma distinguish this entity from other hepatic-based cystic lesions including simple cysts, PCLD, and malignant degeneration. Biliary cystadenomas are multiloculated cysts with an epithelial lining composed of biliary-type cuboidal or nonciliated columnar cells and are surrounded by a stroma that often (85%–90%) mimics ovarian stroma.9 Figure 1 illustrates the typical appearance of a formalin-fixed gross specimen after operative resection. Figures 2 and 3 illustrate the characteristic microscopic findings of a benign biliary cystadenoma, as well as biliary cystadenocarcinoma. The cystadenoma shows the typical features of simple columnar, nonciliated biliary-type epithelium, and a submucosal ovarian-like stroma. This is easily distinguished from the findings typical of a biliary cystadenocarcinoma, namely, a loss of epithelial nuclear stratification, a tubulopapillary architecture, and mild nuclear pleomorphism. The presence of invasion warrants the diagnosis of a cystadenocarcinoma.
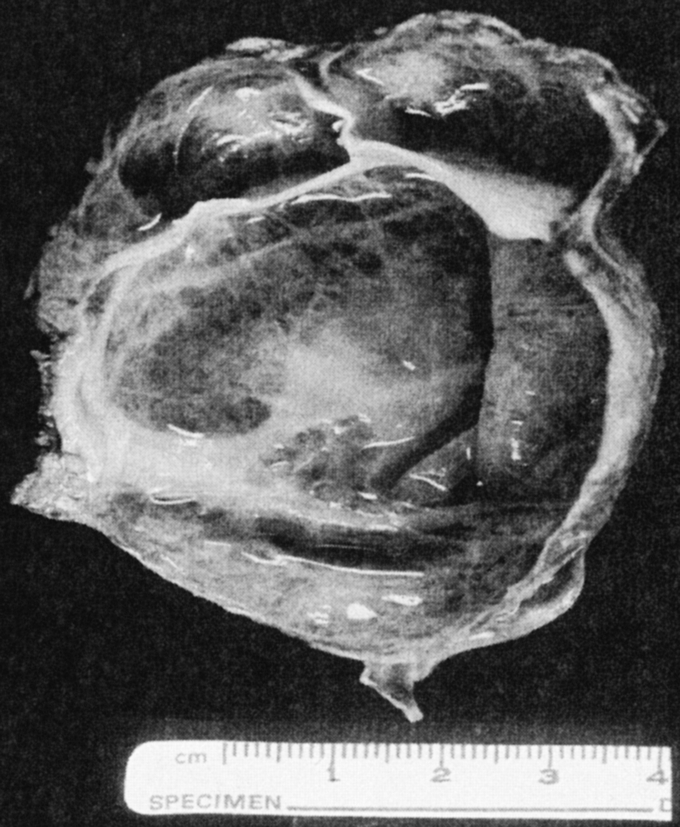
FIGURE 1. Gross photograph of a typical formalin-fixed resection specimen of a biliary cystadenoma, showing a trabecular, multilocular cystic lesion with a thickened wall, and no papillary excrescences, polypoid areas, or significant solid components.
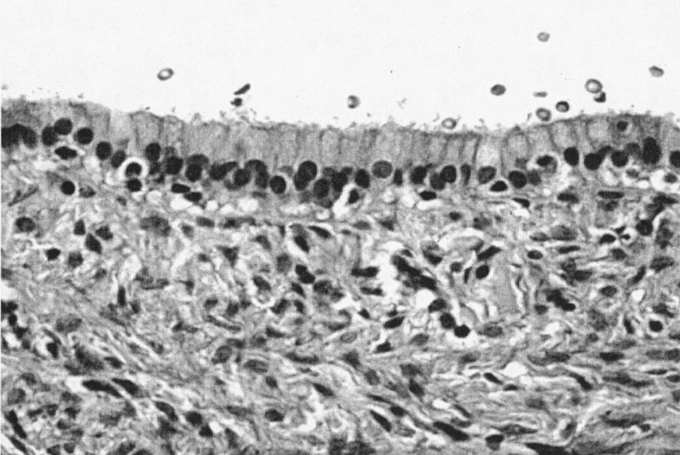
FIGURE 2. Biliary cystadenoma (40× magnification, hematoxylin and eosin stain). Lumen, upper third of frame. The mucosal surface is a simple, orderly, biliary-type columnar epithelium with basally oriented uniform and round nuclei. The mesenchymal stroma is typical of biliary cystadenomas, showing a benign spindle-cell population (“ovarian-like” stroma).
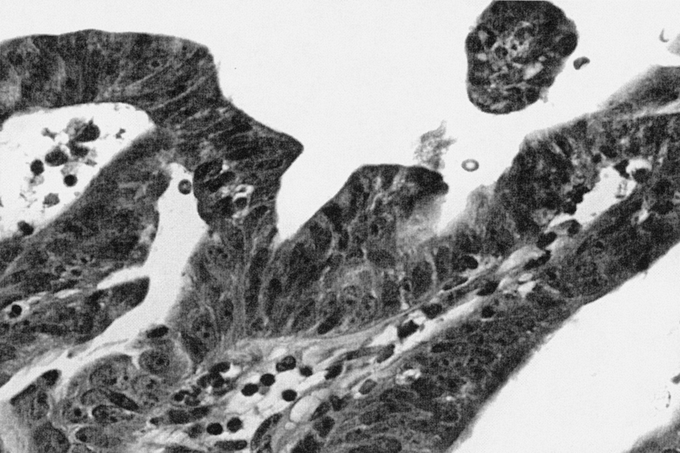
FIGURE 3. Biliary cystadenocarcinoma (40× magnification, hematoxylin and eosin stain). Increased nuclear pleomorphism and chromatin irregularity with increased epithelial cell stratification and tubulopapillary growth as compared with the biliary cystadenoma.
Presenting symptoms are usually those of abdominal pain though jaundice, and signs of cholangitis may be present when significant biliary obstruction is present. Abdominal pain was present in 74% of patients. This mirrors the earlier case series from Lewis et al10 and Ishak et al11 in which abdominal pain was the leading symptom in 80% and 60%, respectively, of their patients. Other less common symptoms such as abdominal fullness and bloating may represent symptoms from the mass effect of the tumor. We had 1 patient that presented with extrahepatic biliary cystadenomas. This patient did present with signs of cholangitis and was found to have a filling defect with ERCP. At the time of her resection, she underwent a left hepatic lobectomy for a 12-cm × 11-cm cystadenoma in the left lobe but also underwent a common bile duct exploration with resection of a second pedunculated cystadenoma of the common bile duct.
The only laboratory studies helpful in the evaluation of patients in this series were markers of biliary obstruction (alkaline phosphatase and total bilirubin) in the one patient that presented with jaundice and cholangitis. Tumor markers were not helpful in this series, though others have reported on the value of carbohydrate antigen 19-9.12–14 The most helpful radiologic studies are CT and transabdominal ultrasound. The findings on CT and US are usually diagnostic.3 CT will reveal well-demarcated cystic lesions, usually with internal septations; the walls are rarely calcified, and the presence of polypoid protrusions or wall excrescences should trigger the concern for cystadenocarcinoma.2 A representative image from one of our patients is shown in Figure 4. Ultrasound will reveal an anechoic mass with sharp demarcations and often with fine internal septations.3,15 We used PET scan in 1 patient with suspected cystadenocarcinoma based on CT findings; it was positive for malignancy.
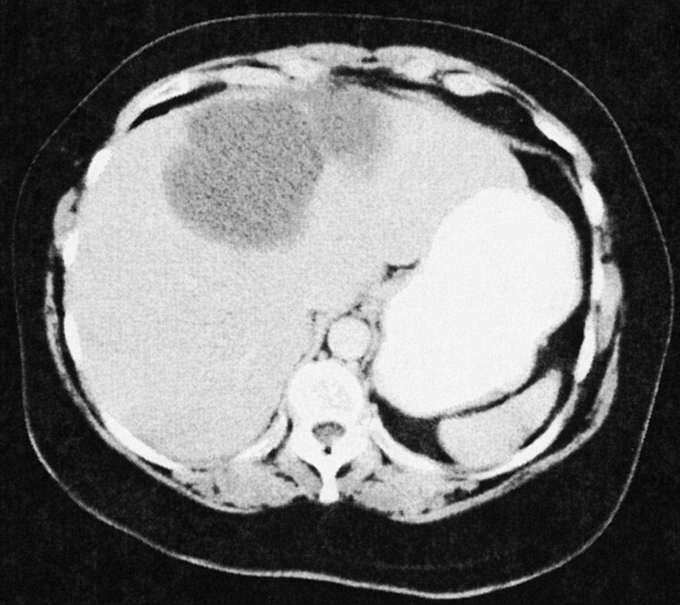
FIGURE 4. Representative CT scan image from a patient with a large biliary cystadenoma of the left lobe of the liver. Several septations are present. The interior illustrates a homogeneous appearance without studding of the internal lining sometimes seen with cystadenocarcinomas.
The malignant potential for cystadenoma supports total ablation as appropriate treatment. The largest pathologic review of cystadenoma to date examined pathologic specimens from 70 patients collected by the Armed Forces Institute of Pathology and the Rhode Island Hospital. Of the seventy specimens, 18 (25%) contained cystadenocarcinomas.16 Other case reports cite varying incidences of cystadenocarcinoma.17–19 Ishak et al11 reported on 14 patients with hepatic cystadenomas, including 6 with cystadenocarcinoma; Tsiftsis et al20 reported on treatment of 2 patients with cystadenoma and 1 with cystadenocarcinoma. Malignancy was an incidental finding in many of these patients. We had 1 patient (5%) with pathologically proven biliary cystadenocarcinoma; imaging studies including CT scan and PET suggested this diagnosis prior to resection. However, due to the difficulty in always accurately predicting malignancy in many of these lesions, complete ablation is the required treatment.
In addition to the malignant potential of these lesions, there is a high likelihood of recurrence with operations other than total ablation. As early as 1971, surgeons realized the difficulty in treating this disease process with anything less than complete removal of the tumor.21 Lewis and coauthors10 reported on surgical treatment of 15 patients with biliary cystadenoma in which resection was used with rare complications and no recurrences. Pinson et al22 previously reported on the use of cystic enucleation without mortality or late recurrence. Due to the large size of many of these lesions, biliary radicles and vascular structures are distorted and may be difficult to separate easily from the wall of the cystadenoma, especially after prior procedures or infection. Because of that finding, enucleation must be applied cautiously. Intraoperative ultrasound is commonly used in all resections.
Two patients presented with large cystadenomas in which fenestration and complete fulguration was used successfully. In one patient with biliary cystadenocarcinoma, a formal resection of the main lesion was performed. However, a satellite lesion was unroofed with fulguration of the internal cystic lining with the argon beam coagulator. Another patient underwent a laparoscopic exploration with fenestration and fulguration of the cyst bed for a 16-cm lesion in the right lobe of the liver. Neither of these patients have had recurrence of their disease, with 2142 and 390 days of follow-up, respectively. This raises the question of whether or not fenestration with complete fulguration of the cystic bed represents a successful treatment option.
Biliary cystadenomas have historically been treated by marsupialization, internal Roux-en-Y drainage, aspiration, sclerosis, or partial resection. All of these methods have been associated with high rates of complication, including sepsis, continued growth, and progress to malignancy. With total ablation, there are reports of lower rates of long-term complications.5,10,11,23–25 Our outcomes following treatment of biliary cystadenoma are similar to other reported series with total ablation.
In conclusion, biliary cystadenoma should be expected when radiologic imaging studies suggest a multilocular cystic hepatic lesion, especially in a woman. Treatment requires excision or enucleation of the cystadenoma. Long-term good outcomes are expected with total ablative treatment.
Discussions
Dr. Mark R. Ghobrial (Los Angeles, California): It is extremely difficult when we are going through very busy surgical practices to focus on lesions that are rarely encountered, such as cystadenomas. Through their diligent efforts, the authors were able to conduct a comprehensive assessment that really pinpointed the correct and appropriate management of those patients.
The authors have been able to treat all patients without recurrence and to achieve complete cure. This work clearly demonstrates that partial excisions are not the correct treatment, but it is total excision, and that when surgery is undertaken, one must be able to undertake major liver resections. In this series, there are about 8 patients who had either a total right or a total left lobectomy without complications, and they were all cured. And that is difficult to achieve.
The other point that was raised in this paper is truly how to manage liver cysts in general. Although benign cystadenomas may be rare, liver cysts in general are common. In one account, about 2.5% of the population in the United States has liver cysts.
So my questions are focused on appropriately managing liver cysts in general that cover a wide range of differential diagnosis from simple benign cysts to neoplastic forms. Dr. Pinson, what do you perceive as the appropriate workup for those cysts? Is an MR or CT sufficient? What is the role of aspiration? Do we have to aspirate those cysts to determine what to do with them?
The second question relates to what appropriate measures should be taken when liver cysts are accidentally encountered during laparotomy or laparoscopy. What should we do then? Should we aspirate the cysts, attempt to resect, or just observe?
Finally, is there a role for follow-up and not operating on somebody with a cystadenoma in particular and for other cysts in general?
Dr. Andrew S. Klein (Los Angeles, California): I would like to commend Dr. Pinson and his team from Vanderbilt for their excellent review of a relatively uncommon but often perplexing hepatobiliary lesion. Although only 1 patient in their series presented asymptomatic at the time of diagnosis, I would submit to you that with the promotion of preemptive whole body scanning and the advertisement of this by our radiology colleagues, we are going to see more incidental biliary cystadenomas in the future.
One of the attractive features of the argon-beam coagulator is that the depth of the tissue necrosis is actually less than that produced by standard electrocautery. Hemostasis of large surfaces can then be achieved while sparing structures that are relatively superficial in relation to the burn. So although the tissue destruction by the argon beam may be appropriate for benign cysts where the epithelium is 1 cell thick, the malignant form of this lesion does tend to have a propensity to penetrate the basal membrane to invade surrounding hepatic parenchyma and even to invade adjacent structures like the diaphragm.
My first question is, how will you determine which patients are appropriate for this type of fenestration and fulguration with the argon-beam coagulator? As a corollary, absent pathological examination of the cyst, which is not possible when you do the fulguration, how do you advise your patients regarding future surveillance, prognosis, and whether or not the lesion you have treated is in fact malignant?
There are several case reports of biliary cystadenomas in young women on oral contraceptives, and a number of investigators have documented the elevated estrogen receptor expression in cystadenomas, suggesting the tumors may be sensitive to estrogens and in fact estrogens may be a tumor promoter. Paradoxically, progesterone receptors have been found in the stroma that often surrounds this epithelium. All but 1 patient in your series were women. So my last question is, did you observe a high incidence of hormone use in the patients that you treated?
Dr. Henry A. Pitt (Indianapolis, Indiana): The 2 main messages are that biliary cystadenomas are frequently misdiagnosed and often mistreated. From my personal experience with 20 of these rare lesions, I agree completely with the author's major points.
Biliary cystadenomas are confused with simple cysts of the liver and inadequately treated with percutaneous or surgical external drainage or with partial excision with marsupialization or internal drainage through a Roux-Y limb. None of these treatments is appropriate because biliary cystadenomas are premalignant lesions, which frequently communicate with the biliary tree near the hepatic duct bifurcation.
Despite my general agreement with the authors, I would like to quibble about their statements regarding the incidence of this problem, ask about the role of cholangiography, and then disagree a little on their final conclusion with respect to fenestration and fulguration.
Current data, especially as we get better and better scanning techniques, suggest that actually 5% of the general population have cysts in their liver. However, the authors have quoted an old paper that states that 5% of hepatic cysts are biliary cystadenomas. If you do this math, the result would be an incidence of 250 biliary cystadenomas per 100,000 population. Now, biliary cystadenomas are more rare than cholangiocarcinomas, which occur in only 1 to 2 per 100,000. So I believe that the author's statement that biliary cystadenomas represent 5% of hepatic cysts is an overestimate. The incidence is probably much less than 1% of all hepatic cysts. However, biliary cystadenomas may represent more than 5% of symptomatic hepatic cysts that are referred for therapy.
Biliary cystadenomas arise from the biliary epithelium, and they usually contain a brown or greenish fluid similar to bile. Because they have malignant potential, the goal of surgery should be complete excision by formal hepatic resection or enucleation. However, these operations are tricky because of the biliary communication. For this reason, I have routinely done either preoperative ERCP or intraoperative cystic duct cholangiography in my patients, and I have found the biliary communication in the vast majority of my patients. Would the authors comment on the role of routine cholangiography either preoperatively or intraoperatively in designing these operations and preventing recurrence?
Again, I would like to disagree with the authors’ final conclusion, even though it was couched in “maybe” and hesitation, that fenestration with complete fulguration may be cautiously applied in selected patients. Nearly one third of their patients did have this treatment prior to referral with recurrence. In addition, the follow-up on their 1 or 2 patients in whom they have applied this treatment is very short, only a year or 2. Clinical recurrence usually takes a few years, and malignant degeneration probably takes at least a decade in these patients. Therefore, to draw this conclusion on the basis of 1 or 2 patients with a short follow-up is premature.
Dr. Joseph B. Cofer (Chattanooga, Tennessee): The problem I have had when I deal with these is the fear that I am going to miss the one that is cancer. So my question to you is this: Is there any septated cystic lesion in the liver that you don't attempt an anatomic resection to start with? Because the problem is that if you try to enucleate it and you get it out and it is cancer, have you done the right operation? So is a PET scan negative septated loculated cyst okay to try to enucleate? My strategy has always been to formally resect the lesion if I thought I could.
Dr. Alan W. Hemming (Gainesville, Florida): Over the 15-year period of the study, the techniques of hepatic imaging have changed dramatically. What do you find the most useful? One of the things we have found with biliary cystadenomas is that septations alone aren't necessarily what make the diagnosis because some simple cysts have septations. But what we found is that if you find see vascularized septations, then it is a cystadenoma. I wonder if you found the same thing.
The second question is, Mike Abecassis from Northwestern recently published a similar series and demonstrated that cyst fluid analysis of CA-19-9 levels could predict cystadenoma in essentially 100% of cystadenomas. In your manuscript, you stated that CA-19-9 levels were not helpful. Could you tell me how many of your patients with cystadenomas had elevated CA-19-9 levels?
The third question is, you propose that laparoscopic cyst fenestration with ablation of the cyst wall may be a reasonable approach—I guess it is 2 cases, although I think in the manuscript you provided it said 1—with very short follow-up. What evidence, if any, do you have for this? In your open techniques, you appear to advocate resection or enucleation. Why the change to the laparoscopic approach? Shouldn't you perform the same technique whether open or laparoscopic?
If you were to perform a laparoscopic cyst marsupialization on what was suspected to be a simple cyst but the frozen section on the wall came back as cystadenoma, would you then open and enucleate the lesion, possibly enucleate it laparoscopically, or would you ablate it with an argon-beam coagulator? We would definitely enucleate it or resect it either open or laparoscopically.
Dr. A. Osama Gaber (Memphis, Tennessee): Some of these cystadenomas that you described have been operated on before and have had complications associated with them. And I would like you to address that management of the cysts that are not just simple. What shapes your decision to excise or enucleate? Is it only anatomic, or [do] prior intervention and complications affect what you do?
Dr. K. Tyson Thomas (Nashville, Tennessee): I appreciate all the very interesting and in-depth questions. I will begin with Dr. Ghobrial.
When evaluating potential biliary cystadenomas, we routinely perform a CT scan. Many of these patients presented at our institution with numerous previous imaging methods. We find with CT scans is very appropriate and we are able to make a diagnosis in nearly 95% of patients.
We think simple cysts can be treated with aspiration and sclerosis. However, if you are treating a simple cyst at the time of an open or a laparoscopic procedure, one should biopsy the cyst wall.
In patients who present with a multiloculated, multiseptated cyst, we do not recommend expectant long-term follow-up. The incidence of cystadenocarcinoma is reported from 25% to 43% of cases. Therefore, we feel any patient with a multiseptated cyst warrants complete resection or enucleation.
In discussing the incidence of cystadenoma, we agree wholeheartedly with Dr. Pitt. There is not a wealth of studies written on the incidence of these tumors, outside of autopsy studies that were performed decades ago. With the advent of better imaging technologies, as well as the application of these modalities to asymptomatic patients, we think we will probably see more of these lesions detected.
We do not routinely perform cholangiography, either intraoperative or preoperatively. In instances where we identify a biliary communication, we oversew this directly at the time of operation. We do agree that complete resection is probably the best treatment, whether that be hepatic resection or cyst enucleation. We did treat a small subset of patients with unroofing combined with fulguration. We do know that these patients have done well for 1 and 6 years, respectively.
Dr. Cofer asked if there were any septated cystic lesions that we would leave alone. Again, if we have a multiseptated lesion, we will recommend to that patient complete ablation.
In patients who we think harbor malignancy, we will consider a PET scan. This applied to only 1 of our patients. We had no patients in our series that had elevated CA-19-9.
Fulguration of cystadenomas was performed in 2 of our patients. One patient underwent laparoscopic unroofing with fulguration of the remaining cyst wall. In 1 patient, it was part of a combined treatment that consisted of a nonanatomic resection, as well as fulguration of the remaining cyst wall after a small satellite lesion was unroofed. We recommend these patients be followed at least yearly for 3 to 5 years as the recurrences we observed routinely occurred within the first 2 years.
Footnotes
Reprints: C. Wright Pinson, MD, MBA, Division of Hepatobiliary Surgery and Liver Transplantation, 3810 The Vanderbilt Clinic, Vanderbilt University Medical Center, Nashville, TN 37232-5545. E-mail: wright.pinson@vanderbilt.edu.
REFERENCES
- 1.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58:626–629. [DOI] [PubMed] [Google Scholar]
- 2.Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895–910. [DOI] [PubMed] [Google Scholar]
- 3.Palacios E, Shannon M, Solomon C, Guzman M. Biliary cystadenoma: ultrasound, CT, and MRI. Gastrointest Radiol. 1990;15:313–316. [DOI] [PubMed] [Google Scholar]
- 4.Buetow PC, Midkiff RB. MR imaging of the liver: primary malignant neoplasms in the adult. Magn Reson Imaging Clin North Am. 1997;5:289–318. [PubMed] [Google Scholar]
- 5.Regev A, Reddy KR, Berho M, et al. Large cystic lesions of the liver in adults: a 15-year experience in a tertiary center. J Am Coll Surg. 2001;193:36–45. [DOI] [PubMed] [Google Scholar]
- 6.Walt AJ. Cysts and benign tumors of the liver. Surg Clin North Am. 1977;57:449–464. [DOI] [PubMed] [Google Scholar]
- 7.Davies W, Chow M, Nagorney D. Extrahepatic biliary cystadenomas and cystadenocarcinoma: report of seven cases and review of the literature. Ann Surg. 1995;222:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramony C, Herrera GA, Turbat-Herrera EA. Hepatobiliary cystadenoma: a study of five cases with reference to histogenesis. Arch Pathol Lab Med. 1993;117:1036–1042. [PubMed] [Google Scholar]
- 9.Ferrell L. Benign and malignant tumors of the liver. In: Odze RD, Goldblum JR, Crawford JM, eds. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. Saunders; 2004:1015–1016. [Google Scholar]
- 10.Lewis WD, Jenkins RL, Rossi RL, et al. Surgical treatment of biliary cystadenoma: a report of 15 cases. Arch Surg. 1988;123:563–568. [DOI] [PubMed] [Google Scholar]
- 11.Ishak KG, Willis GW, Cummins SD, et al. Biliary cystadenoma and cystadenocarcinoma: report of 14 cases and review of the literature. Cancer. 1977;39:322–338. [DOI] [PubMed] [Google Scholar]
- 12.Kim K, Choi J, Park Y, et al. Biliary cystadenoma of the liver. J Hepatobiliary Pancreat Surg. 1998;5:348–352. [DOI] [PubMed] [Google Scholar]
- 13.Thomas JA, Scriven MW, Puntis MC, et al. Elevated serum CA 19-9 levels in hepatobiliary cystadenoma with mesenchymal stroma: two case reports with immunohistochemical confirmation. Cancer. 1992;70:1841–1846. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Chen DR, Pang SC, et al. Mucinous biliary cystadenoma with mesenchymal stroma: expressions of CA 19-9 and carcinoembryonic antigen in serum and cystic fluid. J Gastroenterol. 1996;31:732–736. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita H, Tanimura H, Onishi H, et al. Clinical features and imaging diagnosis of biliary cystadenocarcinoma of the liver. Hepatogastroenterology. 2001;48:250–252. [PubMed] [Google Scholar]
- 16.Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma: a light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078–1091. [PubMed] [Google Scholar]
- 17.Marsh JL, Dahms B, Longmire WP Jr. Cystadenoma and cystadenocarcinoma of the biliary system. Arch Surg. 1974;109:41–43. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Watanabe Y, Tokui K, et al. Hepatobiliary cystadenocarcinoma connected to the hepatic duct: a case report and review of the literature. Hepatogastroenterology. 2003;50:1621–1624. [PubMed] [Google Scholar]
- 19.Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts: a clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56:1434–1445. [DOI] [PubMed] [Google Scholar]
- 20.Tsiftsis D, Christodoulakis M, de Bree E, et al. Primary intrahepatic biliary cystadenomatous tumors. J Surg Oncol. 1997;64:341–346. [DOI] [PubMed] [Google Scholar]
- 21.Short WF, Nedwich A, Levy HA, et al. Biliary cystadenoma: report of a case and review of the literature. Arch Surg. 1971;102:78–80. [DOI] [PubMed] [Google Scholar]
- 22.Pinson CW, Munson JL, Rossi RL, et al. Enucleation of intrahepatic biliary cystadenomas. Surg Gynecol Obstet. 1989;168:534–537. [PubMed] [Google Scholar]
- 23.Cahill CJ, Bailey ME, Smith MG. Mucinous cystadenomas of the liver. Clin Oncol. 1982;8:171–177. [PubMed] [Google Scholar]
- 24.Pelish TL, Roberts JA. Cystadenoma of the biliary system presenting as an abdominal mass in pregnancy. Obstet Gynecol. 1987;70:466–468. [PubMed] [Google Scholar]
- 25.Beretta E, De Franchis R, Staudacher C, et al. Biliary cystadenoma: an uncommon cause of recurrent cholestatic jaundice. Am J Gastroenterol 1986;81:138–140. [PubMed] [Google Scholar]


