Abstract
Objective:
A single institution retrospective analysis of 200 patients with major bile duct injuries was completed. Three patients died without surgery due to uncontrolled sepsis. One hundred seventy-five patients underwent surgical repair, with a 1.7% postoperative mortality and a complication rate of 42.9%.
Summary Background Data:
The widespread application of laparoscopic cholecystectomy (LC) has led to a rise in the incidence of major bile duct injuries (BDI). Despite the frequency of these injuries and their complex management, the published literature contains few substantial reports regarding the perioperative management of BDI.
Methods:
From January 1990 to April 2003, a prospective database of all patients with a BDI following LC was maintained. Patients’ charts were retrospectively reviewed to analyze perioperative surgical management.
Results:
Over 13 years, 200 patients were treated for a major BDI following LC. Patient demographics were notable for 150 women (75%) with a mean age of 45.5 years (median 44 years). One hundred eighty-eight sustained their BDI at an outside hospital. The mean interval from the time of BDI to referral was 29.1 weeks (median 3 weeks). One hundred nine patients (58%) were referred within 1 month of their injury for acute complications including bile leak, biloma, or jaundice. Twenty-five patients did not undergo a surgical repair at our institution. Three patients (1.5%) died after delayed referral before an attempt at repair due to uncontrolled sepsis. Twenty-two patients, having intact biliary-enteric continuity, underwent successful balloon dilatation of an anastomotic stricture. A total of 175 patients underwent definitive biliary reconstruction, including 172 hepaticojejunostomies (98%) and 3 end-to-end repairs. There were 3 deaths in the postoperative period (1.7%). Seventy-five patients (42.9%) sustained at least 1 postoperative complication. The most common complications were wound infection (8%), cholangitis (5.7%), and intraabdominal abscess/biloma (2.9%). Minor biliary stent complications occurred in 5.7% of patients. Early postoperative cholangiography revealed an anastomotic leak in 4.6% of patients and extravasation at the liver dome-stent exit site in 10.3% of patients. Postoperative interventions included percutaneous abscess drainage in 9 patients (5.1%) and new percutaneous transhepatic cholangiography and stent placement in 4 patients (2.3%). No patient required reoperation in the postoperative period. The mean postoperative length of stay was 9.5 days (median 9 days). The timing of operation (early, intermediate, delayed), presenting symptoms, and history of prior repair did not affect the incidence of the most common perioperative complications or length of postoperative hospital stay.
Conclusions:
This series represents the largest single institution experience reporting the perioperative management of BDI following LC. Although perioperative complications are frequent, nearly all can be managed nonoperatively. Early referral to a tertiary care center with experienced hepatobiliary surgeons and skilled interventional radiologists would appear to be necessary to assure optimal results.
A single institution retrospective analysis of 200 patients with major bile duct injuries was completed. Three patients died without surgery due to uncontrolled sepsis. One hundred and seventy-five patients underwent surgical repair with a 1.7% postoperative mortality and a complication rate of 51%.
Calculous biliary disease is a common condition in the United States that affects more than 30 million Americans. Over 750,000 cholecystectomies are performed annually, making gallstone disease one of the most common digestive health problems.1 The treatment of calculous biliary disease has evolved over the last 2 decades. With the development of laparoscopic technology in the late 1980s, new techniques for cholecystectomy were introduced.2–4 By the early 1990s, laparoscopic cholecystectomy (LC) had supplanted open cholecystectomy in the operative management of gallbladder stone disease. Unfortunately, the widespread application of LC has led to a concurrent rise in the incidence of major bile duct injuries (BDI).5–10 Reports have estimated the incidence of BDI has risen from 0.1 to 0.2% to 0.4 to 0.6% between the era of open cholecystectomy11,12 and the age of LC.9,10,13–15
The management of patients following major BDI is a surgical challenge often requiring the skills of experienced hepatobiliary surgeons at tertiary referral centers.16–18 The care of these patients has evolved over the last 14 years by trial and error, as well as by the individual surgeon or institutional philosophy. Collaboration among surgeons, gastroenterologists, and interventional radiologists is imperative in the care of these complex injuries. Despite the frequency of these injuries and their complicated management, the published literature contains multiple studies evaluating the long-term outcomes and management in the patients but few substantial reports regarding the early operative management of BDI.17 The goal of this study is to present the largest single institution experience reporting the perioperative management of BDI following LC from 1990 to 2003.
METHODS
Data Collection
A prospective database of all patients with a major BDI following LC that were treated at the Johns Hopkins Hospital between January 1, 1990, and April 13, 2003, was maintained in accordance with the Johns Hopkins Medicine institutional review board (No. 03-09-25-01). Major BDI included all transections or partial lacerations of the common hepatic duct, common bile duct, or major segmental ducts at the porta hepatis. Minor leaks from the cystic duct or gallbladder bed were excluded. This report includes only injuries and strictures incurred in association with LC, irrespective of whether the operation was completed laparoscopically or converted to an open procedure. Patients with bile duct strictures from trauma or benign inflammatory processes (eg, chronic pancreatitis, gallstones, stenosis of the sphincter of Oddi, biliary tract infections, duodenal ulcers, or primary sclerosing cholangitis), as well as strictures from malignant causes, were excluded. Patients’ electronic and paper charts were retrospectively reviewed to analyze demographics, referring surgeon management, as well as our group's perioperative surgical management and outcomes.
Data Analysis
Comparisons between groups of patients were made using χ2 statistics, the Fisher exact test, the Student t test, and 2-sample test of proportions as appropriate using Intercooled Stata 8.0 (Stata Corp., College Station, TX). Results are reported as mean ± SD, ranges, or percentages of the appropriate denominator. Significance was accepted at the 5% level.
RESULTS
Characteristics of the Entire Cohort (N = 200): Demographics, Prior Management, and Presentation
In the 13 years and 4 months of this study, 200 patients were treated at our institution for a major BDI following LC. The annual distribution of these patients is depicted in Figure 1. Patient demographics, management prior to referral, and initial presentation are listed in Table 1. The mean age was 45.5 ± 16.3 years, with a median age of 44 years. The distribution of age is grouped by decades, as shown in Figure 2. The cohort is notable for 150 women (75%) and 50 men (25%), with a racial distribution of 164 white (82%), 22 black (11%), and 14 other (7%).
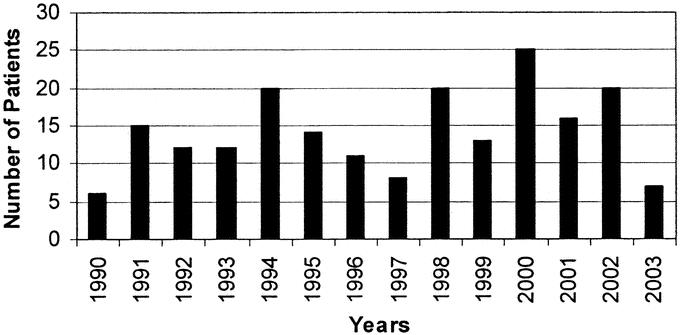
FIGURE 1. Year of presentation of patients with major bile-duct injuries referred for definitive treatment.
TABLE 1. Demographics and Initial Operation
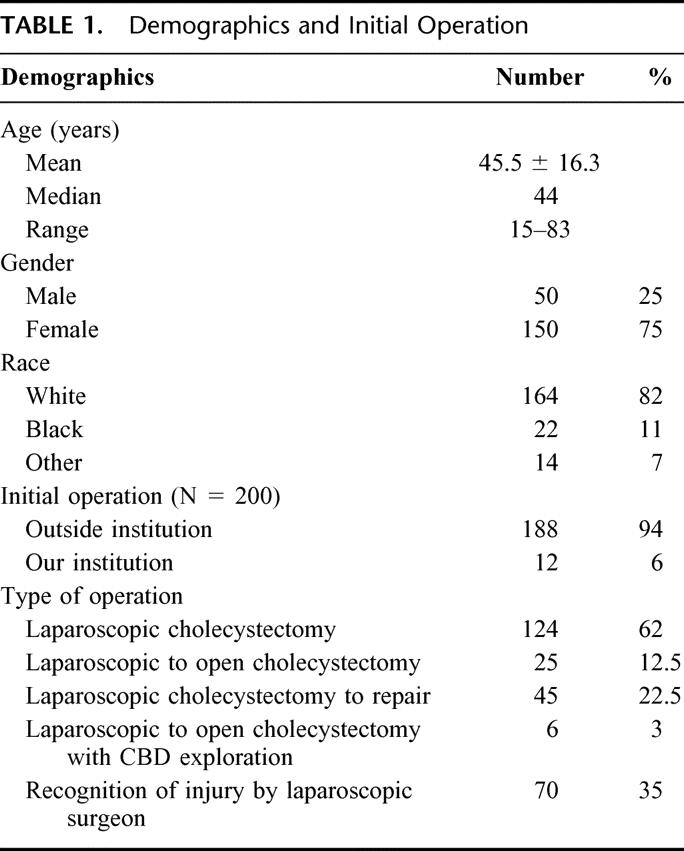
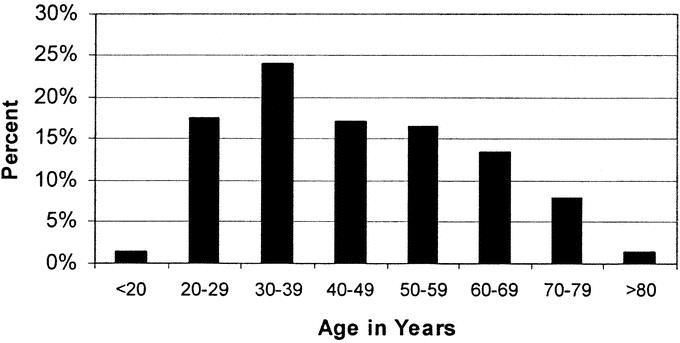
FIGURE 2. Age distribution of patients with major bile-duct injuries referred for definitive treatment.
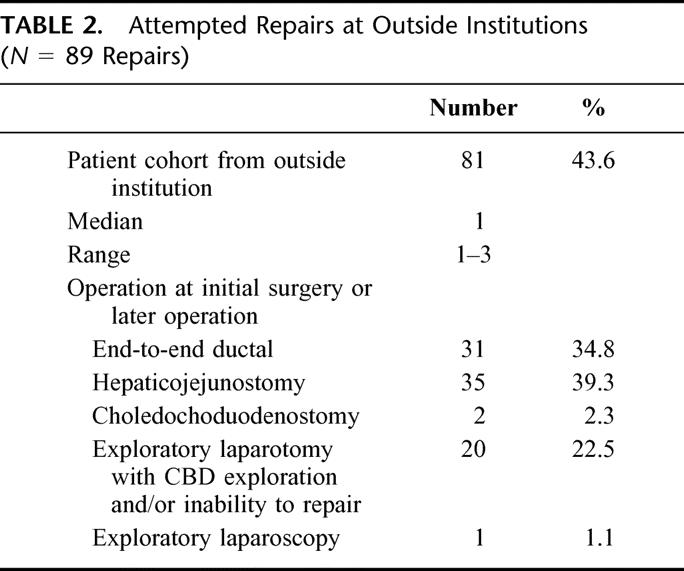
From the cohort of 200 patients undergoing LC, 188 patients (94%) sustained their BDI at an outside hospital. During the performance of the LC, 25 procedures were converted to open cholecystectomy (12.5%) and 6 procedures were converted to open cholecystectomy with common bile-duct exploration (3%). Sixty-one of these patients referred from outside hospitals had their BDI (32.4%) recognized at the original LC, although only 36 patients (19.1%) underwent an attempt at immediate repair. In comparison, 12 BDI occurred at our institution over the 160 months of this study. Nine of the 12 injuries (75%) were identified at the original LC and underwent immediate repair by Roux-en-Y hepaticojejunostomy (HJ) (77.8%) or primary end-to-end ductal anastomosis (22.2%). This rate of recognition of injury at Johns Hopkins was significantly higher (P < 0.01) than at outside hospitals. A total of 81 patients originating at outside hospitals underwent 90 surgical procedures in an attempt to repair the injury either at the time of LC or after the LC but prior to referral (Table 2). These patients underwent a range from 1 to 3 procedures prior to repair (median 1). Repairs by the original laparoscopic surgeon included end-to-end ductal anastomosis in 31 patients (34.8%), HJ in 35 patients (39.3%), and exploratory laparotomy in which the surgeon was unable to repair the injury in 18 patients (22.5%). Of the 89 repairs, ultimately 15 patients did not require reoperation following referral to Johns Hopkins Hospital.
TABLE 2. Attempted Repairs at Outside Institutions (N = 89 Repairs)
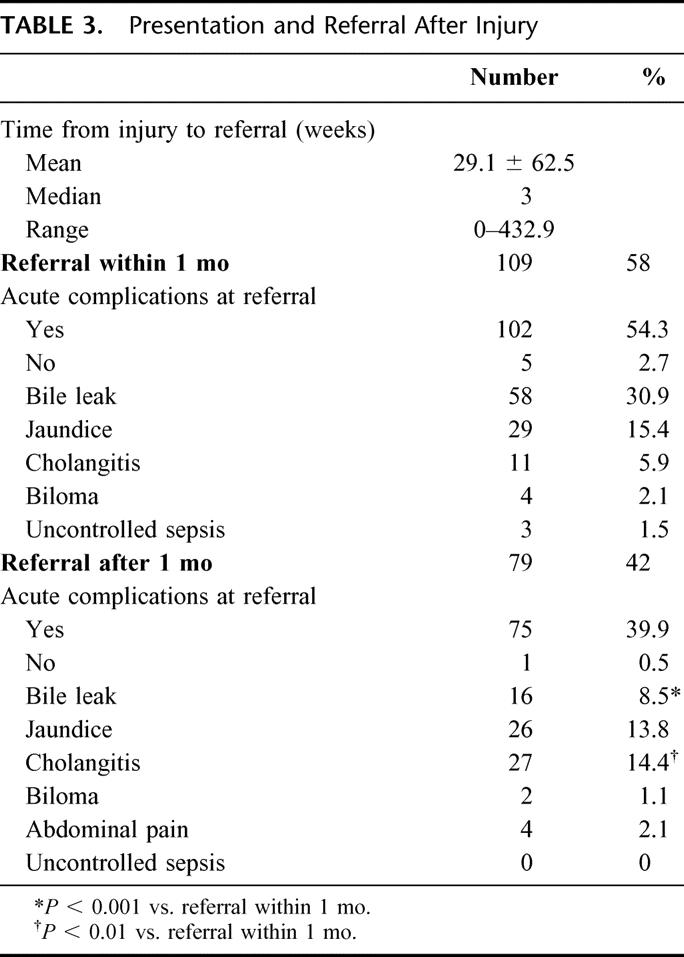
Time to referral was variable, as demonstrated in Table 3. For all 188 patients sustaining their injury at an outside hospital, the mean interval from BDI to referral was 29.1 ± 62.5 weeks (median 3 weeks). One hundred nine patients (58.0%) were referred within 1 month of their injury for acute complications, including bile leak, jaundice, cholangitis, and biloma. Five patients (2.7%) were referred in this period despite being asymptomatic. The remaining 79 patients were referred at least 1 month after their LC for symptoms consistent with biliary stricture. The incidence of cholangitis was significantly greater (P < 0.01) in this group, with a concomitant decrease in the incidence of bile leaks (P< 0.001) when compared with patients referred within 1 month of injury. Finally, only 1 patient that was referred after 1 month was asymptomatic, and no patients presented with uncontrolled sepsis.
TABLE 3. Presentation and Referral After Injury
Nonoperative Management
Twenty-five patients did not undergo a surgical repair at our institution. Included in this subgroup are 3 patients (1.5%) who died before an attempt at repair due to overwhelming sepsis at the time of referral. All 3 patients underwent nonoperative management with percutaneous biliary drainage, as well as drainage of bile collections, but uncontrolled sepsis persisted. The remaining 22 patients (11.2%) had intact biliary-enteric continuity and underwent successful balloon dilatation of an anastomotic stricture.19 The remainder of the patients (N = 175) required operative repair.
Operative Management: Immediate and Delayed Repair
In our cohort of 200 patients, a total of 175 patients underwent definitive biliary reconstruction including 172 Roux-en-Y hepaticojejunostomies (98%) and 3 end-to-end ductal repairs. Of the 3 end-to-end repairs, 2 were performed at the time of injury, and 1 was an incomplete Bismuth 1 transection that was repaired 7 days after BDI. As shown in Table 4, the overall time from BDI to definitive repair was quite variable, depending on the early or late presentation of patients. The overall mean was 42.3 ± 74.4 weeks (median 10.3 weeks). New silastic biliary stents were usually inserted at the time of repair, replacing existing percutaneous biliary catheters placed preoperatively by interventional radiology. Depending on the level of injury and biliary ductal involvement, as depicted in Figure 3, a median of 2 biliary stents was placed intraoperatively, with a range from 0 to 4. To prevent postoperative bilomas or intra-abdominal collections, a median of 3 drains (range 0–4) was placed intraoperatively at the anastomoses or at the liver exit site of the transhepatic stents.
TABLE 4. Definitive Repair (N = 175 Patients)

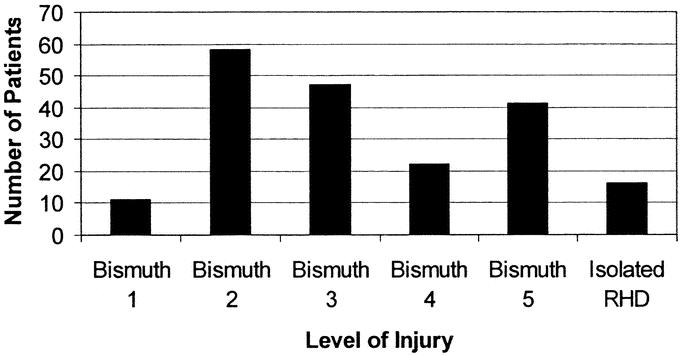
FIGURE 3. Level of bile-duct injury sustained. RHD indicates right hepatic duct.
Of the 175 repairs, 9 were performed at the time of LC at the Johns Hopkins Hospital when the injury was recognized, while 166 patients underwent definitive repair after the original LC. Thirty-four patients underwent repair at the time of the initial hospitalization following referral or at our diagnosis of BDI. This included 33 Roux-en-Y HJ repairs and one end-to-end ductal repair. In contrast, the remaining 132 patients were discharged after initial referral and readmitted for definitive repair 2 to 8 weeks following initial admission (median 5.4 weeks). All underwent an HJ repair.
Postoperative Outcomes
Of the 175 patients who underwent biliary reconstruction, 3 deaths occurred in the postoperative period (1.7%) (Table 5). These deaths included 1 patient who died secondary to a pulmonary embolus on postoperative day (POD) 1, an arrhythmia-induced cardiac arrest on POD 6, and a patient with overwhelming sepsis and multiple-system organ failure who succumbed on POD 57. Seventy-five patients (42.9%) sustained at least 1 postoperative complication (range 1–3). The most common surgical complications were wound infection (8%), cholangitis (5.7%), and intraabdominal abscess (2.9%). Minor biliary stent complications such as partial stent dislodgment or leaks at the skin site occurred in 5.7% of patients. Other nonsurgical complications included cardiopulmonary complications (7.4%), noncholangitis infections (6.9%), and minor gastrointestinal complications, including prolonged ileus and intractable diarrhea (5.1%). The other 24 complications (13.7%) were from miscellaneous causes.
TABLE 5. Postoperative Outcomes

Early postoperative cholangiography was routinely performed in patients with biliary stents in place. One hundred sixty-one (92%) patients who underwent definitive repair had a postoperative cholangiogram performed using preexisting stents (Table 5). The mean time from operation was 5.0 ± 2.5days (median 5 days). Cholangiography revealed an anastomotic leak in 4.6% of patients and extravasation at the liver dome-stent exit site in 10.3% of patients. Postoperative interventions included percutaneous abscess drainage in 9 patients (5.1%) and repeat percutaneous transhepatic cholangiography and new biliary stent placement in 4 patients (2.3%).
The mean length of stay after definitive repair was 9.4± 4.6 days (median 9 days, range 1–57). In the 175 patients that underwent operative repair, none required further operations in the perioperative setting due to anastomotic leak or other indications.
The timing of operation, defined as early (less than 1month after referral), intermediate (1–12 months after referral), and delayed (more than 12 months after referral), presenting symptoms (jaundice, bile leak/biloma, cholangitis, and pain), and history of prior repair did not affect the incidence of the most common perioperative complications noted above or the length of stay in any of the groups.
DISCUSSION
In September 1985, Erich Muhe20 performed the first LC. Although at the time the surgical community was skeptical of his new operation, by the early 1990s, “minimally invasive surgery,” including LC, was prevalent in clinical practice.21 Surgeons around the world were beginning to perform the operation, report case series, and implement guidelines for the procedure.4,22,23 The widespread acceptance and application of LC brought not only the obvious benefits of decreased postoperative pain and length of hospital stay but was associated with a troublesome increase in certain complications and, specifically, BDI.24–27 Over the last decade, BDI following LC has become recognized as a major health problem, as evidenced by studies evaluating the postoperative management and long-term quality-of-life outcomes of patients.16,17,28 Despite expectations that the rate of BDI would decrease over time as the “learning curve” of LC flattened, the rates appear to have reached a plateau, as evidenced by a recent review of nearly 1.6 million cholecystectomies performed among Medicare beneficiaries.18,29 These studies revealed a steady 0.5% incidence of BDI from 1992 to 1999. Unfortunately, BDI appears to be a complication that may continue to exist at rates greater than in the pre-LC era.
Despite improvements in technology, BDI continues to pose a significant clinical challenge. Proper diagnosis and appropriate treatment of BDI are paramount in preventing life-threatening complications of cholangitis, biliary cirrhosis, portal hypertension, end-stage liver disease, and death. Several series have previously reported the long-term outcomes following repair of laparoscopic BDI.16,17,29–36 In a previous report from our institution, a successful outcome was seen in over 94% of a group of 109 patients with laparoscopic BDI, with follow-up approaching 5 years.17 Despite these results, there remains a paucity of data regarding the short-term perioperative management of these clinically challenging patients.
Several studies have begun to provide evidence for the role of an experienced hepatobiliary surgeon in the management of these complex repairs. A major Medicare database search suggested that 75% of primary surgeons attempt to repair the injury themselves.18 But as Stewart and Way30 reported, only 17% of primary repair attempts and no secondary repair attempts performed by the laparoscopic surgeon are successful. Furthermore, Heise et al32 studied 175 patients with BDI and determined that the number of attempted repairs before referral was a significant predictor of poor outcome. The expertise of an experienced hepatobiliary surgeon would appear to be important to insure optimal results following repair of BDI.33 Early referral to tertiary care centers with expertise in biliary surgery may limit further operations, complications, time to definitive repair, and mortality.
Since the early inception and application of LC in the United States, our institution has prospectively maintained a database of patients referred with BDI sustained during LC. To our knowledge, the current series of 200 consecutive patients represents the largest single institution experience reporting the perioperative management of BDI following LC. Our cohort of BDI patients has a median age of 44 years old, with a female predominance, which represents a classic population presenting with symptomatic gallbladder disease. Of the 200 patients presented in this study, 94% of injuries occurred at outside institutions during operations that were initially commenced as an LC.
The current series illustrates the magnitude of the problem resulting from BDI. In our series, 58% of patients were referred within a month of BDI for persistent signs or symptoms of intraabdominal collections, ascending cholangitis, or sepsis. In the postinjury period, 3 patients transferred to our institution succumbed to overwhelming sepsis, representing a mortality rate of 1.5%. This experience attests to the significant impact that BDI may have upon survival.18
The control of sepsis and the ongoing bile leak is the primary goal of the initial management of a BDI. If this can be accomplished, proceeding with surgical reconstruction is not urgent. In fact, reconstruction in the face of peritonitis portends a statistically worse outcome in patients.36 Therefore, our institutional practice is to initially control sepsis via radiologic intervention and antibiotics and generally operate on patients at a later date, at a median of 5.4 weeks after their index admission when the associated inflammation has subsided.17,35
The early perioperative mortality in this series after definitive repair was 1.7%. In a review of 15 studies from 1996 to 2002, including a previous report by our group,17 Flum et al18 showed an overall postoperative mortality of 2.7%. These results are a significant improvement from an earlier era reported in a 1982 review article of more than 5000 patients undergoing 7643 procedures evaluated in 38 series, with a reported operative mortality rate of 8.6%.34
In our series, 42.9% of patients had 1 or more complications in the perioperative period. Most biliary and surgical complications were minor, including wound infections (7.4%), cholangitis (5.7%), anastomotic leak (4.6%), intraabdominal abscesses/bilomas (3.4%), and minor biliary stent complications (5.7%) requiring return to interventional radiology for cholangiography and stent manipulation. The anastomotic leak rate demonstrated by postoperative cholangiography was 4.6%. Most of these complications, although troublesome, can be managed conservatively, and therefore, no patient required reoperation in the perioperative period. In general, a paucity of published data exists on the perioperative complication rates, especially in the situation of BDI repair after LC. Innes and colleagues35 reported 22 patients with biliary strictures undergoing bilioenterostomies, with 1 abscess (4.5%) and 1 biliary fistula (4.5%). Another study by Robinson and colleagues36 evaluated 54 patients with de novo or iatrogenic biliary strictures. They reported a 30-day complication rate of 20% and 0% operative mortality.
Reasons for the relatively high complication rate in this series are unclear. Although a topic of debate, percutaneous biliary catheterization and the use of biliary stents may be partially to blame for these findings. Our group and others believe that this approach has several advantages, including preoperative definition of biliary anatomy and decompression of the biliary tree. Stenting also facilitates intraoperative identification of the proximal bile duct and reconstruction. Long-term stenting may also decrease the incidence of late postoperative stricture.37 In contrast, some argue that the risks and cost of the procedure are real and that stenting may increase postoperative complication rates, especially that of biliary sepsis.37,38
As with our initial management of the acute injury, we employ a multidisciplinary approach with interventional radiologists in management of complications in the early postoperative period. The current cohort of patients had a low anastomotic leak rate, with a slightly higher rate of liver dome-stent exit site extravasation. Only half of these leaks required new percutaneous biliary stent placement. Similarly, only 50% of patients with bilomas or abscesses required further drainage, with no patient requiring reoperation. Despite our high overall incidence of postoperative complication, our postoperative length of stay (mean 9.4 ± 4.6 days) is similar to that reported in other series.39
In this series, the timing of operation, defined as early, intermediate, and delayed, as well as presenting symptoms (jaundice, bile leak/biloma, cholangitis, and pain) or the presence of a prior attempt at repair, did not affect the incidence of the most common perioperative complications or the postoperative length of stay.
In conclusion, over the last 14 years, we have developed an institutional methodology for management of patients with major BDI following LC. Our experience represents the largest experience with patients with major BDI sustained after LC reported by a single institution. This study provides further evidence to the growing body of literature supporting the importance of biliary reconstruction at a tertiary care hospital providing a multidisciplinary approach in biliary tract disease.30,40 Life-threatening complications can occur as a result of delayed referral or, rarely, after surgical repair. Although overall complications are frequent, almost all can be managed nonoperatively. These data support the concept for early referral to a tertiary care center with experienced hepatobiliary surgeons and skilled interventional radiologists to assure optimal short-term and long-term outcomes.
ACKNOWLEDGMENTS
The authors thank the surgical house staff and the surgical nurses of the Johns Hopkins Hospital for their skill and devotion, as well as the institution's interventional radiologists for their expertise and technical abilities.
Discussions
Dr. Selwyn M. Vickers (Birmingham, Alabama): Since the advent of laparoscopic cholecystectomies in the 1980s, we have all had to deal with the real but infrequent complication of bile-duct injuries. Dr. Lillemoe, Dr. Pitt, Dr. Cameron, Dr. Talamini, and Dr. Yeo are to be commended for outlining the full gambit of evaluation, perioperative management, and outcomes in patients with bile-duct injuries. In this series, they evaluated 200 patients, actually 175, that underwent operative management. They again are to be saluted for excellent results. This group defines a safe and effective methodology for perioperative management of these patients with a 1.7% mortality and a remarkably zero rate of reexploration. However, I have a few questions that I would like to ask them, and actually related to the overall management of the patients.
In the study, there were about 30 patients, actually 34 patients, who did not get delayed for their operation. They in fact were operated upon their transfer to your institution. Can you give us any indication about the timing which you favor for operating on these patients? We have often believed delaying their surgery was beneficial. Can you indicate about anything other than the length of stay and the complication rate, which you state was no different. Can you comment on operative time and intraoperative complications? Were they different in this group of patients who received immediate operation? And are there any data that would help us determine whether you would favor early or delayed operative intervention?
Secondly, at the core of your operative management is the stent placement. And as you know, the literature has documented a number of areas where stent placement is controversial and their benefits debatable. I would say that some people swear by stents and some people swear at stents. Could you comment on 3 areas regarding the stent use in your repairs?
In addition, you have a reasonably high complication rate of 41%, although no major complications. Does the placement of these stents participate in increasing your complication rate? You have shown previously in relation to pancreatic cancer patients that stent placement actually increases wound infections, and in this series the most common complication is actually wound infections. Are the same factors at play in this group of patients?
Secondly, you have a remarkably low (0%) rate for reoperation. Do stents play a role in this result or do they require you to be dependent on your colleague interventional radiologists, who may or may not feel comfortable manipulating these stents in the postoperative setting?
Thirdly, you obviously have a number of authors who are excellent surgeons who have participated in this series. What role do the stents and the management of these patients reflect on the reproducibility of your data, as well as the training of your house staff who participate in these operations?
Finally, 96% of your patients had no clinical or radiological evidence of a leak. Do you still recommend routine postoperative cholangiography?
Dr. Reid B. Adams (Charlottesville, Virginia): This is a terrific collection of cases regarding a bedeviling problem. Bile-duct injuries continue to be a major source of morbidity for patients and a major source of concern for us as surgeons. Despite continued efforts, the rate of injury has leveled off and appears to be even at about a half percent for patients undergoing laparoscopic cholecystectomy.
This paper is important as it provides additional insight into the management of these patients and the perioperative issues that will require redress to improve their care. I have several specific issues that I wanted to address from the manuscript.
First, I notice in the manuscript no mention of endoscopic evaluation or therapy, except possibly in the group undergoing dilation. I see from the discussion and the talk that those were done by interventional radiology. While I know your preferred approach is PTC, does endoscopic evaluation and intervention play a role in these patients, and if so, when? Or since your preference is to have preoperative stents in all of these patients, have you eliminated this modality from your treatment paradigm?
Secondly, I also note that no patient required hepatic resection for treatment. I am somewhat surprised in this very large series of 200 patients that none of the patients had injuries up into the right hilum or had a combined major vascular injury at the time of their presentation that would require hepatectomy rather than reconstruction. Do you always reconstruct this type of injury? And if not, when would you consider resection?
In a similar vein, the manuscript notes that 15 isolated right hepatic duct injuries were seen. I presume this refers to an aberrant posterior sectorial duct draining segments 6 and 7. Were all of these repaired? Have you encountered the asymptomatic patient with an occluded sectorial duct and considered allowing these segments to atrophy when they are more than several weeks out from their initial operation? I ask that as this may be less of a risk for those patients in light of the 41% complication rate that you reported for those patients that were repaired.
Finally, not to belabor the point that has been discussed many times in the past regarding the practice of PTC placement in these patients, but this issue clearly represents an opportunity for improvement in the care of these patients. By my estimation it appears that approximately 15% of the reported complications in this series are directly related to the PTC tubes themselves, and likely, as Dr. Vickers suggested, some of the other complications, such as wound infections, may be related to their presence. Do you have any idea as to how to minimize or eliminate their use in these patients?
Dr. A. Osama Gaber (Memphis, Tennessee): I am very impressed with the series. In what we see in our program in terms of referrals after laparoscopic cholecystectomies, the incidence of continued biliary leakage is so much higher and the incidence of sepsis is also very high.
One of the solutions that we have developed that I didn't see referred to in this paper is an earlier operation of patients with sepsis and exteriorization of the bile duct then coming back again and doing the repair at a later date after the sepsis is abated. I see you have no reexplorations, which makes your techniques probably different because we have to reexplore the patient again to do the reconstruction. Can you tell me what you do with the patient that comes in with very extensive abdominal sepsis that you would have to explore? Would you still continue to do the primary repair right away?
Dr. Thomas R. Gadacz (Augusta, Georgia): What is your approach to those patients who have had a bile-duct injury, undergone instrumentation, and now present with an infected biloma? What is your timing for reconstruction in these patients?
Dr. Joseph B. Cofer (Chattanooga, Tennessee): Dr. Lillemoe, this was a very nice series. My experience with these types of injuries nowhere approaches yours, but in my neck of the woods I am fortunate to get referred many of these. And I was struck by the fact that you had a significant percentage of dome of the liver abscesses, and also it looks like you have about 3 drains in all these people. And you mention that you put a drain over the dome of the liver. I assume the drains are near the exit site of the PTH tubes. Recent data, as I am sure you are aware, looking at the use of drains in pancreaticojejunum anastomoses seems to indicate that maybe that use of drains is associated with a higher incidence of abscess.
In my personal series, I never see this particular problem with abscess formation over the dome of the liver. But then I don't put a drain there. Have you thought that maybe the drain was the cause of that abscess? And do you have enough people that you didn't drain over the dome of the liver that you could compare with ones you had drained to see if the drain might be the problem?
Dr. Keith D. Lillemoe (Indianapolis, Indiana): First, to Dr. Vickers, as well as Drs. Gaber's and Gadacz's question concerning the timing of our reconstruction: Indeed, only 34 patients had their repair done at a time near their injury, whereas the remaining patients were usually discharged for a period of time and brought back for elective repair.
Our belief, and again getting to Dr. Gaber's question, is most of these patients who have an ongoing bile leak or bile collections need to be managed initially with the interventional radiologist both controlling the biliary fistula by transhepatic stent replacement and draining the biloma or infected collections associated with the leak. In 100% of our patients, we were able to accomplish this noninvasively, with none of our patients requiring early operation after transfer. We did have a couple of patients who died after transfer, but all had controlled bile leaks, and they were in multisystem organ failure at the time of transfer. Notably, all of those patients had already undergone either 1 or 2 operations prior to transfer to our institution.
So in the optimal situation, we control the sepsis, we send the patients home, usually with their biliary system completely externalized, for a period of 3 to 4 weeks, bring them back at that time, expecting that the inflammation associated with the bile leak has resolved. Those patients that we chose to operate on early usually had a controlled leak associated with an operatively placed drain, and we thought the inflammation was minimal and felt we could go back in at that early time.
Dr. Vickers and Dr. Adams both raised the question of the role of the stents. As most people know, in the Hopkins experience, these stents play a very valuable role. Our intervention radiologists, who are tremendous, are very aggressive and are persistent in trying to get access to these nondilated biliary systems. They are almost always able to control the biliary fistula to allow to us to do the definitive repair in an elective setting.
However, do stents contribute to some complications? Most of our complications associated with the stents were minor. These include dislodgements or leakage at the exit site or over the dome of the liver. We experienced no major complications such as hemobilia. In contrast, stents provide such an advantage both in the short term in controlling the biliary fistula, at the time of repair in finding the transected duct which is always retracted high up into the liver hilum, and finally postoperatively for control of any leak that may occur at the anastomosis.
Concerning postoperative anastomotic leaks, in most patients when we have a drain in that area, we see bile and so we are pretty sure that there is a leak. The interventional radiologist doesn't necessarily have to diagnose the leak, but they can tell us the magnitude and make sure we have the system adequately drained. Due to the presence of stents, no patients required reoperation for an anastomotic leak.
Finally, to address the stents as they contribute to the development of complications: It has been shown by both data from our institution and from Memorial Sloan-Kettering following Whipple resection that stents do increase the rate of infectious complications, likely because they allow the biliary tree to be colonized with bacterial. Yet despite this, I still think in the long run these stents are very valuable, and I would hate to try to repair these patients without them in place.
The question was asked concerning the reproducibility of the data. I think that the key for this is not just the experience of the surgeons, but the essential role should be played by interventional radiologists. I really think that any experienced biliary surgical group with a high-quality interventional radiologist should be able to employ his approach for the management of their patients and have comparable outcomes.
Dr. Adams asked about the role of endoscopic treatment. Some of these patients did come to us having failed attempts at endoscopic balloon dilatation. Dr. McFee talked about that original report from the Duke describing the so-called classic lap chole injury with bile duct transaction. In almost all of these patients, there is discontinuity of the proximal biliary tree and the GI tract. Therefore, access would be impossible by the endoscopic route. The one exception is those cases in which they have an end-to-end repair at the time of injury, which has subsequently developed a stricture; then the endoscopist may have a role. Many of these patients had ERCPs before they got to us, but we don't consider an ERCP alone to provide adequate anatomic information and in almost all cases need percutaneous access to define the proximal anatomy and for placement of a stent.
Dr. Adams spoke of the role for hepatic resection. Our incidence of recognized major arterial injuries appears to be lower than most series that have been reported. I know of only 1 patient in the series that had to have a liver debridement for necrosis, and that was done before transfer to our institution. So we didn't have to deal with dead livers that required resection.
Now, would a very high injury well up into secondary branches of the right hepatic duct be better managed with a right hepatic lobectomy? I think our data say that the level of the injury really doesn't make a difference. So I think we are still doing these patients a favor to avoid resection and to perform a reconstruction, although some of these patients will require 3 or more stents.
Concerning isolated right isolated hepatic duct injury, which usually involves the posterior segment: The vast majority of these presented with a bile leak. Often these are very hard to diagnose. We actually reported a series of isolated hepatic duct repairs at the SSAT and published in The Journal of GI Surgery a few years ago. During the discussion of the paper, the question was asked: Would they be better off with resection as opposed to reconstruction because of the small duct? I think that resection might be indicated, but actually if the patient is asymptomatic we might leave them alone.
I think I addressed the questions of Drs. Gaber and Gadacz about the timing of the repair and how we manage septic patients. The control of sepsis is very important, and in almost all cases this can be accomplished nonoperatively. I always say to our residents that the last place a patient with a major bile duct injury and a bile leak needs to be is in the operating room, and I think the radiology suite is the best place for them.
Finally, Dr. Cofer, let me explain the leaks over the dome of the liver. These silastic stents have multiple side holes throughout about a third of the length of the stent. It is key at operation that you leave the last side hole about a centimeter or 2 below the surface of the liver parenchyma. Sometimes, however, during closing and positioning of the stents, or maybe just postoperatively, these stents will slip back so the last side hole is outside the parenchyma of the liver over the dome, resulting in a bile leak over the dome of the liver. We place drains almost routinely over the dome so that only a handful of our patients required another instrumentation to drain a collection. In most cases, the radiologist is able to advance the stent back into the liver parenchyma and the leak resolves.
Footnotes
Reprints: Keith D. Lillemoe, MD, Department of Surgery, Indiana University School of Medicine, 545 Barnhill Drive EH 203, Indianapolis, IN 46202-5124. E-mail: klillemo@iupui.edu.
REFERENCES
- 1.Nakeeb A, Comuzzie AG, Martin L, et al. Gallstones: genetics versus environment. Ann Surg. 2002;235:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko ST, Airan MC. Review of 300 consecutive laparoscopic cholecystectomies: development, evolution, and results. Surg Endosc. 1991;5:103–108. [DOI] [PubMed] [Google Scholar]
- 3.Peters JH, Ellison EC, Innes JT, et al. Safety and efficacy of laparoscopic cholecystectomy: a prospective analysis of 100 initial patients. Ann Surg. 1991;213:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadacz TR, Talamini MA, Lillemoe KD, et al. Laparoscopic cholecystectomy. Surg Clin North Am. 1990;70:1249–1262. [DOI] [PubMed] [Google Scholar]
- 5.A prospective analysis of 1518 laparoscopic cholecystectomies: the Southern Surgeons Club. N Engl J Med. 1991;324:1073–1078. [DOI] [PubMed] [Google Scholar]
- 6.Ponsky JL. Complications of laparoscopic cholecystectomy. Am J Surg. 1991;161:393–395. [DOI] [PubMed] [Google Scholar]
- 7.Voyles CR, Petro AB, Meena AL, et al. A practical approach to laparoscopic cholecystectomy. Am J Surg. 1991;161:365–370. [DOI] [PubMed] [Google Scholar]
- 8.Lepsien G, Ludtke FE, Neufang T, et al. Treatment of iatrogenic common bile duct injury during laparoscopic cholecystectomy through the laparoscopic insertion of a T-tube stent. Surg Endosc. 1991;5:119–122. [DOI] [PubMed] [Google Scholar]
- 9.Deziel DJ, Millikan KW, Economou SG, et al. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9–14. [DOI] [PubMed] [Google Scholar]
- 10.Wherry DC, Marohn MR, Malanoski MP, et al. An external audit of laparoscopic cholecystectomy in the steady state performed in medical treatment facilities of the Department of Defense. Ann Surg. 1996;224:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roslyn JJ, Binns GS, Hughes EF, et al. Open cholecystectomy: a contemporary analysis of 42,474 patients. Ann Surg. 1993;218:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101–125. [PubMed] [Google Scholar]
- 13.Orlando R 3rd, Russell JC, Lynch J, et al. Laparoscopic cholecystectomy: a statewide experience: the Connecticut Laparoscopic Cholecystectomy Registry. Arch Surg. 1993;128:494–498. [DOI] [PubMed] [Google Scholar]
- 14.Wherry DC, Rob CG, Marohn MR, et al. An external audit of laparoscopic cholecystectomy performed in medical treatment facilities of the department of Defense. Ann Surg. 1994;220:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go PM, Schol F, Gouma DJ. Laparoscopic cholecystectomy in The Netherlands. Br J Surg. 1993;80:1180–1183. [DOI] [PubMed] [Google Scholar]
- 16.Melton GB, Lillemoe KD, Cameron JL, et al. Major bile duct injuries associated with laparoscopic cholecystectomy: effect of surgical repair on quality of life. Ann Surg. 2002;235:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lillemoe KD, Melton GB, Cameron JL, et al. Postoperative bile duct strictures: management and outcome in the 1990s. Ann Surg. 2000;232:430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flum DR, Cheadle A, Prela C, et al. Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA. 2003;290:2168–2173. [DOI] [PubMed] [Google Scholar]
- 19.Misra S, Melton GB, Geschwind JF, et al. Percutaneous management of bile duct strictures and injuries associated with laparoscopic cholecystectomy: a decade of experience. J Am Coll Surg. 2004;198:218–226. [DOI] [PubMed] [Google Scholar]
- 20.Muhe E. [Laparoscopic cholecystectomy: late results]. Langenbecks Arch Chir Suppl Kongressbd. 1991:416–423. [DOI] [PubMed] [Google Scholar]
- 21.Litynski GS. Erich Muhe and the rejection of laparoscopic cholecystectomy (1985): a surgeon ahead of his time. JSLS. 1998;2:341–346. [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgibbons RJ Jr, Schmid S, Santoscoy R, et al. Open laparoscopy for laparoscopic cholecystectomy. Surg Laparosc Endosc. 1991;1:216–222. [PubMed] [Google Scholar]
- 23.Goodman GR, Hunter JG. Results of laparoscopic cholecystectomy in a university hospital. Am J Surg. 1991;162:576–579. [DOI] [PubMed] [Google Scholar]
- 24.Hansen OH, Hakansson TU, Jensen LP. [Complications of laparoscopic cholecystectomy]. Ugeskr Laeger. 1991;153:3235–3236. [PubMed] [Google Scholar]
- 25.Parish KL, Chapman WC, Williams LF Jr, et al. Are new treatment methods of gallbladder stones the death-knell for gallstone surgery? Am Surg. 1991;57:634–641. [PubMed] [Google Scholar]
- 26.Bailey RW, Zucker KA, Flowers JL, et al. Laparoscopic cholecystectomy: experience with 375 consecutive patients. Ann Surg. 1991;214:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters JH, Gibbons GD, Innes JT, et al. Complications of laparoscopic cholecystectomy. Surgery. 1991;110:769–777. [PubMed] [Google Scholar]
- 28.Savader SJ, Lillemoe KD, Prescott CA, et al. Laparoscopic cholecystectomy-related bile duct injuries: a health and financial disaster. Ann Surg. 1997;225:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvete J, Sabater L, Camps B, et al. Bile duct injury during laparoscopic cholecystectomy: myth or reality of the learning curve? Surg Endosc. 2000;14:608–611. [DOI] [PubMed] [Google Scholar]
- 30.Stewart L, Way LW. Bile duct injuries during laparoscopic cholecystectomy: factors that influence the results of treatment. Arch Surg. 1995;130:1123–1128. [DOI] [PubMed] [Google Scholar]
- 31.Lillemoe KD, Martin SA, Cameron JL, et al. Major bile duct injuries during laparoscopic cholecystectomy: follow-up after combined surgical and radiologic management. Ann Surg. 1997;225:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heise M, Schmidt SC, Adler A, et al. [Management of bile duct injuries following laparoscopic cholecystectomy]. Zentralbl Chir. 2003;128:944–951. [DOI] [PubMed] [Google Scholar]
- 33.Melton GB, Lillemoe KD. The current management of postoperative bile duct strictures. Adv Surg. 2002;36:193–221. [PubMed] [Google Scholar]
- 34.Warren KW, Christophi C, Armendari ZR. The evolution and current perspectives of the treatment of benign bile duct strictures: a review. Surg Gastroenterol. 1982;1:141–146. [Google Scholar]
- 35.Innes JT, Ferrara JJ, Carey LC. Biliary reconstruction without transanastomotic stent. Am Surg. 1988;54:27–30. [PubMed] [Google Scholar]
- 36.Robinson TN, Stiegmann GV, Durham JD, et al. Management of major bile duct injury associated with laparoscopic cholecystectomy. Surg Endosc. 2001;15:1381–1385. [DOI] [PubMed] [Google Scholar]
- 37.Mercado MA, Chan C, Orozco H, et al. To stent or not to stent bilioenteric anastomosis after iatrogenic injury: a dilemma not answered? Arch Surg. 2002;137:60–63. [DOI] [PubMed] [Google Scholar]
- 38.Hochwald SN, Burke EC, Jarnagin WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261–266. [DOI] [PubMed] [Google Scholar]
- 39.Bauer TW, Morris JB, Lowenstein A, et al. The consequences of a major bile duct injury during laparoscopic cholecystectomy. J Gastrointest Surg. 1998;2:61–66. [DOI] [PubMed] [Google Scholar]
- 40.Pitt HA, Murray KP, Bowman HM, et al. Clinical pathway implementation improves outcomes for complex biliary surgery. Surgery. 1999;126:751–756. [PubMed] [Google Scholar]


