Abstract
Objective:
To determine the impact of pathologic response following preoperative chemoradiation (CRT) on the AJCC esophageal cancer staging system.
Summary Background Data:
Increasing numbers of locoregionally advanced esophageal cancer patients are treated with preoperative CRT prior to surgical resection.
Methods:
Five hundred ninety-three pts from 1985 to 2003 with esophageal cancer who underwent surgery with (n = 239) or without CRT (n = 354) were reviewed. Resected esophageal tumors were assessed for pathologic response by determining extent of residual tumor following CRT (P0, 0% residual; P1, 1%–50% residual; P2, >50% residual).
Results:
After CRT down-staging, pTNM specific survival was similar, irrespective of treatment group (P = 0.98). The pTNM stage distribution was more favorable in the CRT group (P < 0.001) despite a more advanced initial cTNM stage distribution (P < 0.001). Following CRT, the pathologic response (pP) at the primary tumor as defined by extent of residual tumor predicted overall survival (3 years: P0, 0% residual = 74%; P1, 1%–50% residual = 54%; P2, >50% residual = 24%, P < 0.001) and stage specific survival with greater accuracy than pTNM stage alone.
Conclusions:
Our analyses demonstrate that following CRT, pTNM continues to predict survival. The extent of pathologic response following CRT is an independent risk factor for survival (pP) and should be incorporated in the pTNM esophageal cancer staging system to better predict patient outcome in esophageal cancer.
Following preoperative chemoradiation (CRT), pathologic stage (pTNM) continues to predict survival in esophageal cancer. The extent of pathologic response following CRT is an independent risk factor for survival and should be incorporated in the pTNM esophageal cancer staging system to better predict patient outcome in esophageal cancer.
Carcinoma of the esophagus and gastroesophageal junction (GEJ) is an aggressive disease with a poor prognosis.1–3 Because of poor outcomes with surgery alone, an increasing number of patients with locoregionally advanced esophageal cancer are treated with preoperative chemoradiation (CRT) and surgery.4,5 The impact of this treatment paradigm shift on the pathologic stage (pTNM) esophageal cancer staging system has not been clearly defined. When surgery alone is the primary therapy, the best predictor of survival and long-term survival is the surgical pathologic stage according to the American Joint Committee on Cancer (AJCC) staging criteria.6,7 Because of pathologic down-staging, the possibility exists that CRT-treated patients may have different survivals than patients treated with surgery alone. Additionally, it is well known that a complete pathologic response (pP) (path CR, 0% residual cancer) following CRT is associated with improved long-term survival.8–10 We have recently demonstrated that a partial pP is also prognostic following CRT, with improved outcomes in patients who have partial pPs (1%–50% residual cancer) compared with no pP (>50% residual cancer).11 This manuscript extends these initial observations and evaluates the impact of complete and partial pPs on the pTNM esophageal cancer staging system. Our study shows that pP is an independent predictor for long-term survival in patients treated with CRT. In this manuscript, we propose a modified pTNM esophageal cancer staging system to incorporate this prognostic factor (pP: P0, 0% residual; P1, 1%–50% residual; P2, >50% residual) into the pTNM staging system to better predict long-term outcome for esophageal cancer patients treated with CRT.
METHODS
Patients
This study included 239 consecutive patients with histologically confirmed invasive squamous cell carcinoma or adenocarcinoma of the esophagus and GEJ with available pathologic materials out of a total of 295 patients, who were treated with preoperative CRT followed by esophagogastrectomy between 1985 and 2003 and 354 consecutive patients treated with surgery alone in the same period used as a control group at The University of Texas M. D. Anderson Cancer Center (MDACC). The study was approved by the MDACC institutional review board.
Preoperative Staging and Treatment
Pretreatment clinical staging involving computed topographic (CT) scans, endoscopic ultrasonography, or positron emission tomography (PET) was performed at admission. In patients treated with preoperative CRT followed by surgery, the clinical stages were II, III, or IVA (celiac lymph node involvement); patients with systemic metastasis (stage IVB) were excluded. Preoperative chemotherapy included 3 major chemotherapeutic agents; 5-FU-based in 223 patients, CIS-based in 167 patients, and Taxol-based in 117 patients. Radiation treatment used concurrent treatment with chemotherapy and 45 to 50.4 Gy of treatment over a 4- to 6-week period. Four to 6 weeks after completion of neoadjuvant therapy, patients underwent surgical esophageal resection. Surgical treatment included Ivor-Lewis esophagectomy (abdominal-right thoracic approach), 3-field or McKeown esophagectomy (right thoracic abdominal-cervical approach), or transhiatal esophagectomy (abdominal-cervical approach). Patients treated with surgery alone received surgery without preoperative CRT.
Assessment of Residual Tumor Status
Residual cancer status in patients treated with preoperative CRT was determined in esophagogastrectomy specimens as previously described.11 The extent of residual cancer was assessed based on an estimation of the percentage of residual cancer in relation to the total tumor area, including the amount of radiation-induced tissue injury, as described before.11 The extent of residual cancer in the esophagectomy specimen was semiquantitatively evaluated and assigned to 1 of the 3 categories: 0% residual cancer (P0); 1% to 50% residual cancer (P1); and more than 50% residual cancer (P2), as previously described.11 All H&E sections from resected esophagogastrectomy specimens, including lymph nodes, were retrospectively reviewed without knowledge of patient treatment or follow-up status. Each specimen was evaluated for extent of residual cancer, depth of invasion, and lymph node metastasis and staged according to the current AJCC staging system for esophageal cancer.7 PP was arbitrarily assigned a pP factor (pP: 0% residual cancer (P0); 1%–50% residual cancer (P1); and more than 50% (P2) residual cancer) based on residual tumor at the primary to allow assessment of the pP and its impact on the pTNM staging system.
Statistical Analysis
Survival probability analyses were performed using the Kaplan-Meier method and were calculated from the date of surgery to the date of death or most recent follow-up. Operative mortality was excluded from survival analyses to allow determination of long-term outcome rather than short-term morbidity. The prognostic significance of potential parameters was determined by univariate analysis. Cox proportional-hazards models were fitted for multivariable analysis. Differences between groups were considered statistically significant if the P values were less than 0.05 in a 2-tailed test. After examining interactions between variables, backward stepwise procedure was used to derive the “best-fitting” model. The statistical analysis was performed with SPSS Software for Windows (version 11.5.2.1; SPSS, Chicago, IL).
RESULTS
Patient and Tumor Characteristics
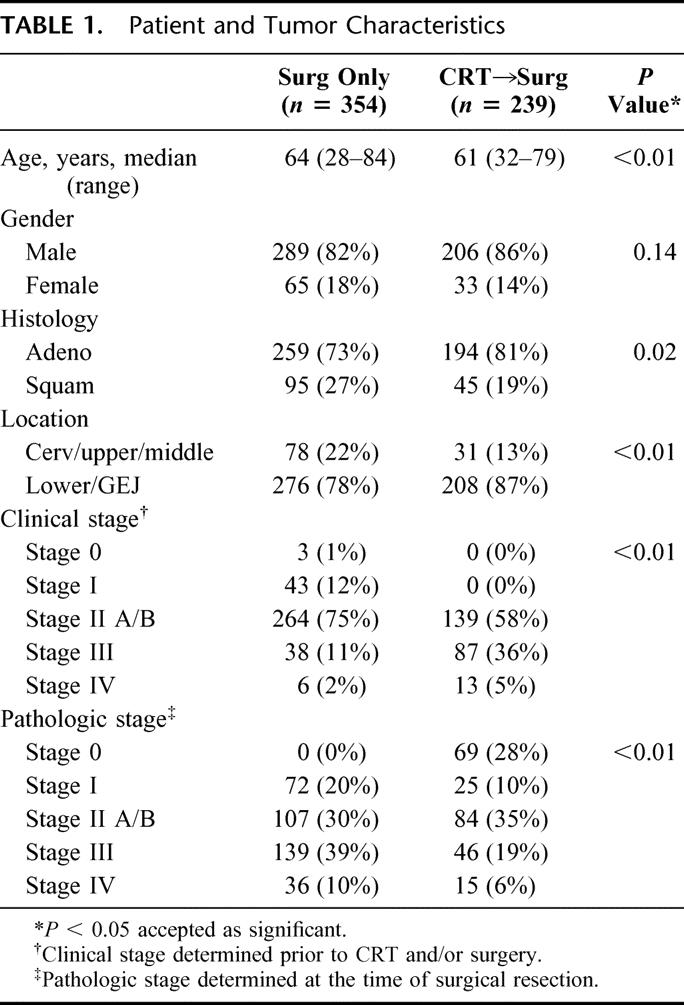
The study population included all patients with squamous cell carcinoma or adenocarcinoma treated between 1985 and 2003 at MDACC who underwent esophageal resection. Patients were assigned to the different treatment groups according to surgeon and patient preference and the clinical stage at initial evaluation. As evidenced by Table 1, esophageal cancer patients with earlier clinical stage were usually treated with surgery alone while more advanced tumors were usually treated with CRT and surgery. Despite this imbalance in clinical stage, significant pathologic down-staging was observed in those treated with CRT, as evidenced by the earlier pathologic stage at the time of surgery. Other differences noted in the study populations included a tendency for CRT patients to have a higher proportion of adenocarcinoma and lower esophageal locations. ASA performance status, preoperative comorbidities, and operative mortality (3% versus 4%) did not differ significantly being groups (data not shown).
TABLE 1. Patient and Tumor Characteristics
Impact of CRT on pTNM Stage Specific Survival
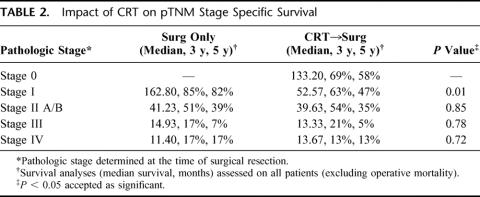
As Table 2 demonstrates, despite pathologic down-staging, overall survival was still well predicted by the pTNM staging system. CRT patient outcome was determined by their final pathologic pTNM stage and did not reflect their original clinical stage. Interestingly, the survival between groups was also very similar except in stage I patients. This suggests that the pTNM staging system works well after CRT and reflects the long-term outcome of patients.
TABLE 2. Impact of CRT on pTNM Stage Specific Survival
Impact of pP on pTNM Stage Specific Survival
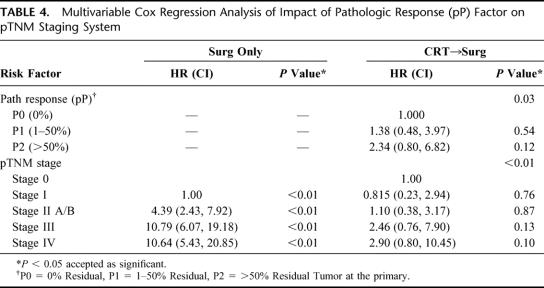
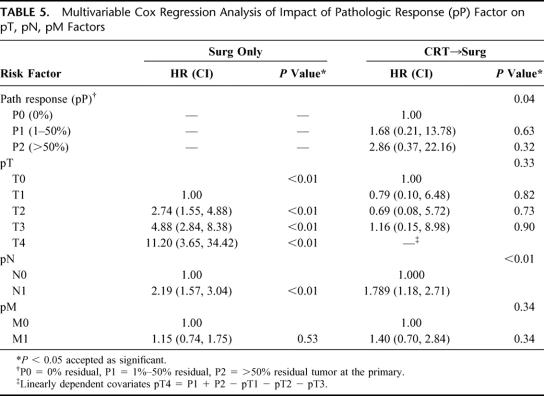
We then evaluated the impact of pP on the standard pTNM staging system in CRT-treated patients (Table 3) to determine if this predictive factor would further subclassify patients. As Figure 1 demonstrates, pP (pP) predicts long-term survival (3 years: P0 = 0% residual = 74%; P1= 1%–50% residual = 54%; P2 = >50% residual = 24%, P < 0.001). The data also suggest that pP impacts on each pTNM stage, allowing a further prognostic subclassification (Table 3, Figs. 2 and 3). A patient with involved nodes (N1) with a significant pP (P1, 1%–50% residual) has a similar survival to a patient with noninvolved nodes (N0) and a poor pP (P2, >50% residual) (Fig. 2). Additionally, stage II patients without a significant pP (P2, >50% residual) have similar survival to stage III patients with a partial pP (P1, 1%–50% residual) (Fig. 3). Multivariable Cox regression analysis demonstrates that pP is an independent predictor of survival even when controlled for pathologic stage (Table 4) or each of the pT, pN and pM factors (Table 5). In fact, Table 5 suggests that pP and nodal status (pN) are the most important predictors of survival in the CRT-treated patients.
TABLE 3. Impact of Pathologic Response (pP) at Primary Tumor on pTNM Stage Specific Survival Following CRT
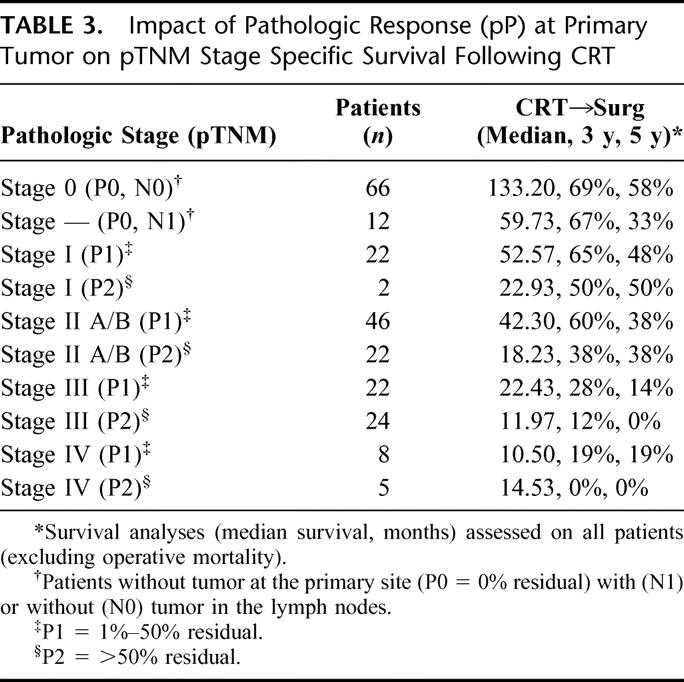
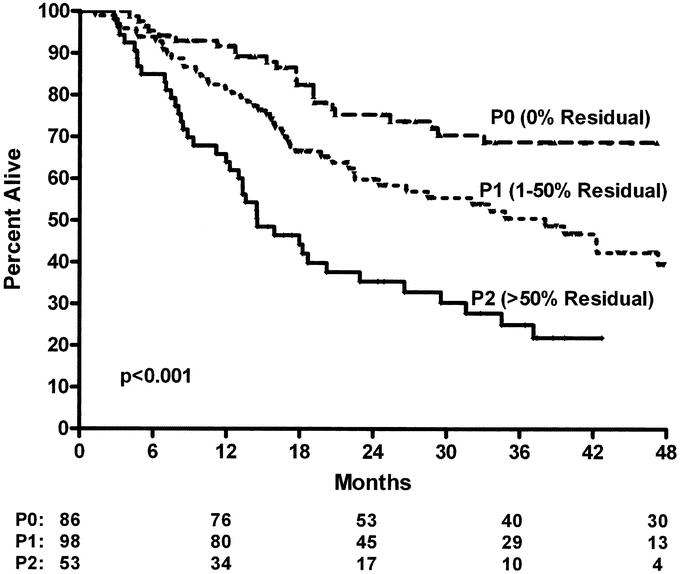
FIGURE 1. Overall survival of resected esophageal cancer patients treated with preoperative chemoradiation and surgery according to pathologic response at primary tumor (pP) (3 years: P0 = 0% residual = 74%; P1 = 1%–50% residual = 54%; P2 = >50% residual = 24%, P < 0.001).
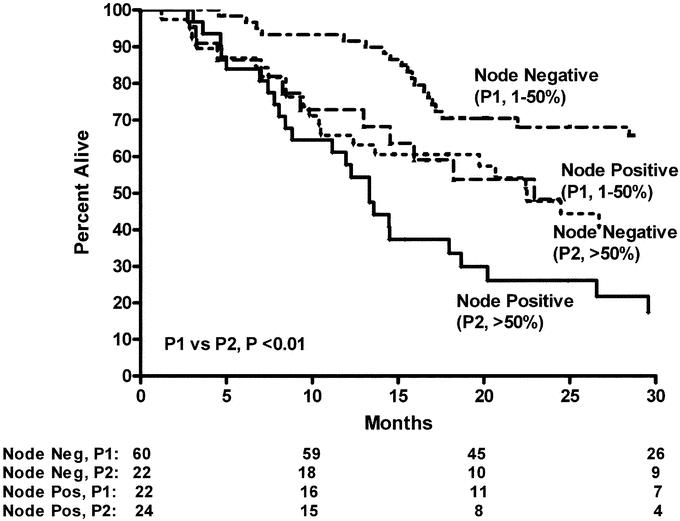
FIGURE 2. Overall survival of resected esophageal cancer patients treated with preoperative chemoradiation and surgery according to pathologic response at primary tumor (pP) and lymph node status at time of surgery (P1 versus P2, P < 0.01).
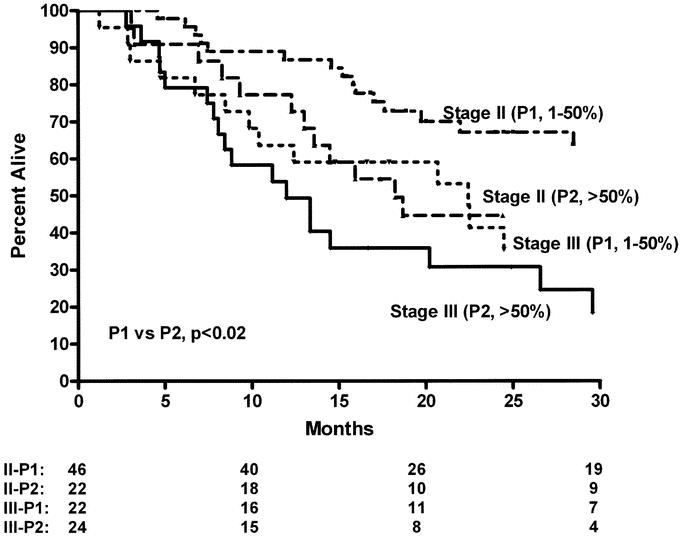
FIGURE 3. Overall survival of resected esophageal cancer patients treated with preoperative chemoradiation and surgery according to pathologic response at primary tumor (pP) and pTNM pathologic stage (P1 versus P2, P < 0.02).
TABLE 4. Multivariable Cox Regression Analysis of Impact of Pathologic Response (pP) Factor on pTNM Staging System
TABLE 5. Multivariable Cox Regression Analysis of Impact of Pathologic Response (pP) Factor on pT, pN, pM Factors
Modified Esophageal Cancer Staging System
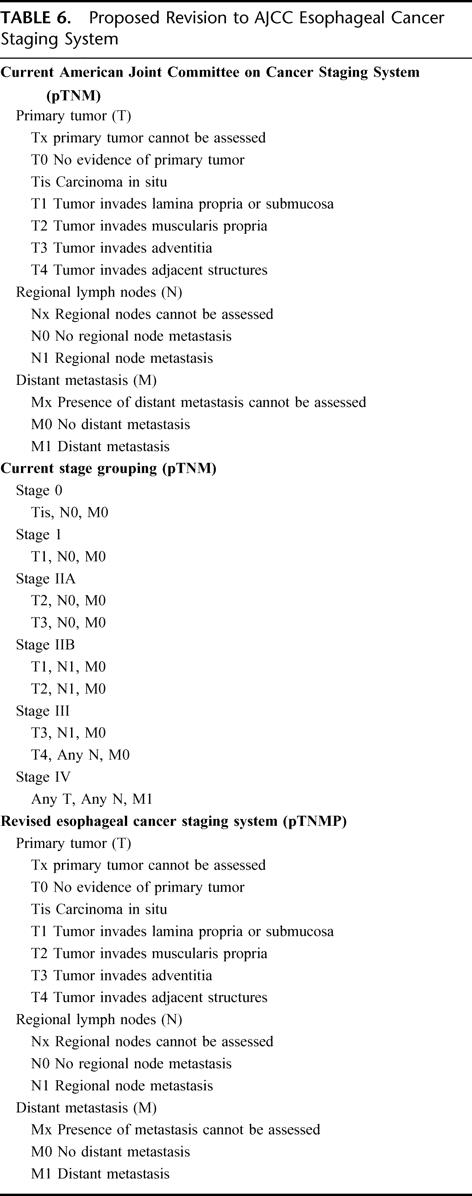
Table 6 proposes a modified pTNMP esophageal cancer system to accommodate the independent predictive factor of pP. The current AJCC pTNM staging system classifies patients into different stages according to pT (tumor depth), pN (nodal status), and pM (metastases) status.7 Our proposed modification incorporates a fourth factor of pP for CRT patients. This status would allow patients who were not assessed for pP or were treated with surgery alone to be designated as PX (pP cannot be assessed or no preoperative CRT). Additionally, the staging system accommodates the group of patients treated with CRT who are found to have a complete pP at the primary but involved nodes in the specimen (T0, N1, M0, P0). As Table 2 suggests, this group should be classified with a favorable stage II prognosis or a stage IIA, P0 designation suggesting optimum outcome within stage IIA. Patients with no tumor at the primary and no involved lymph nodes (T0, N0, M0, P0) would have the best prognosis and would be classified as stage 0, P0 to distinguish them from patients with carcinoma in situ treated with surgery alone (stage 0, PX).
TABLE 6. Proposed Revision to AJCC Esophageal Cancer Staging System
DISCUSSION
The current AJCC esophageal cancer staging system is based on a T (tumor depth), N (regional nodal statues) and M (nonregional nodes or systemic metastases) staging classification.7 In esophageal cancer patients treated with surgery alone, the pTNM stage accurately predicts long-term survival based on the above-defined pTNM factors.6,12 An increasing number of patients, however, with locoregionally advanced esophageal cancer (stages II-IVA) are currently being treated with CRT prior to surgery because of poor long-term outcomes with surgery alone.4,5 The impact of pathologic down-staging from this preoperative treatment on the pTNM staging system has not been fully evaluated.9 This manuscript attempts to evaluate the impact of pP following CRT on the AJCC esophageal cancer pTNM staging system.
The first important point to emphasize about this study is that it is a retrospective review of all patients treated at our institution from 1985 to 1993 with surgery alone or CRT followed by surgery. Patients were selected for treatment based on protocol availability, surgeon preference and clinical stage. As noted in Table 1, patients treated with CRT tended to have more advanced tumors that were felt less likely to be cured with surgery alone and were therefore enrolled in institutional protocols evaluating CRT followed by surgery. Additionally, patients treated with CRT tended to be younger and more often of adenocarcinoma histology (Table 1). These differences between the groups emphasize the fact that that this study is not randomized and cannot be used to assess which treatment modality (surgery alone or CRT followed by surgery) is best since multiple selection biases were used to select patients for each treatment. It is nevertheless intriguing that patients treated with CRT followed by surgery tended to have a lower overall pTNM pathologic stage despite a higher initial cTNM stage, suggesting pathologic down-staging. The first question, then, is whether survival is similar for the 2 groups based on pTNM stage since many CRT-treated patients started out at a higher initial cTNM stage. Table 2 suggests that for most groups, except stage 1, the pTNM stage is similar, regardless of treatment modality. This simplifies the use of pTNM after CRT since it suggests that the long-term survival is dictated by the pathologic stage and is not invalidated by the use of CRT, regardless of the initial clinical stage or the amount of pathologic down-staging achieved.
The second important point demonstrated by this paper is that the pTNM staging system can be further refined in CRT-treated patients by knowledge of the amount of pathologic response (pP) to CRT. CRT-treated patients appear to have survival dependent on the pP and the amount of residual tumor remaining in the surgical specimen after CRT (Table 3, Fig. 1). In fact, as Figures 2 and 3 suggest, the pP dictates outcome on an equal level with lymph-node status so that lymph-node-positive patients with a P1 (1%–50% residual tumor) pP have a similar outcome compared with lymph-node-negative patients with a P2 (>50% residual tumor) pP (Fig. 2). This observation is borne out by our multivariate analysis since pP remains an independent predictor of survival following CRT, even when controlled for pTNM stage (Table 4) or the individual pT, pN and pM criteria (Table 5). In fact, pP, along with nodal status (pN), appears most predictive of outcome following CRT (Table 5). These findings suggest that CRT-treated patients should have an additional factor added to the standard esophageal pTNM staging system, which we have designated as pP. Additionally, the importance of pP for prognosis may explain the recent observations that decreased activity on FDG-PET scans following CRT correlates with survival since decreased FDG-PET activity is associated with increased pP and lower residual tumor volume.13–15
In an effort to refine the AJCC esophageal cancer staging system to better predict outcome following CRT, we propose the addition of a new factor (pP) for pP. As suggested in Table 6, pP would be similar to pT, pN and pM in that various levels would exist determined by pP or residual tumor following CRT (P0, 0% residual; P1, 1–50% residual; P2, >50% residual). This proposal would allow refinement of the AJCC staging system but would not change the pTNM stage if pP had not been assessed or the patient had not received CRT through designation of a pPX status (Table 6). We also suggest that the group of patients with no residual tumor at the primary but involved lymph nodes be designated with a stage IIA, P0 (pT0, pN1, pM0, pP0) to accommodate predicted survival (Tables 2 and 3). Other authors have proposed modifications of the AJCC esophageal cancer staging system to accommodate complete surgical resection, regional and nonregional nodal designation, and to refine the classification of tumor depth in stage I patients.16–18 This is the first study, however, to suggest modifying the AJCC esophageal cancer staging system to accommodate preoperative CRT that has increasingly become the “standard of care” in many esophageal centers despite conflicting randomized results.4,5 Other prognostic factors are included in the AJCC esophageal staging manual (pG: histologic grade; pR: residual tumor-ie, margins at resection; pL: lymphatic vessel invasion; pV: venous invasion) but none of these factors have been put forward as strong enough to modify the current pTNM staging classifications.7 Many centers have also been searching for biologic markers that could help prognosticate outcome but at the present time there is not a consensus on these factors.19,20 At the present time, our best determinant of outcome following preoperative CRT is the pP. Interestingly, the type of chemotherapy or radiation therapy used to achieve the response does not appear to be critical in outcome (data not shown), which suggests that we may be able in the future to improve survival by increasing pP with novel chemotherapeutic or biologic agents.
Our observation that pP translates into survival may be able to be used in upcoming phase II trials designed to evaluate novel biologic agents since it will allow a firm end point that predicts survival immediately at the end of surgery. This early end point would be very useful because it will allow trials to be completed in a shorter time period than would be required if the trial had to wait for mortality and will allow more studies to be performed with the limited number of patients available for esophageal cancer trials. Additionally, the pP end point would be an efficacy end point that could be compared with other regimens simultaneously in multiple centers. As suggested by several authors, the use of FDG-PET may allow the prediction of pP prior to surgery. In the future, clinicians may be able to use FDG-PET to optimize different therapies to individuals to achieve high pPs in all patients.14–16,21 Such a strategy might allow selection of nonresistant regimens prior to surgery to maximize pP prior to surgical resection. This strategy would allow multimodality regimens to be tailored to individual patients to maximize efficacy while minimizing toxicity.
In conclusion, our study suggests that the current AJCC pTNM staging system accurately predicts survival following CRT despite pathologic down-staging. We suggest that following CRT the amount of pP at the primary tumor (pP) is an independent predictor of long-term survival that should be included in the current AJCC pTNM staging system to better predict long-term survival following CRT. Our proposed staging modification allows subclassification of patients who have been treated with CRT according to their pP status (P0, 0% residual; P1, 1%–50% residual; P2, >50% residual). The proposal puts forward a pPX designation for patients who have not been treated with CRT or whose pP has not been evaluated. Additionally, we propose that the subset of patients with a complete response at the primary tumor but involved lymph nodes (pT0, pN1, pM0, pP0) should be classified as stage IIA, P0 patients. These modifications of the AJCC staging would allow a more accurate prediction of long-term survival in the large number of patients currently being treated with preoperative CRT and surgery and would allow a comparison of efficacy of various preoperative treatment regimens used at different esophageal cancer centers.
Discussions
Dr. Kelly M. McMasters (Louisville, Kentucky): I would like to congratulate the authors on an excellent and thought-provoking study. In this retrospective analysis of patients treated with preoperative chemoradiation, there were 2 main conclusions. First, that the current AJCC TNM staging system maintains prognostic significance even after preoperative chemoradiation. The second conclusion was that the degree of pathological response to chemoradiation is an independent predictor of response. As a result of these conclusions, the authors propose that the AJCC staging system should be changed to include a category to assess pathological response to preoperative chemoradiation therapy in addition to just the TNM staging.
First my comments. I will take the devil's advocate position (which appears to suit me quite well) about changing the staging system.
As a general principle, I think the AJCC staging system can't be all things to all people. It cannot be on the one hand simple, intuitive, and easy to remember while on the other hand be exquisitely prognostically significant and incorporate every prognostic factor that has ever been shown to predict survival on a multivariate analysis. Therefore, every staging system strikes a balance between these factors, some more than others.
Complex staging systems may make more sense for diseases such as breast cancer, where we have a variety of treatment options that might be affected by stage. For esophageal cancer, I will argue that we presently have no treatment options that are affected by response to preoperative chemoradiation, therefore little reason to change the staging system. The patients with a complete pathological response have a relatively better prognosis; everyone else has a prognosis that varies from bad to worse. I have a few questions.
First, did histology (squamous cell versus adenocarcinoma) make any difference in response or survival? Were there other factors, including preoperative staging factors, that predicted response?
How did clinical staging prior to chemoradiation compare to pathological staging after chemoradiation? What is your standard staging workup for patients with esophageal cancer at the present time: PET scan, CT scan, endoscopic ultrasound, or all 3?
In your proposed revision to the staging system, a pathological complete responder is called stage 0, which is the equivalent of an in situ cancer. Yet the prognosis of complete responders is relatively poor (58% 5-year survival) compared to even stage I patients with surgery alone (82% 5-year survival). Does it make sense to call a patient who has invasive cancer stage 0, even after chemoradiation? Similarly, the prognosis of the patients with stage I cancers after chemoradiation was not as good as that of stage I surgery-alone patients (47% versus 82% 5-year survival). So for the patients with stages II and III cancer after preoperative treatment, there appears to be no difference in survival compared to those who had surgery alone. While you have concluded that the AJCC staging system still maintains prognostic significance after preoperative treatment compared to surgery alone, the prognosis is clearly not the same, at least, not for stage 0 and 1 patients. Please comment on that.
Although many centers are using preoperative radiation as “standard of care,” there really is not convincing evidence that this offers a survival advantage. In the absence of a clear survival advantage, should preoperative chemoradiation be considered investigational? Should it be offered outside of clinical trials for resectable tumors? If it is investigational, why should it be included in the staging system? This seems to lend an air of legitimacy to a therapy that has not yet been proven to be beneficial.
Please do not mistake my reservations about changing the staging system to indicate that the findings of this study are not fundamentally important. There is no question that this study will be heavily referenced in the literature and provides important information for all of us who treat patients with esophageal cancer. It has important implications for stratification of patients in clinical trials. I congratulate the authors for a very fine study.
Dr. Joseph Locicero III (Mobile, Alabama): I wish to congratulate the authors for presenting a very provocative proposal. They nicely demonstrate that posttherapy pathologic staging correlates with prognosis. For pretherapy screening, however, they used a variety of techniques, including CT scan, PET scanning in the US, but they do not elaborate on how many patients got each.
Both PET and CT scan correlate with stage but are not prognostic of pathologic staging. The ultrasound (EUS) is better, but it is only tissue diagnoses that tell us whether we have an accurate pathologic staging. My first question for the authors is how many patients had pretherapy pathologic staging?
The current staging system, which has already been mentioned, shows that patients have a major decline in prognosis once a tumor gets a little larger or they have lymph-node involvement. This leads to a jumble of stages that have poor prognosis. Other authors, including Drs. Ellis, Lerut, and Steven and Thomas Demeester, have suggested that changing the staging system to include more stratification by the number of involved nodes might be beneficial. So my second question to the authors is do they feel that we have reached sufficient maturity in the AJCC staging system prior to their suggested change? In other words, should we be doing something on the number of the nodes involved?
While neoadjuvant therapy is becoming popular, it has not proven to be beneficial. In fact, the 2004 National Comprehensive Cancer Center Working Group for Esophageal Cancer, which includes one of the authors who is a member of this Association, recommended neoadjuvant fixed therapy only for cervical cancers. However, despite this, the authors do show differences with a variety of regimens, as has been pointed out.
As I look at the second graph in the program on page 56, I am reminded of one of my cardiac surgical colleagues, a former Division I basketball player who developed a foolproof pregame comment for the press: “If we do all right tonight, we'll be okay.” So my final question to the authors is how can we identify those patients who are going to respond before we touch them with chemotherapy and radiation?
Dr. Gerard M. Doherty (Ann Arbor, Michigan): First of all, this is a new type of analysis that we haven't had experience with: assessing the primary tumor in terms of its amount of response. So my first question deals with the reproducibility and reliability of this assessment both within M. D. Anderson and as we try to broaden this at other institutions. Has Dr. Swisher in his previous investigations done any kind of interobserver reliability for this?
The second thing has to do with some of the other work that Dr. Swisher has done in esophageal cancer looking at FDG PET scans and their ability to predict the amount of response from the preoperative systemic therapy. The PET scans done after that therapy and prior to resection seem to have some predictive capability for the final pathologic stage, and I wonder if there is a correlation between these 2 measures.
Finally, I think that while this appears to be an important distinction among patients who have this terrible disease, it may be premature to propose broadening it to the staging system. However, I think it would be appropriate to include this in our stratification for clinical trials.
Dr. Nipun Merchant (Nashville, Tennessee): We have recently presented our data on complete pathologic response to neoadjuvant therapy for esophageal cancer from Vanderbilt, and we actually found the exact opposite results.
While we showed that neoadjuvant therapy achieved good local regional control, we found that patients recurred with metastatic disease, not only of metastatic disease, but metastatic disease in unusual locations, suggesting that by giving neoadjuvant therapy we may be changing the biology of the disease somewhat.
Can you tell us what the incidence of metastatic disease was in your patients who recurred and was that the cause of death in these patients? Do you have a sense or idea of where this metastatic disease was occurring?
Dr. Edward M. Copeland III (Gainesville, Florida): Dr. Swisher, I do not think your colleagues are going to agree to changing the AJCC Staging System if they do not agree that esophageal cancer down-staged to pTO has an improved survival over the original lesion prior to treatment with chemoradiation. Dr. Kirby Bland and I introduced this concept of improved survival after down-staging of rectal cancer with preoperative radiation to the members of the Southern Surgical Association in 1992, and Dr. Steven Vogel did the same with esophageal cancer down-staged with chemoradiation before the Southern in 1994. Both of these studies obviously originated from the University of Florida. Dr. Swisher, if I read your survival graphs in the abstract booklet correctly, the patients who down-staged to pTO had a 3-year survival of about 74% and with almost as good a response if a node remained positive. This remarkable survival is hard to overlook even in the absence of a randomized prospective trial. Why are other institutions not able to achieve the same improvement in survival with these diseases that the M. D. Anderson and the University of Florida seem to do with the use of neoadjuvant therapy? I suspect that we will hear from the Memorial Sloan-Kettering group later today that they are now confirming this increase in survival with down-staging to pTO with neoadjuvant therapy for locally advanced rectal cancer.
Dr. Frederick L. Greene (Charlotte, North Carolina): I rise in my capacity as chair of the AJCC and as editor of the sixth edition of the TNM Manual. It is always nice to hear a staging paper at our Association meeting.
Since the 1980s, we have advocated using a subscript to identify neoadjuvant therapy when you put your cases in cancer registries. The “Y” subscript is placed in front of the pTMN, which indicates the use of neoadjuvant therapy in any site. Unfortunately, this subscript is not used very frequently. My question to the authors is at M. D. Anderson, are they using the “Y” subscript so they can at least follow these patients in their registry?
We have recognized for years that response to neoadjuvant therapy is the most robust of the prognostic indicators along with TNM. For the 2009 seventh edition, we are going to change some of the staging for esophageal cancer, and we may well advocate some of the things the authors are talking about. I enjoyed the paper.
Dr. Stephen B. Vogel (Gainesville, Florida): I very much enjoyed your presentation and certainly agree with your results. I had the honor of presenting a paper at this meeting in the mid-1990s documenting the down-staging of esophageal cancer following preoperative chemoradiation and our feeling that this resulted in significant survival advantage. As part of that review, we commented on the number of patients with positive lymph nodes. Although the study was nonrandomized, the incidence of positive lymph-node metastases in the neoadjuvant chemoradiation group was approximately one half of the incidence of positive lymph node (55%) in the group undergoing surgery without previous chemoradiation. I would like to ask the authors if they feel that preoperative chemoradiation can actually sterilize or destroy tumor in lymph nodes.
Dr. Stephen G. Swisher (Houston, Texas): I would like to thank all the discussants for some excellent questions.
Dr. McMasters, with regards to whether histology made any difference, we analyzed that in a multivariate analysis, and histology did not seem to make a difference.
How did clinical staging compare with pathologic staging? In patients who had surgery alone clinical stage was very predictive of survival. But interestingly, if you looked at clinical stage for the group that received preoperative chemoradiation, clinical stage was not predictive of survival, except in the single group that had EUS-identified celiac axis lymph nodes. For the most part, pathologic staging but not clinical stage is predictive after preoperative chemoradiation.
What is our standard preoperative workup? Our standard workup at M. D. Anderson is to do an endoscopic ultrasound to start with. If the tumor is T-1 or less and without evidence of lymph nodes involved, these patients are treated with surgery alone. If they are more advanced, a CT PET is obtained to rule out distant metastatic disease. These patients are then usually treated on protocol with preoperative chemoradiation and surgery.
With regards to the observation that it doesn't make sense to call patients stage 0 if they have invasive cancer and have had a complete pathologic response, we agree with this observation and therefore feel this would be another advantage to the modification of the AJCC stage system as proposed in our study.
As noted, the majority of clinicians do not use the subscript ypTNM, although they should. Our modification would allow P0 to be placed in the AJCC staging, which would allow us to know which patients were stage 0 patients because of preoperative chemoradiation response and which patients were stage 0 patients because of in situ cancer.
With regards to the question of whether preoperative chemoradiation should be considered investigational, this, of course is a very controversial topic. One of the problems in esophageal cancer, unlike breast or lung cancer, is that there are a far smaller number of patients to evaluate in randomized trials. Most of the esophageal randomized trials have only 100 patients in them, and many of the earlier trials have been plagued by very high treatment-related morbidity and mortality. Our group believes that there is a benefit for preoperative chemoradiation in locoregionally advanced cancer when performed at a high-volume esophageal referral center. But this is a very controversial topic.
Dr. LoCicero commented on how few of these patients had pretherapy pathologic staging. Some people have advocated thoracoscopic staging to pathologically stage patients prior to treatment. Our institution uses EUS and FNA of enlarged nodes for staging. Interestingly, though, as I have mentioned, the clinical stage does not correlate with long-term survival after preoperative chemoradiation, even in EUS patients that have been biopsied.
To the question about whether the number of lymph nodes should be added to the AJCC esophageal cancer staging system, certainly the number of involved lymph nodes is a very important prognostic factor. In our study in patients who received preoperative chemoradiation, the number of involved lymph nodes also correlated with survival, so this may be another factor to include in a revised AJCC staging system.
To the question about how we could identify responders prior to treatment, currently we are not able to do that, but hopefully in the future, with the biologic identification of different genetic subgroups, we may be able to pick out patients who are more sensitive to chemoradiation. Additionally, I think the CT PET may be very valuable since there is evidence that if the SUV of the primary tumor drops significantly with treatment, patients have a pathologic response. There is a recent study from Germany suggesting that 2 weeks after the beginning of therapy, one can predict those patients who are going to pathologically respond with CT PET. This may be very helpful since in the future, we may be able to start a treatment and identify those patients who are not responding, and we could then alter the treatment by adding additional chemotherapy or biologic agents until the CT PET demonstrates response.
Dr. Merchant asked what the incidence of recurrent metastatic disease was? I didn't include this data, but when we also analyzed recurrence rates, they correlated with pathologic response as well.
Dr. Copeland commented on the very impressive survival results of the study. It is important to be cautious because this study was retrospective. This study evaluated only patients who underwent surgery. So those patients who were treated with preoperative chemoradiation but progressed are not included in this analysis, and that is about 5% of all patients treated.
Additionally, the data that I was showing you here excluded operative mortality in an attempt to look only at long-term tumor related mortality. The overall operative mortality rate was 4%, so one would have to take this into account as well.
The final reason for these very good results is that they were performed at an experienced esophageal referral center in which there was minimal treatment-related morbidity.
In fact, if one does the treatment at a center that is not used to treating esophageal cancer, the treatment-related morbidity both from operation as well as from the chemotherapy and radiation therapy may [increase and] mask any potential benefit of combining the modalities. Therefore, one may need to consider just doing a single modality in a nonreferral center.
Finally, Dr. Greene commented, Are we using subscripts for staging? This is a very important point. At M. D. Anderson, we do indeed use the yPTNM subscript. I think this gives us additional knowledge. It gives a marker of response. And as we have future trials that come forth, we can compare agents from different institutions according to the pathologic response.
Finally, Dr. Vogel commented on the total number of nodes in patients undergoing preoperative chemotherapy and the biologic effect of this. Certainly the number of nodes in patients treated with preoperative chemoradiation does have some impact on survival and may have an impact on response. We have noted in patients with celiac involvement that there is a decreased pathologic response to preoperative chemoradiation.
Footnotes
Support for this study was obtained in part from the George Sweeney and George Swank Esophageal Research Fund.
Reprints: Stephen G. Swisher, MD, The University of Texas M. D. Anderson Cancer Center, Department of Thoracic and Cardiovascular Surgery, Director, Esophageal Cancer Program, 1515 Holcombe Blvd., Box 445, Houston, TX 77030. E-mail: sswisher@mdanderson.org.
REFERENCES
- 1.Muller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845–857. [DOI] [PubMed] [Google Scholar]
- 2.Hulscher JBF, van Sandick JW, de Boer AGEM, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. [DOI] [PubMed] [Google Scholar]
- 3.Hofstetter W, Swisher SG, Correa AM, et al. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. [DOI] [PubMed] [Google Scholar]
- 5.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. [DOI] [PubMed] [Google Scholar]
- 6.Steup WH, De Leyn P, Deneffe G, et al. Tumors of the esophagogastric junction: long term survival in relation to the pattern of lymph node metastasis and a critical analysis of the accuracy or inaccuracy of pTNM classification. J Thorac Cardiovasc Surg. 1996;111:85–95. [DOI] [PubMed] [Google Scholar]
- 7.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. Philadelphia: Lippincott-Raven; 2001:91–98. [Google Scholar]
- 8.Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993;11:1118–1123. [DOI] [PubMed] [Google Scholar]
- 9.Donington JS, Miller DL, Allen MS, et al. Tumor response to induction chemoradiation: influence on survival after esophagectomy. Eur J Cardiothorac Surg. 2003;24:631–637. [DOI] [PubMed] [Google Scholar]
- 10.Swisher SG, Ajani JA, Komaki R, et al. Long-term outcome of phase II trial evaluating chemotherapy, chemoradiotherapy, and surgery for locoregionally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;57:120–127. [DOI] [PubMed] [Google Scholar]
- 11.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2004. In press. [DOI] [PubMed]
- 12.Holscher AH, Bollschweiler E, Bumm R, et al. Prognostic factors of resected adenocarcinoma of the esophagus. Surgery. 1995;118:845–855. [DOI] [PubMed] [Google Scholar]
- 13.Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21:428–432. [DOI] [PubMed] [Google Scholar]
- 14.Brucher BL, Weber W, Bauer M, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001;233:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swisher SG, Erasmus J, Maish M, et al. 2-Fluoro-2-deoxy-D-glucose positron emission imaging is predictive of imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–1785. [DOI] [PubMed] [Google Scholar]
- 16.Korst RJ, Rusch VW, Venkatraman E, et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg. 1998;115:660–670. [DOI] [PubMed] [Google Scholar]
- 17.Rice TW, Blackstone EH, Rybicki LA, et al. Refining esophageal cancer staging. J Thorac Cardiovasc Surg. 2003;125:1103–1113. [DOI] [PubMed] [Google Scholar]
- 18.Dickson GH, Singh KK, Escofet X, et al. Validation of a modified GTNM classification in peri-junctional oesophago-gastric carcinoma and its use as a prognostic indicator. Eur J Surg Oncol. 2001;27:641–644. [DOI] [PubMed] [Google Scholar]
- 19.Gibson MK, Abraham SC, Wu TT, et al. Epidermal growth factor receptor, p53 mutation, and pathologic response predict survival in patients with locally advanced esophageal cancer treated with perioperative chemoradiotherapy. Clin Cancer Res. 2003;15:6461–6468. [PubMed] [Google Scholar]
- 20.Ishibashi Y, Matsumoto T, Niwa M, et al. CD147 and matrix metalloproteinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer. 2004;101:1994–2000. [DOI] [PubMed] [Google Scholar]
- 21.Wieder HA, Brucher BL, Zimmerman K, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–908. [DOI] [PubMed] [Google Scholar]