Abstract
Objective:
The largest series in the literature dealing with redo fundoplication was presented and published in 1999 and included 100 patients. Herein we update this initial series of 100, with 207 additional patients who have undergone redo fundoplication (n = 307).
Summary Background Data:
Increasing numbers of patients are failing esophagogastric fundoplication and requiring redo procedures. Data regarding the nature of these failures have been scant.
Methods:
Data on all patients undergoing foregut surgery are collected prospectively. Between 1991 and 2004, 307 patients underwent redo fundoplication for the management of anatomic complications or recurrent GERD. Statistical analysis was performed with multiple χ2 and Mann-Whitney U analyses, as well as ANOVA.
Results:
Between 1991 and 2004, 1892 patients underwent primary fundoplication for GERD (1734) or paraesophageal hernia (158). Of these, 54 required redo fundoplication (2.8%). The majority of failures (73%) were managed within 2 years of the initial operation (P = 0.0001). The mechanism of failure was transdiaphragmatic wrap herniation in 33 of 54 (61%). In the 231 patients who underwent fundoplication elsewhere, 109 had transdiaphragmatic herniation (47%, P = NS). In this group of 285 patients, 22 (8%) required another redo (P = NS). The majority of the procedures were initiated laparoscopically (240/307, 78%), with 20 converted (8%). Overall mortality was 0.3%.
Conclusions:
Failure of fundoplication is unusual in experienced hands. Most are managed within 2 years of the initial operation. Wrap herniation has now become the most common mechanism of failure requiring redo. Redo fundoplication was successful in 93% of patients, and most could be safely handled laparoscopically.
Fundoplication is widely accepted as the gold standard in operative management of GERD. Failure requiring reoperation is unusual, but when it occurs the outcomes of redo surgery can be quite good. This paper details an experience with 285 patients undergoing 305 redo fundoplications including operative and long-term outcomes.
Since its introduction in 1991,1 laparoscopic Nissen (360 degree) fundoplication has become the most widely applied antireflux procedure’ accounting for 87 of every 100,000 hospital discharges in 1999, according to the National Inpatient Sample.2 This represents a near 8-fold increase for this procedure over a 10-year period. The best outcomes with 5-year or longer follow-up after Nissen fundoplication report patient satisfactions of 86% to 96%, making the laparoscopic Nissen fundoplication the gold standard for antireflux procedures.3–8
Laparoscopic fundoplication has recently been called into question.9–11 The rate of failure following fundoplication for gastroesophageal reflux disease (GERD) varies from 2%–30%, depending on how “failure” is defined; for example, failure requiring resumption of medical therapy versus failure requiring reoperation. Failure following Nissen fundoplication for paraesophageal hernia also ranges from 7%–33%, depending on whether failure is defined symptomatically or anatomically.12–14 In select cases, fundoplication failure requires revisional fundoplication (redo).
In 1996, we reported our experience with redo fundoplication in 100 consecutive patients, detailing the pattern of failure and outcomes with redo fundoplication.15 Herein, we detail our updated experience with over 300 consecutive redo fundoplications.
MATERIALS AND METHODS
Patients
The institution's institutional review board approved this study. Data on all foregut patients undergoing surgery are collected prospectively and maintained in a computer database (Microsoft Access, Microsoft Corp, Seattle, WA). Details on preoperative presentation and symptoms, results from objective testing (typically, barium swallow, esophagogastroduodenoscopy, ambulatory esophageal pH testing, esophageal motility, and gastric emptying), operative findings including surgeon-documented mechanism of failure (herniated fundoplication, disrupted fundoplication, slipped fundoplication, crural stenosis/tight wrap, misplaced fundoplication, and twisted fundoplication), and postoperative course. When more than 1 failure mechanism was identified intraoperatively, the most prominent or causative mechanism was recorded as the mechanism of failure (eg, fundoplication herniation accompanied by fundoplication disruption was categorized as a herniated fundoplication).
Postoperative symptom assessment was performed 1 month after surgery and annually thereafter. Symptoms of heartburn, dysphagia, and chest pain were assessed using a 5-point scale (0, none; 1, mild; 2, moderate; 3, severe; 4, intolerable).
Revisional surgery (redo fundoplication) is offered to patients who have persistent, recurrent, or new foregut symptoms (heartburn, dysphagia, chest pain, regurgitation, asthma, hoarseness, chronic cough, or laryngitis) and confirmed physiologic abnormalities or a definable anatomic defect. Potential candidates for redo fundoplication are evaluated for anatomic and physiologic evidence of failure by selective use of barium swallow, upper endoscopy, esophageal motility, esophageal pH testing, and gastric emptying study. For this study, preoperative diagnoses were assigned based upon these objective evaluations, and all types of fundoplication (partial or complete) and operative approaches (open, laparoscopic, converted, or thoracic) were included. Patients were excluded if their initial operation was for the diagnosis of achalasia.
From October 1, 1991, to April 1, 2004, 1892 patients underwent primary fundoplication at Emory for GERD (n = 1734) or paraesophageal hernia repair (n = 158) (Fig. 1). Of these, 54 patients required redo fundoplication (2.8%). For purposes of comparison and data analysis, these 54 patients have been grouped together (internal primary fundoplication group, n = 54). During this same time period, 231 patients underwent fundoplication elsewhere and subsequently underwent redo fundoplication at Emory. All patients who underwent any redo fundoplication, either first redo or multiple redos, prior to referral to Emory are grouped (external fundoplication group, n = 231). From the combined group of 285 patients (54 Emory patients and 231 external patients), more than 1 redo fundoplication was necessary in 22 patients (multiple redos group, n = 22) bringing the total number of redo fundoplications performed during this time period to 307 redos in 285 patients. Stratified by number of redos, 241 patients underwent 1 redo, 59 underwent 2 redos, 6 underwent 3 redos, and 1 underwent 4 redo fundoplications (Fig. 2). Again, for comparing preoperative presentation and operative findings for the first redos, those referred after failure of primary fundoplication externally are grouped (external primary fundoplication group, n = 187).
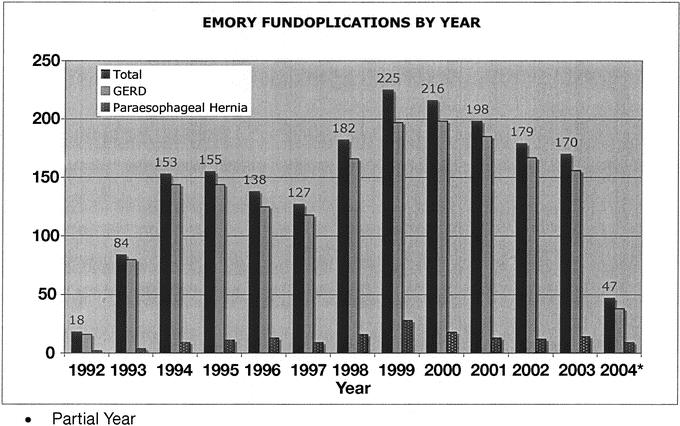
FIGURE 1. Antireflux surgery at Emory from 1992 through 2004.
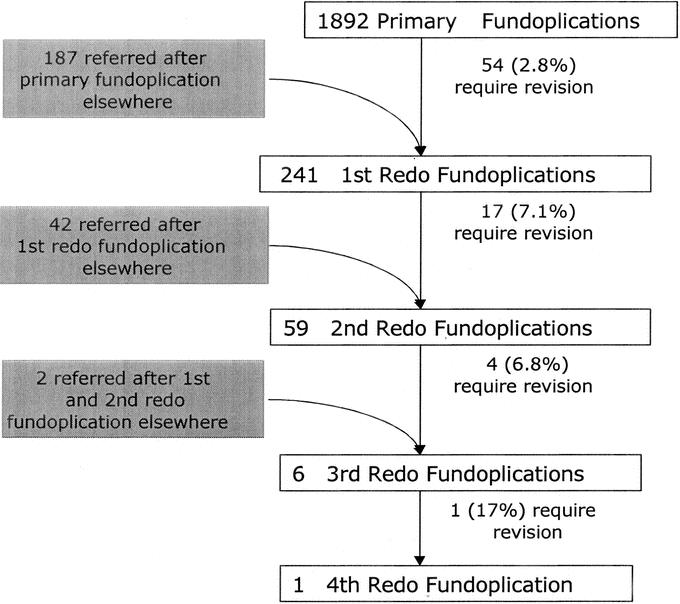
FIGURE 2. Breakdown of patients undergoing redo fundoplication.
Follow-up
For the past 10 years, a full-time research nurse has been maintaining Emory's foregut database. In addition to collecting data from office visits, the research nurse also contacts patients every 2–3 years by phone or mail and has them complete a follow-up questionnaire. When a patient cannot be found through the contact information maintained in the database, an Internet search for the patient's contact information is conducted. This is a paid service and claims that if “an individual cannot be found, they do not want to be found.”
With this follow-up strategy, follow-up information is available on 88% of the study group (269/307). However, over time the rate of follow-up decreases significantly (Fig. 3). One year or longer follow-up data are only available on 54% of patients who are more than 1 year postoperative (150/278). Median follow-up for the overall group is 1.2 years (range, 9–3042 days).
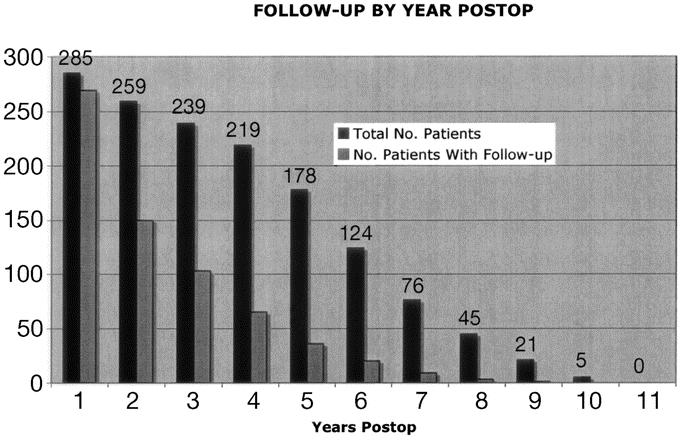
FIGURE 3. Number of patients with follow-up data available by year.
Statistics
Statistical analysis was performed with multiple χ2 and Mann-Whitney U analyses, as well as ANOVA. Comparisons of preoperative and postoperative data were made with the Wilcoxon signed-rank test. Statistical significance was set at P < 0.05 for each symptom.
RESULTS
Redo Clinical Presentation
Internal Primary Fundoplication (n = 54)
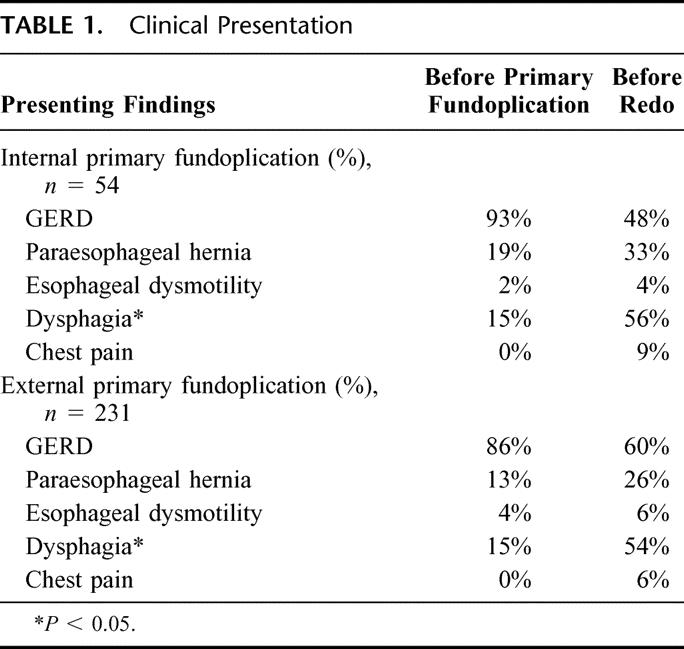
Fifty-four patients undergoing redo fundoplication had their primary fundoplication at Emory for either paraesophageal hernia (n = 10) or GERD (n = 40). In this group, very few patients were experiencing dysphagia (n = 8) or chest pain (n = 0) before their first fundoplication (Table 1). While the new onset of chest pain following fundoplication occurred in several patients (n = 5), the most common new clinical finding in patients requiring redo fundoplication was dysphagia (15% prior to first fundoplication versus 56% prior to redo; P < 0.05).
TABLE 1. Clinical Presentation
The majority of failures were managed within 2 years of the initial operation (73%). Five patients underwent a redo within 1–4 days after their primary fundoplication. All 5 suffered immediate postoperative nausea and retching followed by severe dysphagia, and all underwent an immediate contrast swallow, which revealed wrap herniation above the diaphragm. All were immediately returned to the operating room for reduction and repair of the hernia. None of these patients went on to require another redo fundoplication.
External Fundoplication (n = 231)
The majority of patients in this series undergoing redo fundoplication were referred from externally (n = 231) after fundoplication for GERD (n = 198) or paraesophageal hernia (n = 31). Forty-two patients underwent 1 redo externally before referral, and 2 underwent 2 redos prior to referral (Fig. 2). Results in patients undergoing multiple redos are detailed later in the “Multiple Redos” section.
Presenting findings are detailed in Table 1. Compared with the internal primary fundoplication group, patients referred for redo fundoplication were more likely to have recurrent GERD as the clinical presentation for redo (60% of external versus 48% internal primary fundoplication). Patients referred were more likely to have a delayed presentation for redo fundoplication, with 66% of patients in this group having their redo fundoplication within 2 years of the primary operation.
Patterns of Failure: Operative Findings
Internal Primary Redos (n = 54)
In the 54 patients from Emory undergoing redo fundoplication, 52 were initiated laparoscopically (96%). Two were converted to open procedures due to dense adhesions in the operative field, resulting in 50/54 patients successfully undergoing laparoscopic revisions (93%).
The reasons for failure as documented in operative reports are detailed in Table 2. The majority of failures fell into the categories of fundoplication herniation, fundoplication disruption, slipped fundoplication or tight wrap/crural stenosis. Only 2 failures were felt to be due to technical errors during the first operation (1 twisted wrap and 1 misplaced wrap).
TABLE 2. Patterns of Failure (%)
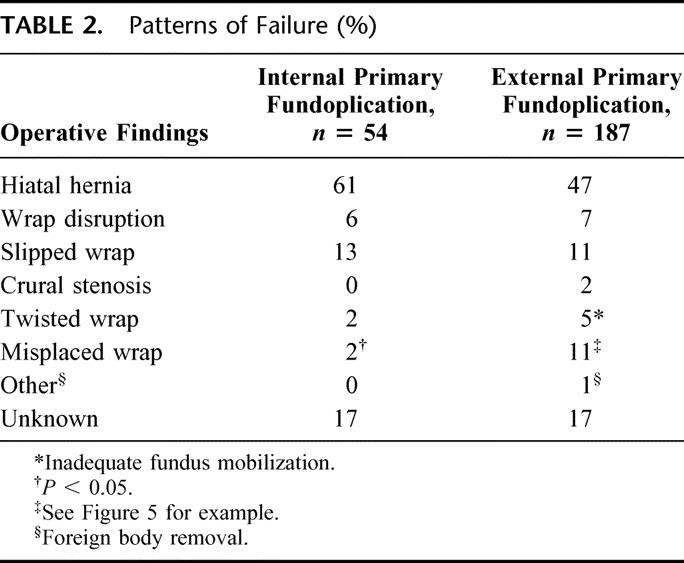
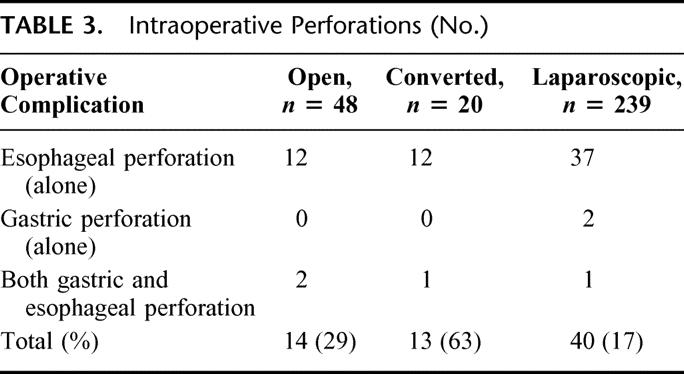
Eleven intraoperative complication were encountered in 9 patients (17%) (Table 3). Two of the 4 patients undergoing an open redo experienced an intraoperative complication, and in both cases this was gastric perforation.
TABLE 3. Intraoperative Perforations (No.)
External Primary Fundoplication (n = 187)
A laparoscopic approach was chosen for the 151 patients whose initial fundoplication was performed laparoscopically. Thirteen of these 151 laparoscopic redos were converted to open procedures (8.6%). In 9 of the 27 patients whose initial operation was open, the redo was initiated laparoscopically, with 2 of these 9 being converted to open procedures (conversion rate when first operation open, 22%). In this group, 77% of the redos were successfully completed laparoscopically, and the overall conversion rate was 9.4% (15/160). Combining this with the internal primary fundoplication group, the overall conversion rate was 8% (20/240). All conversions were for dense adhesions at the operative site.
As in the internal primary group, the most common pattern of failure was fundoplication herniation. There were also comparable other reasons for failure in all categories except for misplaced fundoplications. Significantly more patients who underwent fundoplication externally were found to have a misplaced wrap (2% internal versus 11% external; P< 0.05). Figure 4 depicts the most common configuration of the misplaced fundoplication, using the gastric body for the wrap instead of the fundus.
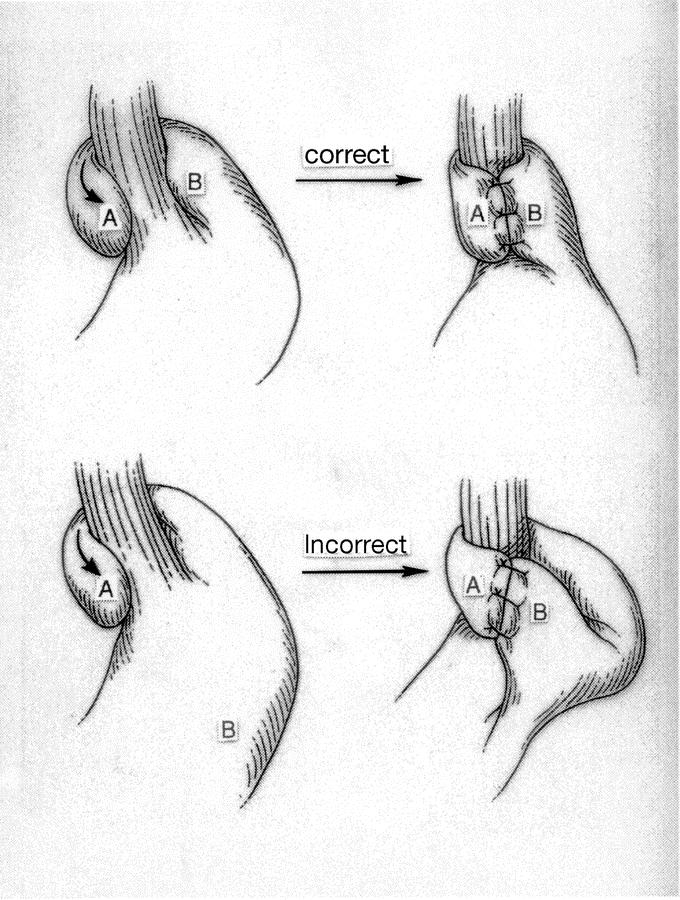
FIGURE 4. Most common configuration of a misplaced wrap.
Forty-three operative complications occurred in 39 patients undergoing their first redo. Intraoperative complications were more likely when the procedure was converted from laparoscopic to open than for open or laparoscopic alone (62% versus 17% and 17%; P < 0.05) and in the overall group of 307 redos, gastric perforation was the most common intraoperative complication (Table 3).
Immediate Postoperative Outcomes (Total Group, n = 307)
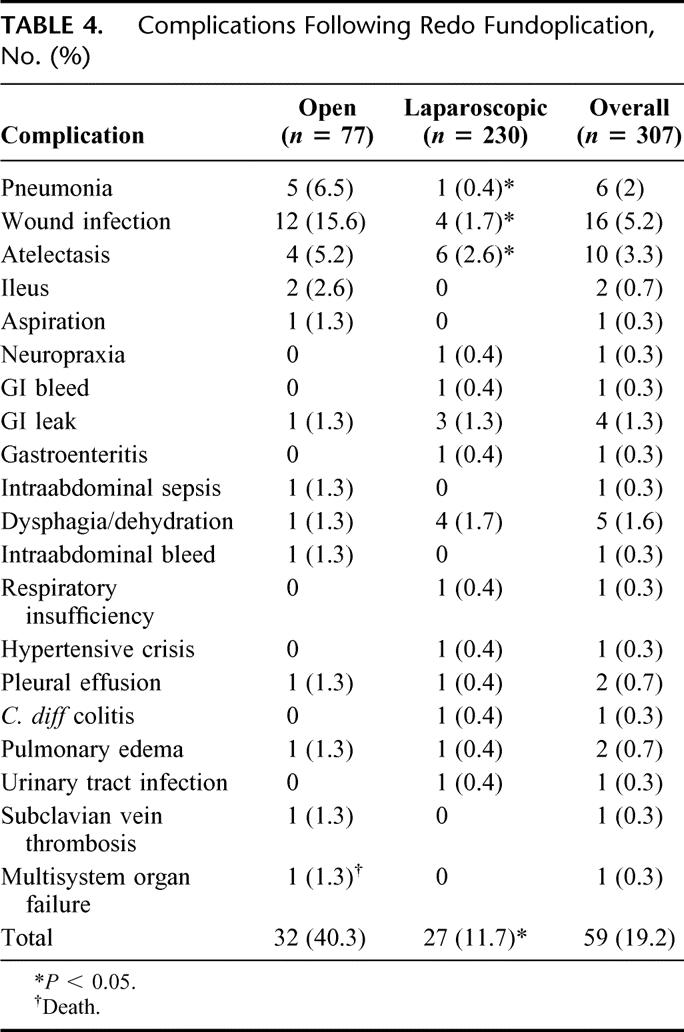
Postoperatively, 48 patients experienced 59 complications (14.7%). Complications are detailed in Table 4. Complications were more common in patients undergoing open redo fundoplication compared with those undergoing laparoscopic redo (32.5% versus 10%, P < 0.05). Forty percent of those who underwent conversion from laparoscopic to open redo suffered a complication versus only 13% of those who had their operation completed laparoscopically (8/20 versus 40/287; P < 0.05). One patient died of sepsis and multisystem organ failure from pneumonia after an open redo. There was a trend toward an increased complication rate as patients underwent multiple redos (see section below on Multiple Redos).
TABLE 4. Complications Following Redo Fundoplication, No. (%)
Long-term Outcomes
Symptom Response
All patients underwent pre- and postoperative symptom assessment for heartburn, dysphagia, and chest pain. In addition, use and dosage of antisecretory medications were recorded, as well as the need for any postoperative interventions (EGD with dilation or more surgery related to foregut problems).
Between 73% and 89% of patients reported their postoperative symptoms of heartburn, dysphagia, and chest pain to be absent or mild. Similarly, only 3%–8% of patients reported their symptoms postoperatively to be severe, and no patients rated their symptoms as intolerable. These postoperative findings were significantly different than preoperative symptoms (Fig. 5). While the majority of patients were satisfied with their results, 16% were unsatisfied and 14% were undecided.
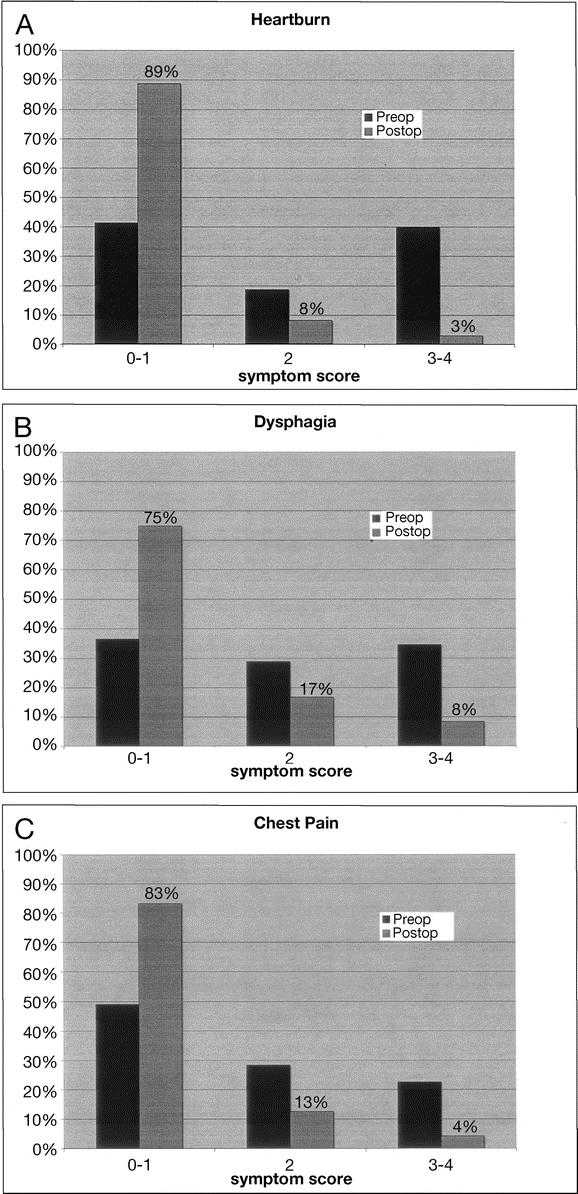
FIGURE 5. Pre- and postoperative symptom scores for A, heartburn, B, dysphagia, and C, chest pain.
At last follow-up, 17% of patients were using antisecretory medications for “GI symptoms.” Eleven percent underwent dilations postoperatively, and at least 5 patients underwent redo fundoplication or takedown of their fundoplications elsewhere. No specific data are available for these patients.
Multiple Redos
Twenty-two patients who underwent a redo fundoplication at Emory went on to have multiple redos at Emory (Fig. 2). Five patients underwent another redo externally. Since data on these elsewhere redos are not available, they are not included in this group's analysis. The rate of second and third redo for patients was 7.1% and 6.8%, respectively (P = NS), more than twice the rate of revision for our primary fundoplication group (2.8%, P < 0.05).
Mean time from first redo to second redo was 24 ± 33 months, and from second to third redo, 12 ± 7 months. While 5 of the first redos were done for acute wrap herniation during the same hospitalization as the initial fundoplication, all of the remaining second redos were for chronic symptomatic failures.
Based upon objective testing, preoperative diagnoses were compared between the first redo and second redo groups. Findings of transdiaphragmatic wrap migration and Barrett esophagus were more common preoperatively in the second redo group. There was also a trend away from recurrent GERD as a preoperative diagnosis. Nearly one third of patients had different preoperative findings at the time of second redo than at the time of previous operation, suggesting a new diagnosis and surgical indication for the second redo procedure (Fig. 6).
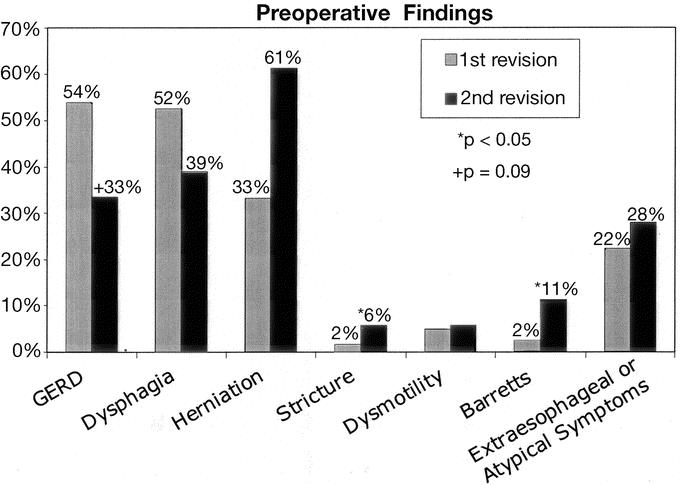
FIGURE 6. Preoperative findings comparing the first failure with the second failed fundoplication.
Operative details, including operative times, type of approach, type of fundoplication, operative findings, and complications, were compared between the first and second redo groups. There were no significant differences between groups in approach or fundoplication type. A laparoscopic approach predominated (74% in first redo versus 67% in second redo), with conversion rates of 8% and 6%, respectively. Conversions were usually due to adhesions or, less commonly, enterotomy. The majority were 360-degree fundoplications in both groups, with approximately one fifth of patients having partial fundoplications, reflecting the preoperative incidence of esophageal-body dysfunction. A gastrostomy tube, either operative or PEG, was used in 17% of second redos, and a Heller myotomy was used in 3.7% of second redos.
The gold standard for determining the cause of fundoplication failure is the pattern of failure as seen at the time of redo. A second revision in those patients previously revised at our institution was associated with a significantly higher rate of fundoplication herniation compared with the first redo group (72% versus 50%, P < 0.05). The finding of a shortened esophagus was similarly elevated in the second redo (2% versus 17%, P < 0.05).
Those undergoing multiple redos did not experience an increased risk of gastric perforation (14% first redo versus 17% in second redo, P = NS) or esophageal perforation (1.4% versus 5.6%, P = NS). There was no perioperative mortality in the multiple redo group.
Length of stay increased with each revision, from 4.5 ± 3.4 days for the first redo to 5.8 ± 3.0 days for second redo and 8.6 ± 3.7 days for those undergoing a third redo fundoplication. There was no difference in the rates of postoperative dysphagia or use of dilation, but those undergoing a second redo did have a higher rate of antisecretory use after their surgery (12% versus 23%; P < 0.05).
Using a univariate logistic regression, we looked for risk factors at the time of first redo that may predict the need for future surgery. The presence of fundoplication herniation at the time of first redo was the only significant predictor of the need for another redo fundoplication, with an odds ratio of 4.0 (95% CI 1.2–12.4).
DISCUSSION
Indications for Redo
Patients who present after a fundoplication with persistent or recurrent foregut symptoms represent a unique challenge. Increasing numbers of patients are being diagnosed with GERD or paraesophageal hernia, and with this, significant numbers of patients who in the past would have lived with their foregut symptoms are now receiving interventions. Since 1991, laparoscopic antireflux surgery, in particular, the most widely studied and applied operation, the Nissen (360-degree) fundoplication, has seen an 8-fold increase in use.2 While largely successful when performed by surgeons experienced in both laparoscopy and esophageal surgery, even in the most experienced hands, surgery fails to control the patient's symptoms or results in new symptoms or anatomic problems in 3%–16% of patients.5,11,16 Some type of revisional surgery may be required in select patients.
In our practice, we consider redo fundoplication for patients with persistent or recurrent foregut symptoms, or in those who develop new foregut symptoms not present before their initial fundoplication. Redo surgery is offered to those who then have objective evidence of failure based on physiologic studies or an anatomic abnormality. Using this approach, we have had to redo nearly 3% of our own fundoplications. We have documented in other publications a higher redo rate in those with more complicated GERD (Barrett, esophagitis, and stricture).15–17 We have also documented a symptomatic failure rate of 7% in those undergoing fundoplication for paraesophageal hernia and an anatomic failure rate of 33% in these same patients.13,14 We do not offer a redo for patients with anatomic failure in the absence of foregut symptoms. In particular, we do not feel that a recurrent paraesophageal hernia with its associated mediastinal scarring has the same natural history as a primary paraesophageal hernia. In our experience, we have never seen an acute presentation for a recurrent paraesophageal hernia not occurring in the immediate postoperative period as a result of postoperative retching! The most challenging patients have been those with dysphagia, an anatomically normal-appearing wrap, a normal barium swallow with a 12.5-mm tablet, and normal esophageal pH and motility. These can be very desperate patients whose desperation has led us to reoperate. In fact, over half of the redos reported in this paper were on patients with dysphagia. While most of these patients also had anatomic reasons for their dysphagia, several presented as described above, and we have had some dramatically positive outcomes in these patients, as well as some frustrating negative outcomes. The Emory patient who underwent 4 fundoplications was one of these patients. He continues to have dysphagia. Currently, we would not recommend reoperation for a patient with dysphagia if an anatomic or physiologic reason for dysphagia cannot be defined preoperatively.
Another challenging patient group has been those with recurrent heartburn or atypical symptoms, only mildly abnormal esophageal pH studies, and no symptom improvement with antisecretory medication. While redo fundoplication may be warranted, there is a significant potential to leave these patients with even worse foregut symptoms. Before considering a redo in these patients, we will document failure of acid suppression with maximum-dose PPIs. The patient with heartburn symptoms and appropriate acid suppression on PPIs should not have a redo unless they cannot tolerate PPIs due to severe side effects. The new endoluminal approaches may offer an attractive alternative to redo fundoplication for these patients. We have used this approach in several patients, with satisfactory early results.
Choice of Approach
In our paper published in 1999, we detailed comparable outcomes whether the redo operation was performed using an open or laparoscopic approach. Our more recent experience continues to support the utility of a laparoscopic approach for redos, even when the initial fundoplications was open. The conversion rate in those in whom a prior open fundoplication was approached laparoscopically was 22%, and overall 77% of redos in this series were completed laparoscopically. At times a primary open redo is selected, especially for patients whose prior foregut surgery was complicated (eg, GI perforation with perioperative abdominal sepsis or prior transthoracic approach with chest complications). There was no increase in intraoperative complications or postoperative complications in the laparoscopic group. In fact, gastric and esophageal perforation was more common in those undergoing either primary open redo or converted to open redo. This most likely reflects the complexity of the cases that need to be performed with an open approach. However, the laparoscopic group realized a lower incidence of the postoperative complications of pneumonia, wound infection, atelectasis and ileus. As our experience with these operations has increased, we are infrequently using an open approach, and over the past 23 months we have used a primary open approach in only 4 of 48 patients (8%).
Technical Challenges
Reoperative esophageal surgery can be one of the most challenging procedures that a GI surgeon will face. Anatomy can be severely distorted by scarring, fundoplication herniation, and unexpected findings. Experience and knowledge of normal and abnormal anatomy is critical to not only a safe operation but also effective resolution of the patient's problems. This experience documents a low rate of significant complications and death despite the challenges of reoperative foregut surgery.
The preoperative reason for failure is not always what is found during surgery. In the external primary fundoplication group undergoing redo fundoplication, the preredo diagnosis was GERD in the majority of patients. This would suggest that a loose wrap would be found during surgery. In fact, several patients were found to have twisted wraps without an appropriately mobilized gastric fundus (short gastric vessels intact) or misplaced or loose wraps (Fig. 5A, B). Very few had simply a disrupted fundoplication. In all cases, we take down the prior fundoplication and redo the entire operation. In one patient with dysphagia and a very normal-appearing wrap, after complete mobilization of the fundoplication, a Penrose drain was found wrapped around the distal esophagus. In several other patients, unexpected foreign material was found, including hernia tacks used for the crural approximation and fundoplication and mesh when the first operation mentioned nothing about hiatal hernia or the use of mesh.
Diminishing Success With Each Redo
Perhaps most significant is the data revealing a higher rate of failure with a redo when compared with a first-time fundoplication. While it is intuitive that the best outcome will be with the first fundoplication, it is perhaps surprising that the failure rate with 1 redo is only 7.1% and not higher. This is, however, nearly 3 times the redo rate after a first-time fundoplication. With a third redo, the failure rate does not appear to increase, but after 3 redos, it looks like the chance of subsequent failure becomes prohibitive. While not detailed here, we now seriously consider esophagectomy after 4 failures (primary and 3 redos). Over the past 4 years, this has been necessary in fewer than 10 patients. The only exception to this is the patient who has a “redo” during which very little is done (ie, more of an exploratory procedure than a true attempted redo). Many patients have undergone a redo, but at the time of another redo, virginal tissue plans are encountered, suggesting a limited dissection in the previous procedure.
Mechanism of Failure: Future
Fundoplication herniation was the most common mechanism of failure in our 1999 series of 100 redos, and it remains the most common mechanism of failure, even in our own patients requiring redo fundoplication. Clearly there is a need to better understand the mechanics the esophageal hiatus and its reconstruction. The etiology of this mechanism of failure is unclear. Some have suggested that esophageal shortening predisposes to fundoplication herniation and that an esophageal lengthening should accompany all hiatal hernia repairs. We have looked carefully at our own experience with both the short esophagus and esophageal lengthening and have found true esophageal shortening to be unusual (<2% when assessed intraoperatively). Esophageal lengthening, while providing an esthetically pleasing intraoperative result, has resulted in uncontrolled esophagitis or pathologic esophageal acid exposure in 80% of patients postoperatively.18 Except in the extreme situation, esophageal lengthening should rarely be indicated. Another often-discussed issue is the idea that hiatal hernias should be subjected to a tension-free repair, as is now widely accepted for abdominal wall hernias. Many are advocating routine use of mesh for reconstruction of the esophageal hiatus. Several prospective studies have documented improved outcomes with mesh hiatoplasty; however, numbers are small and follow-up is short.9 We have avoided mesh except for the largest of hiatal defects due to concerns about placing an inflammatory foreign body next to the esophagus and our anecdotal experience in several patients where mesh eroded into the esophagus or caused significant esophageal stenosis. Clearly, work needs to focus on improving our management of the esophageal hiatus during fundoplication to improve the results of this operation.
Finally, a comment about follow-up. As detailed earlier, we have made a commitment to maintaining contact with our patients through our full-time research nurse and follow-up strategy. Even with such efforts, follow-up is incomplete and extremely difficult to maintain. After 1 year, just over half of our patients respond to requests for follow-up. The optimist and fundoplication enthusiast offers the theory that in such a mobile society, most patients are doing so well after this operation that they cannot be bothered with follow-up. On the other hand, many surgery enthusiasts have announced this theory only to be dashed by a colleague in the room claiming to be caring for increasing numbers of patients with failures. As a quaternary referral center for foregut conditions, we join the optimist group, hoping that if significant numbers of our patients were requiring redo surgery, the patients would be coming back to us or we would hear about this from our colleagues. That being said, the lack of objective long-term follow-up, combined with an unclear definition of failure (back on medication versus need for more surgery), continues to fuel the debate over the role of fundoplication in the management of the GERD patient, and this series does not resolve the debate.
In summary, carefully selected patients who have recurrent or persistent problems after fundoplication can safely undergo redo fundoplication with good results. In experienced hands, most patients can be approached laparoscopically. Ideally, documented long-term follow-up will allow continued evolution of the care for the foregut patient, thereby providing ever improving outcomes, but follow-up remains problematic.
Discussions
Dr. William O. Richards (Nashville, Tennessee): Drs. Smith, Hunter, and colleagues are to be congratulated on the significant work they have accomplished with this report with redo Nissen fundoplications. I want to emphasize several important aspects about their experience.
Their results after redo operations are really outstanding. They were able to perform the majority of these redo operations laparoscopically with excellent results and very low morbidity. In the manuscript, the authors report five patients from their own experience who presented with acute herniation of the intact wrap in the early postoperative period that was related to nausea and retching. Could the authors expand on these cases and tell us if they were in any way related to inadequate crural closure and if they recommend any changes in the crural closure or postoperative care that can decrease the risk of herniation?
Second, about half of their redo cases were related to dysphagia in an angulated route, but 3.7% of the patients undergoing a second redo operation underwent Heller myotomy in the manuscript. Secondary achalasia caused by the wrap occurs in a small number of patients undergoing fundoplication at Vanderbilt. We believe that takedown of the Nissen and performance of a Heller myotomy is the best operation for these patients with secondary achalasia. I would like to hear how you handle the patients who have manometric findings of achalasia after Nissen fundoplication.
Finally, about half the redo operations were performed to alleviate recurrent GERD, but redo Nissen, even with your excellent results, was associated with a significant conversion rate, increased hospitalization, and increased risk of another reoperation compared to your primary operations. The new endoluminal therapies may be able to improve the antireflux barrier significantly and avoid performing a redo Nissen fundoplication.
In patients with a partially disrupted wrap and recurrent GERD but with no significant dysphagia, I would preferentially perform a Stretta procedure or another completely endoluminal therapy rather than attempting a redo Nissen. I would be interested in your thoughts as to which patients are best suited for this approach.
Dr. Blair A. Jobe (Portland, Oregon): I wish to congratulate Dr. Smith and his colleagues on this excellent study and for assembling what is now the largest series of reoperations for failed fundoplication. The opportunity to gain insight into the mechanisms by which our procedures “fall down” is both valuable and rare.
The tremendous success of Nissen fundoplication has been founded in the tenets of proper patient selection and an adherence to the technical fundamentals of reconstruction, that is, division of the short gastric vessels, return of the esophagogastric junction into the abdominal cavity, crural closure, and the creation of a short floppy fundoplication around the distal esophagus. Despite these advances in technique, a small percentage of these patients will develop recurrent symptoms and the need for revision as the result of incompetence of the cardia, esophageal obstruction, or both.
This study brings several important points to light. First, similar to the findings of others, the most common cause of failure resulting in reoperation was herniation of the esophagogastric junction (with or without the fundoplication) into the chest. Second, the presence of fundoplication herniation at the time of the first reoperation was a significant risk factor for a subsequent failure, which would require another operative repair. Third, the laparoscopic approach to fundoplication failure results in good short-term clinical outcomes and low morbidity in experienced hands. Finally, failures, which are rare, occur within 2 years of the primary operation. Based on these findings, I have several questions.
Perhaps we have been too simplistic in how we conceptualize herniation after fundoplication. The age-old debate is whether herniation occurs as a result of an unrecognized short esophagus, crural dehiscence, or elements of both. Is it unrealistic to think that we can place these dynamic and continually moving structures adjacent to each other and not have some element of herniation? The esophagus shortens with each swallow and the diaphragm flattens with each breath. This is bound to place stress on the crural closure and fundoplication. With this in mind, should Collis gastroplasty be used more liberally in the primary operation, or is the risk for continued esophageal injury too great? Did the majority of patients that herniated have complicated reflux (Barrett metaplasia, stricture) or a diaphragmatic stressor? In light of your results, will you modify your approach when herniation after a primary repair is encountered?
From your data, it appears as if the incidence of the malformed wrap is decreasing. In your study of fundoplication failure in 1999, the most common cause of failure in patients who were referred to Emory was a twisted or slipped wrap. In the current study, the referral consists primarily of patients with herniation. Why do you think this pattern has shifted?
Finally, as Dr. Richards brought up, is there a role for endoscopic antireflux techniques in the “tune-up” of an anatomically intact fundoplication which allows reflux?
Dr. J. David Richardson (Louisville, Kentucky): What do you do with the occasional patient at the far end of the spectrum who has had sort of a disastrous failure? I have operated on several of these patients recently where the entire fundoplication is in the chest and nothing is working. Often, the wrap cannot be anatomically returned to the abdomen with a laparoscope or run open. What do you do with those patients who are quite challenging? The authors stressed that experienced hands are important in managing these patients, but there is a lot of esophageal surgery being done by relatively inexperienced surgeons.
Dr. Robert V. Rege (Dallas, Texas): I would like to congratulate you on your paper. When you are done with all of the redos and multiple redos, there is a significant number of patients left over which are troublesome for us because they show up at referral centers. And those are the patients that do not have physiologic or anatomic reasons to redo the wrap again but continue to be dissatisfied with the results. I would like to know how you handle these. Do you ever take the wrap apart and give up on the reflux procedure in those patients?
Dr. C. Daniel Smith (Atlanta, Georgia): Thank you for these wonderful questions. As you might suspect, with this experience we have struggled with a lot of these same issues for years and years in managing these patients.
Dr. Richards asked questions about the 5 patients who had acute wrap herniation and what we have done, if anything, to try to manage that, and in particular, do we do anything different with the crura?
In each of these cases, the patients experienced acute postoperative retching. The first thing we have done is implemented a policy of antinausea control and antiemetics. We are very aggressive about all patients getting preemptive nausea control. With that, in the last 2 years we have not had any patients who have had acute herniation and needed to go back to the operating room. So I think retching prevention and nausea prevention is number 1.
When you go back in on those few who have acute herniation, it is hard to know what may have been etiologic, if anything, at the time of the first operation. Usually, there is a stitch that has popped loose in the crura as the wrap has herniated. We have not been able to determine any issues related to the crura and how we handled the crura in those patients.
The second question had to do with myotomy and the proposal of more liberal use of myotomy. We will do a myotomy, but only if we can't find an anatomic basis at the time of the second operation for esophageal outlet obstruction, and the patient has aperistalsis on their preoperative motility. In contrast, if we have any element of preserved motility or body function and we find a bit of a twist or tightness of the wrap, we will not do a myotomy. That is why our myotomy rate is fairly low. Again, we will apply it for the aperistaltic esophagus when everything else looks fairly normal.
Regarding the use of Stretta or endoluminal therapy endoluminal therapy; absolutely, to both Drs. Jobe and Richards. I think this is an application of endoluminal therapy that I am probably most eager to see developed, and we are doing it. Our preliminary results are fairly favorable. Even if you can only achieve acid control in 50% of patients with an endoluminal therapy, that is half of the patients who may not go on to the risks of a redo operation. However, I think the endoluminal therapies are really only appropriate for the patients who have recurrent GERD and clear anatomic evidence of a disrupted wrap. It is a fairly small number of patients, but I think for those patients this is very appropriate.
Regarding Dr. Jobe's question about complicated GERD, I think the answer is yes, no question, the patients who have Barrett esophagitis or stricture have an 8% failure rate. But we have patients in this group who also had herniation because of things like abdominal trauma or episodes of reaching years after surgery and even some patients where we have no explanation for why they have this problem. So you certainly should suspect it in the patients with complex reflux.
Regarding liberal use of the Collis gastroplasty, we looked at our own experience. We found that 80% of patients who have undergone a Collis gastroplasty have persistent esophageal acid exposure after that operation. I believe it is a good operation. But it has to be done correctly.
You cannot get unlimited length with a Collis gastroplasty. You can probably only get 2 cm of length by creating the wrap around the neoesophagus. If you do that, you will probably avoid the persistent esophageal acid exposure. I really don't think we should be looking at more liberal use of the Collis because there is a considerable risk of continued acid exposure.
Why are we seeing fewer misplaced wraps or malformed wraps? I think there are probably 2 answers to that. Number 1, people are learning how to do this operation. They are being more aggressive about taking down the short gastric vessels and those other principles that we all come to understand. Number 2, I think fewer surgeons are doing this operation. More of these operations are now coming to referral centers and are consolidating in centers of excellence, in part because of the reports 3 and 4 years ago calling into question the success of this operation.
Dr. Richardson asked about the disaster patient. We do a combined abdominal chest approach. We will leave the patient without a wrap if we have to. We have not left any wraps in the chest. We have had concerns about reported incidents of perforation in those situations. We have done some esophagectomies. If you have the end-stage patient, you just take their esophagus out.
Dr. Rege asked about the patient in whom you really cannot find any anatomic basis for failure, what do we do, counseling, sit on our hands? Every time we do a redo, we take a wrap entirely apart. We have a few patients that we have not rewrapped. I will tell you hands down, for the patient who does not have an objective basis for reoperation, you should not reoperate. Those patients will inevitably do very poorly.
Footnotes
Reprints: C. Daniel Smith, MD, Department of Surgery, Emory University School of Medicine, 1364 Clifton Road, NE (H124), Atlanta, GA 30322. E-mail: csmit27@emory.edu.
REFERENCES
- 1.Dallemagne B, Weerts JM, Jehaes C, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc. 1991;1:138–143. [PubMed] [Google Scholar]
- 2.Morton J, Lucktong T, Behrns KE, et al. National trends in fundoplication utilization and outcomes from 1989 and 1999. J Am Coll Surg. 2002;195:S55. [Google Scholar]
- 3.Anvari M, Allen C. Five-year comprehensive outcomes evaluation in 181 patients after laparoscopic Nissen fundoplication. J Am Coll Surg. 2003;196:51–59. [DOI] [PubMed] [Google Scholar]
- 4.Bammer T, Hinder RA, Klaus A, et al. Five- to eight-year outcome of the first laparoscopic fundoplications. J Gastrointest Surg. 2001;5:42–48. [DOI] [PubMed] [Google Scholar]
- 5.Catarci M, Gentileschi P, Papi C, et al. Evidence-based appraisal of antireflux fundoplication. Ann Surg. 2004;239:325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dassinger MS, Torquati A, Houston HL, et al. Laparoscopic fundoplication: 5-year follow-up. Am Surg. 2004;70:691–694. [PubMed] [Google Scholar]
- 7.Lafullarde T, Watson DI, Jamieson GG, et al. Laparoscopic Nissen fundoplication: five-year results and beyond. Arch Surg. 2001;136:180–184. [DOI] [PubMed] [Google Scholar]
- 8.Peters JH, DeMeester TR, Crookes PF, et al. The treatment of gastroesophageal reflux disease with laparoscopic Nissen fundoplication: prospective evaluation of 100 patients with “typical” symptoms. Ann Surg. 1998;228:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arguedas MR, Heudebert GR, Klapow JC, et al. Re-examination of the cost-effectiveness of surgical versus medical therapy in patients with gastroesophageal reflux disease: the value of long-term data collection. Am J Gastroenterol. 2004;99:1023–1028. [DOI] [PubMed] [Google Scholar]
- 10.Spechler SJ. Medical or invasive therapy for GERD: an acidulous analysis. Clin Gastroenterol Hepatol. 2003;1:81–88. [DOI] [PubMed] [Google Scholar]
- 11.Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease. JAMA. 2001;285:2331–2338. [DOI] [PubMed] [Google Scholar]
- 12.Hashemi M, Peters JH, DeMeester TR, et al. Laparoscopic repair of large type III hiatal hernia: objective followup reveals high recurrence rate. J Am Coll Surg. 2000;190:553–560. [DOI] [PubMed] [Google Scholar]
- 13.Mattar SG, Bowers SP, Bradshaw WA, et al. Laparoscopic repair of paraesophageal hernia is subject to recurrence but rarely requires reoperation. Gastroenterology. 2001;120:A479. [Google Scholar]
- 14.Mattar SG, Bowers SP, Galloway KD, et al. Long-term outcome of laparoscopic repair of paraesophageal hernia. Surg Endosc. 2002;16:745–749. [DOI] [PubMed] [Google Scholar]
- 15.Hunter JG, Smith CD, Branum GD, et al. Laparoscopic fundoplication failures: patterns of failure and response to fundoplication revision. Ann Surg. 1999;230:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry M, Smith CD, Branum GD, et al. Outcomes of laparoscopic fundoplication for gastroesophageal reflux disease and paraesophageal hernia. Surg Endosc. 2001;15:691–699. [DOI] [PubMed] [Google Scholar]
- 17.Bowers SP, Mattar SG, Smith CD, et al. Clinical and histologic follow-up after antireflux surgery for Barrett's esophagus. J Gastrointest Surg. 2002;6:532–538. [DOI] [PubMed] [Google Scholar]
- 18.Lin E, Swafford V, Chadalavada R, et al. Disparity between symptomatic and physiologic outcomes following esophageal lengthening procedures for antireflux surgery. J Gastrointest Surg. 2004;8:31–39. [DOI] [PubMed] [Google Scholar]


