Abstract
Introduction:
Gastrointestinal (GI) complications following heart operation may be life-threatening. Systematic analysis of risk factors to allow early identification of patients at risk for GI complication may lead to the development of strategies to mitigate this complication as well as to optimize management after its occurrence.
Methods:
Of 8709 consecutive patients undergoing heart operation during 7 years (1997–2003), 46 (0.53%) developed GI complications requiring surgical consultation. Preoperative, intraoperative, and postoperative predictors of complication and death were identified and compared with a control group.
Results:
Significant (P < 0.05) preoperative predictors of complication were prior cerebrovascular accident (CVA), chronic obstructive pulmonary disease (COPD), type II heparin-induced thrombocytopenia, atrial fibrillation, prior myocardial infarction, renal insufficiency, hypertension, and need for intra-aortic balloon counter-pulsation. The most frequent serious GI complication was mesenteric ischemia, which developed in 31 (67%) patients. Twenty-two (71%) of these patients were explored, and 14 (64%) died within 2 days of heart operation. Of the 9 patients with mesenteric ischemia who were not explored, 7 (78%) died within 3 days of heart operation. Other complications included diverticulitis (5), pancreatitis (4), peptic ulcer disease (4), and cholecystitis (2). The mortality rate in this group of other diagnoses was lower (40%), and death occurred later (32 days) after heart operation (P = 0.03 compared with mesenteric ischemia). Predictors of death from GI complication included New York Heart Association (NYHA) class III and IV heart failure, smoking, chronic obstructive pulmonary disease, history of syncope, aspartate aminotransferase (AST) >600U/L, direct bilirubin >2.4mg/dL, pH < 7.30, and the need for >2 pressors.
Conclusions:
The most common catastrophic GI complication after cardiac surgery is mesenteric ischemia, which is frequently fatal. This complication may be a result of atheroembolization, heparin-induced thrombocytopenia, or hypoperfusion. Techniques to reduce the occurrence of and/or preemptively diagnosis postcardiotomy mesenteric ischemia are necessary to decrease its associated mortality.
Gastrointestinal complications following heart operation may be life-threatening. Of 8709 consecutive patients undergoing heart operation, 46 (0.53%) developed gastrointestinal complications requiring surgical consultation. Preoperative, intraoperative, and postoperative predictors of complication and death were compared with a control group to better identify patients at risk and to optimize management after occurrence.
Gastrointestinal (GI) complications after heart operation are rare but carry significant morbidity and mortality even when recognized early and treated appropriately. Although several centers have reported retrospective reviews of GI complications after heart operation, few have undertaken systematic analyses of preoperative, intraoperative, and postoperative variables that contribute to this entity.1–18 Risk stratification based on identification of such variables may influence cardiac surgeons to alter their operative strategy or early postoperative management decisions. Identification of risk factors for GI complication should heighten the index of suspicion of the treating physician when confronted with a postoperative course, which deviates from the expected norm. Finally, identification of risk factors for death after GI complication provides general surgeons with clinically relevant features indicating subsets of patients most likely to benefit from and survive laparotomy. Such information would help to direct more rational utilization of resources and may provide a framework for providing patients and their families with realistic expectations should such complications occur. Accordingly, we reviewed the case records of 46 patients suffering GI complication from 8709 consecutive patients that underwent heart operation at the Massachusetts General Hospital (MGH), searching specifically for the influence of 89 variables cross-matched with the Society of Thoracic Surgery database on the odds of development of GI complication as well as on outcome after complication.
PATIENTS AND METHODS
Permission was obtained from the Institutional Review Board. A total of 8709 patients underwent heart operation at the MGH between January 1, 1998 and December 31, 2003. The Cardiac Surgery Morbidity and Mortality database was reviewed over the same period, and any patient that developed a GI complication requiring general surgical consultation was included in the study cohort. Forty-six such patients were identified. Eighty-nine variables were listed (Table 1), and the charts of study cohort were reviewed for the presence of these variables. Primary outcomes were presence of a GI complication and death. To identify preoperative predictors of GI complication and subsequent outcome, univariate and multivariate analyses were performed against a control group of 250 patients (cohort:control, 1:5 ratio) randomly selected from the MGH case volume that is submitted annually to the Society of Thoracic Surgery database. To identify predictors of outcome after GI complication, univariate analyses were performed on the cohort group. The 2-tailed Student t test was used for noncategorical variables and χ2 test was used for categorical variables. Multivariate and univariate analyses were performed using SAS 9.0 (SAS Institute Inc, Cary, NC).
TABLE 1. Variables

TABLE 1. (continued) Variables

RESULTS
Patient Demographics
Twenty-eight of 46 patients (61%) were male. The median age was 74 years (range, 35–86 years). The median ejection fraction was 48%. Twenty-five of 46 cases (54%) were performed urgently, and an additional 7 (15%) were performed emergently. Forty-one operations were done on cardiopulmonary bypass. Twenty patients underwent coronary artery bypass graft operation (CABG) only (median number of grafts, 3; mean number of grafts, 3.18) and of these, 5 underwent off-pump CABG; 9 underwent combined CABG and mitral valve operation; 6 underwent combined CABG and aortic valve operation; 5 underwent isolated aortic valve replacement; 2 underwent isolated mitral valve replacement; 2 underwent aortic root replacements; 1 underwent aortic arch replacement; and 1 underwent cardiac transplantation. There was no significant difference between the case and control groups in such general demographic data as age, sex, and type of operation. The median perfusion time was 144 minutes (mean, 152.9 minutes; range, 63–252 minutes), and median ischemic time was 101 minutes (mean, 108.6 minutes; range, 40–207 minutes). Four patients underwent operation with the heart fibrillating. The remaining 42 underwent cardioplegia arrest (antegrade only in 18 patients and both antegrade and retrograde in 19 patients). Six patients underwent epiaortic scanning. Of these, 1 had grade III atheroma, 2 had grade IV atheroma, and 3 had grade V atheroma. Forty patients underwent intraoperative transesophageal echocardiography. Twelve patients required preoperative cardiac support with intra-aortic balloon pump, and an additional 8 required intraoperative intra-aortic balloon pump, for a total of 43.4% of patients in the study group, compared with 27 of 250 (10.8%) in the control group (P < 0.0001). Twelve patients (26%) required reexploration for postoperative bleeding, as compared with 14 of 250 patients (5.8%) in the control group (P < 0.0001). These data suggest that hemodynamic lability and hypoperfusion may, in part, contribute to the development of GI complications in the postoperative period.
Categories of Intra-abdominal Event and Outcome
Thirty-one patients (67%) developed mesenteric ischemia after heart operation. Twenty-two were explored and 9 were not explored.
Of the 22 explored (71%), 14 (64%) died with 2 days of heart operation. Of the 14 that died, 13 had massive and diffuse necrosis of the small and large intestine. One had foregut necrosis in addition. No patient underwent resection for cure. Among the 8 survivors, 6 patients underwent segmental colonic resection, 1 underwent subtotal colectomy with end ileostomy, and 1 underwent superior mesenteric artery bypass with concomitant small bowel resection. Salvage survival rates for patients with isolated colonic ischemia were significantly improved over those with small bowel ischemia (P = 0.023). Median time to discharge was 52 days.
Nine patients were not offered abdominal exploration. Seven (78%) were not explored because of a presumptive preoperative diagnosis of unsalvageable mesenteric infarction These 7 patients died within 3 days of heart operation. The remaining 2 patients survived. Both these patients complained of abdominal pain 6 and 16 days after heart operation, had nonfocal abdominal examination, had an isolated leukocytosis (20,600 and 28,000) with no abnormalities in pH, serum AST, or bilirubin. Abdominal computer tomogram was remarkable for nonspecific thickening of the colon with small amounts of free fluid. One patient underwent superior mesenteric artery (SMA) angiography that was normal, and the other underwent placement of a cholecystostomy tube for coincidentally diagnosed acalculous cholecystitis. These 2 patients were discharged from the hospital a median of 46 days after heart operation. Both patients were therefore thought to have suffered regional mesenteric ischemia from which they recovered without laparotomy, suggesting that there are subgroups that may benefit from conservative management. There were no statistically significant differences in preoperative, intraoperative, or immediate postoperative variables between these 2 survivors and the 7 patients that did not survive, except for the earlier onset of abdominal pain (the 7 patients who died complained of pain 1.8 days after heart surgery, whereas this group complained an average of 12 days after heart operation, P < 0.05).
Overall mortality in patients who were thought to have developed postoperative mesenteric ischemia was 68%. Clearly, however, subgroups of patients with regional intestinal ischemia (ie, isolated colonic ischemia) benefit from laparotomy and resection, and constitute a group that is distinct from patients who develop massive intestinal ischemia and necrosis.
The remaining 15 patients comprised a more diverse group who developed conditions that included diverticulitis (5 patients), pancreatitis (4 patients), peptic ulcer disease (4 patients), and cholecystitis (2 patients). The mortality rate in this group was 40% (P = 0.003 versus patients who develop mesenteric ischemia), and death occurred 32 days after heart operation (P = 0.03 versus patients who develop mesenteric ischemia). Of the 5 patients that suffered from diverticulitis, 2 died a median of 26 days after heart operation. The remaining 3 were discharged from the hospital a median of 41 days after heart operation. Of the 4 patients who developed pancreatitis, 1 died on postoperative day 17. The remaining 3 required pancreatic debridement for infected pancreatic necrosis but survived to discharge at a median of 38 days after heart operation. Both patients with perforated peptic ulcer survived to hospital discharge at a median of 52 days after heart operation. Notably, both had a known preoperative history of peptic ulcer. Both patients with bleeding ulcers died after surgical management (laparotomy and oversewing of bleeding duodenal ulcer in one and subtotal gastrectomy in the second). Two patients who developed symptomatic cholecystitis died, despite placement of cholecystostomy drain in one and cholecystectomy in the other at a median of 32 days after heart operation. A third patient (previously mentioned) who was noted to have findings on CT suggestive of acalculous cholecystitis survived after placement of a cholecystostomy tube.
Predictors of Intra-abdominal Event
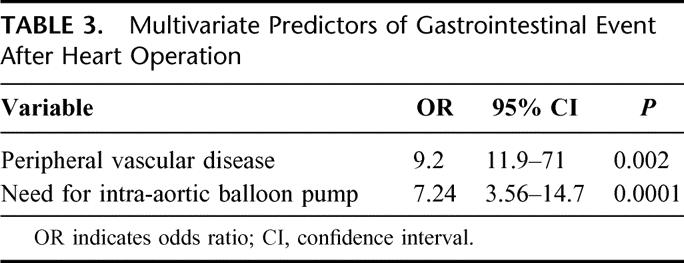
Preoperative variables that emerged as predictors of intra-abdominal complication on univariate analysis are presented in Table 2. These included chronic obstructive pulmonary disease, need for intra-aortic balloon counter-pulsation (whether placed preoperatively or intraoperatively), type II heparin-induced thrombocytopenia (only 2 of 8 patients who developed type II heparin-induced thrombocytopenia HIT, and an abdominal complication survived the hospitalization), prior cerebrovascular accident, atrial fibrillation, renal insufficiency with a creatinine of ≥1.4 mg/dL, peripheral vascular disease, hypertension, and prior myocardial infarction. On multivariate analysis, peripheral vascular disease (odds ratio, 9.2) and need for intra-aortic balloon pump (odds ratio, 7.24) were strongly independently predictive of GI complication after heart operation as presented in Table 3. Intraoperative variables such as cardiopulmonary bypass time, off-pump operation, type of heart operation (CABG, valve, aortic), and myocardial protection strategy were not predictive of GI event after heart operation. Similarly, none of the variables studied in the immediate postoperative period emerged as predictive of intra-abdominal event after heart operation.
TABLE 2. Univariate Predictors of Gastrointestinal Event After Heart Operation
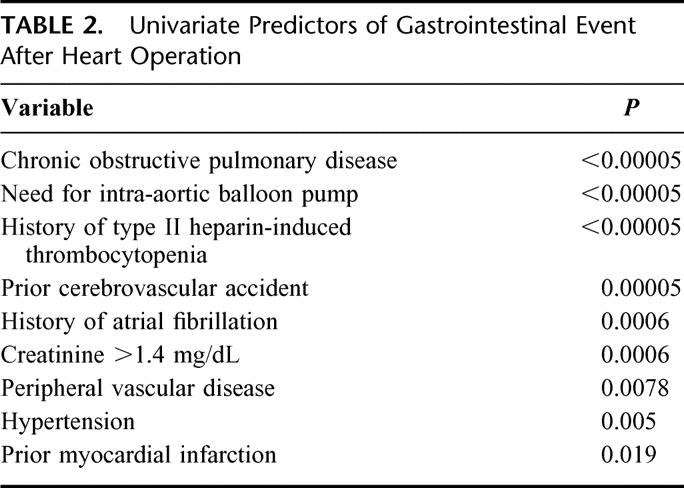
TABLE 3. Multivariate Predictors of Gastrointestinal Event After Heart Operation
Predictors of Death After Intra-abdominal Event
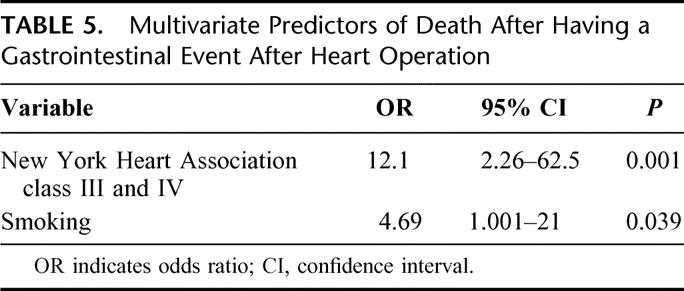
Variables that were predictive of increased risk of death in patients suffering intra-abdominal catastrophe on univariate analysis are presented in Table 4. These included NYHA class III and IV failure, a history of smoking, chronic obstructive pulmonary disease, or syncope at the time of presentation. Intraoperative variables such as cardiopulmonary bypass time, off-pump operation, type of heart operation (CABG, valve, aortic), myocardial protection strategy, or degree of postoperative hemorrhage did not exert any influence on the probability of death after GI event. At the time of general surgical consultation in the immediate postoperative period, AST ≥600 U/L, bilirubin ≥2.4 mg/dL, an arterial pH ≤ 7.30, or the need for 2 or more pressors were strongly predictive of death. On multivariate analysis, New York Heart Association class III and IV (odds ratio, 12.1) and smoking (odds ratio, 4.69) emerged as the strongest independent predictors of death after GI complication as presented in Table 5.
TABLE 4. Univariate Predictors of Death After Having a Gastrointestinal Event After Heart Operation
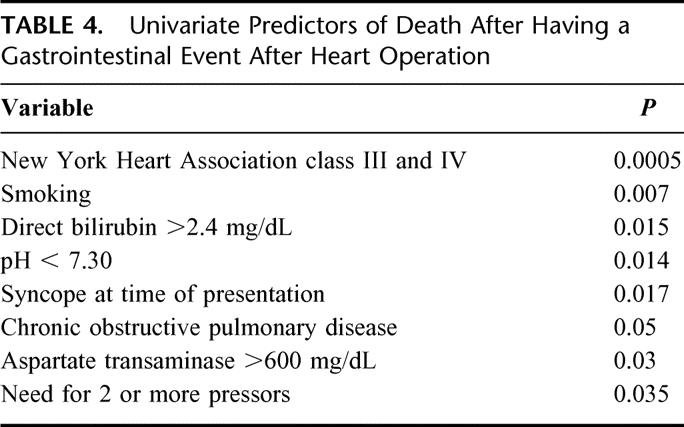
TABLE 5. Multivariate Predictors of Death After Having a Gastrointestinal Event After Heart Operation
DISCUSSION
Gastrointestinal complications following heart operation are a rare (46 of 8709 patients; 0.53%) but often fatal (61% of all presentations) event. Previous series have published higher rates of complications (0.6%–2.1%) with lower mortality rates (13.9%–63%)3–7,11,19,20 than our current data. Interestingly, the mortality rates cited in these studies vary inversely with incidence, suggesting that as the criteria for complications broaden, the percentage of those complications that are life-threatening decreases. This study used a retrospective analysis of the Cardiac Surgery Morbidity and Mortality database to locate those patients on whom a general surgical consult was requested. This implies that patients with complications that did not involve a consult from a GI surgeon were excluded. Accordingly, patients with such commonly cited postbypass GI events as mild to moderate pancreatitis or those suffering from upper or lower GI bleeding able to be sufficiently controlled by transfusion or endoscopic intervention were not necessarily all accounted for. Consequently, the severity of the complications reported and the attendant mortality rate may be overstated.
These complications can be broken down broadly into 2 subgroups: those secondary to mesenteric ischemia and those secondary to nonischemic causes. Mesenteric ischemia, clinically documented or suspected in the majority (31 of 46; 67%) of patients requiring postoperative surgical consultation, augurs a particularly ominous outcome (68% fatal). The etiology of mesenteric ischemia may also be conceptually divided into 2 distinct subsets: low flow (low perfusion) states and embolic related events, although in clinical practice these etiologies may overlap in the same patient.
Low Flow
Patients with a predilection toward postcardiotomy pump failure (and, thus, subsequent low flow states) tend to be at higher risk for developing GI complications. In our series, patients with renal failure (Cr > 1.4), prior myocardial infarction, and those requiring intra-aortic balloon counter-pulsation were at significantly higher risk for the development of GI complication when compared with an equivalent control group. Moreover, within the category of those who do develop GI complication, it is those with the most disabling underlying heart disease (NYHA III/IV failure) who are most likely to suffer a fatal event. This may represent a selection bias by identifying a subpopulation in whom revascularization alone is not sufficient to reverse underlying cardiomyopathy in the early postoperative period.
Cardiopulmonary bypass has been implicated in causing mesenteric ischemia by effecting regional differences in intestinal blood flow. Ohri et al21 demonstrated that, although there is typically no change in SMA flow rate, flow to the jejunal mucosa decreases by 40%, and flow to the serosa decreases by almost 50% upon the institution of hypothermic cardiopulmonary bypass. Commensurate decreases in oxygen delivery and consumption are noted. However during rewarming, oxygen delivery falls approximately 50% further while oxygen consumption increases dramatically, with resultant decreases in intestinal mucosal pH.21 Tao et al have demonstrated a similar phenomenon during normothermic cardiopulmonary bypass and suggest that this may be in part due to a diversion of blood flow away from the ileum and colon toward the foregut.22 These changes in flow patterns are accompanied by cardiopulmonary bypass-induced dysfunction in mesenteric endothelial cells with consequent hyperreactivity to alpha agonists.23 These differences may be amplified in patients with NYHA III/IV failure and with atrial fibrillation, who emerge from operation in postcardiotomy failure and may be further accentuated by the need for escalating pressors (especially as evidenced by the need for >2 pressors).
Embolic
Mesenteric ischemia following heart operation resulting from embolic disease can be secondary to either macrovascular embolism or thrombosis (ie, proximal SMA embolism) or microvascular disease (ie, embolic cholesterol showers presumably secondary to aortic manipulation or HIT-induced platelet aggregation). Those patients with isolated large vessel disease requiring SMA embolectomy had a favorable prognosis (2 of 3 requiring embolectomy or bypass survived) once revascularized when compared with those with small vessel disease, which was manifested as diffuse intestinal ischemia. While our small case number prevented the identification of a “signature” profile of variables that categorically identified patients at risk for GI complication following heart operation, it is significant that those patients with multiple preoperative risk factors for a prothrombotic state (history of smoking or COPD) or embolic event (type II HIT, history of atrial fibrillation) or preoperative indicators of diffuse microvascular disease (prior cerebrovascular accident, underlying peripheral vascular disease) are those most likely to suffer GI complications.
Outcome Based Upon Stratification of Cause of Ischemia
Stratification of patients into the categories of low-flow ischemia or occlusive ischemia related to an embolic event failed to demonstrate any clinically or statistically significant differences in presentation. Salvage survival rates in these 2 subsets were similar. On review of final pathology of those patients submitted for autopsy, there did not appear to be any histopathologic correlates of one etiology versus the other. This may be a result of a small sample size, significant overlap of etiologies within a single patient (as evidenced by significant overlap in risk factors), and the occurrence of diffuse intravascular coagulation, which accompanies end-organ failure. A subgroup of patients with segmental colonic necrosis constituted a group of patients that enjoyed a significantly more favorable outcome when operated upon early. None of the surviving patients with segmental colonic ischemia had any evidence of liver failure by liver enzymes, while half of those who died demonstrated significant elevations in bilirubin or AST indicating more a more global ischemic insult.
Other Causes
Other GI complications such as diverticulitis, peptic ulcer disease, cholecystitis, and pancreatitis were also observed. Notably, these events occurred much later in the hospitalization (weeks rather than hours to days after operation) and augured a less ominous clinical course. Patients suffering from these entities should be managed with condition-specific strategies.
Implications for Clinical Management
The morbidity and mortality associated with the development of postcardiotomy mesenteric ischemia impose an imperative for the identification of preventative strategies as well emphasizing the need for early recognition of cases that do occur. The technique of off-pump CABG has successfully reduced the vascular complication rates in patients with stroke, renal failure, and peripheral vascular disease.24 Theoretically, the avoidance of cardiopulmonary bypass-induced mesenteric hemodynamic lability as well as the avoidance of cannulation-related embolic events should also diminish the likelihood of bypass-related GI complications. Unfortunately, 2 recent retrospective studies have failed to demonstrate this protective effect.25,26 This may be in part due to the selection bias inherent in studies investigating outcome and off-pump operation and by the already low percentage of overall patients suffering GI complications. Other strategies that may help prevent mesenteric ischemia include routine epiaortic ultrasonography to identify and avoid debris in the aorta; institution of a screening program for HIT and active prophylaxis against HIT with iloprost and dipyridamole;27 the use of internal mammary-based pedicled grafts;28 and proximal anastomotic devices29 to avoid clamping the aorta in such patients; and paying meticulous attention to hemodynamic management in the perioperative period. Experimental studies in animal populations have noted modest success in pharmacologic modulation of cardiac bypass induced α1-mediated hyperactive vasoconstriction through the administration of dopexamine or dopamine.30–32 These agents are thought to contribute to splanchnic vasodilation through the stimulation of mesenteric β2 and DA-1 receptors, but clinical efficacy in humans undergoing bypass remains unproven.33 Moreover, at least one study suggests that dopamine directly acidifies colonic crypts through an intercellular mechanism independent of blood supply or tissue oxygenation and may not only increase the metabolic demand of splanchnic tissue but also, through mucosal acidification, contribute to bacterial translocation and/or endotoxemia.34
Survival from the insult of mesenteric ischemia is, in part, dependent upon early recognition.35 Postoperative predictors of death identified in this study were laboratory values indicative of multisystem end-organ failure and, therefore, late indicators of ischemic insult. The patient's early complaint of abdominal pain in the appropriate clinical setting is the most sensitive indicator of a significant GI event and allows the physician to intervene prior to laboratory manifestation of end-organ damage. As the overwhelming majority of cases of mesenteric ischemia occur during the initial hours to days following operation, fast-track extubation pathways and/or minimal sedation36 theoretically may enable earlier recognition of a catastrophic GI event. This opens a window for both more sophisticated diagnostic imaging (CTA or MRA) and for intervention. Early laparotomy guided by the clinical scenario may therefore result in early intraoperative recognition of a correctable ischemic event: isolated SMA/large vessel occlusion or segmental colonic necrosis. In settings in which early extubation may not be feasible, the use of invasive monitoring systems such as continuous gastric wall pH monitoring has been advocated,37 but the efficacy and role of such strategies in routine postoperative care remain untested.
The prognosis associated with postoperative GI complication following heart operation remains grim. Mesenteric ischemia follows cardiotomy in susceptible patients at a period of hours to days, whereas a more diverse group of intra-abdominal disease (diverticulitis, cholecystitis, pancreatitis, peptic ulcer) occurs days to weeks following operation. Routine surgical management is indicated in this latter group of patients, but perioperative preventative strategies are likely to be of greater benefit in the former. Laboratory markers of widespread ischemic insult, often used to make the diagnosis of mesenteric ischemia, unfortunately appear after the closure of a window for therapeutic intervention. These values may help to provide a better framework for surgical decision making and family counseling, but ultimately techniques to reduce the occurrence of and/or preemptively diagnosis postcardiotomy mesenteric ischemia will be necessary to decrease its associated mortality.
ACKNOWLEDGMENTS
The authors thank Elkan Halpern, PhD, of the Institute for Technology Assessment, Boston, Massachusetts, for his assistance with the multivariate analysis.
Discussions
Dr. Keith D. Lillemoe (Indianapolis, Indiana): I want to congratulate Drs. Mangi and Warshaw and their colleagues for an excellent presentation. This is an excellent paper with a very complete analysis of their database. I have a few questions and a couple of comments.
First, the number of consults seems very low, with only 46 patients seen over a 6-year period including over 8700 patients undergoing cardiac procedures. I don't have my own database, but this seems from memory that at Hopkins when I was still seeing these patients, the number of consults we had was clearly greater.
Also, when you saw patients, they generally were very sick and, as your data showed, most needed operations and many died. Weren't you ever consulted with someone who was less than with a life-threatening situation, or even a false alarm? The bottom line: are you sure your data are correct with respect to the number of surgical consults? Obviously, this may affect the recommendations and conclusions that we might draw from your paper.
Along those lines, I recall previous publications from the Massachusetts General Hospital from the 1980s and 1990s reporting what I considered a very high incidence of post-pump pancreatitis. This really didn't match my own personal experience, but I am surprised in this series that you only report 4 patients with pancreatitis, 1 of which died and 3 of which required going to the operating room for pancreatic debridement. What has changed at your institution since that time with respect to post-pump pancreatitis?
Similarly with respect to cholecystitis, this seems like a very low incidence with only 3 patients, and again 2 of 3 patients died. Why does this series appear to have such an extreme disease when you would have hoped that some of these patients would have survived even in the post-cardiac surgery situation?
Finally, with respect to the problem of most concern, that is, mesenteric ischemia: it would appear that, although you were seeing these patients early, actually usually within 2 days postoperatively, your mortality rate was extraordinarily high, certainly suggesting that the die was cast by the time you were involved. If this is indeed the case, can you tell us what, other than by recognizing the risk factors that you have observed of peripheral vascular disease and the use of an intra-aortic balloon pump, can cardiac surgeons and the general surgeons learn from this presentation that can help us better take care of these patients? Specifically, what clues might there be for early postoperative diagnosis that might provide the opportunity to intervene during a window of opportunity when we may be able to salvage these patients?
It is a very good paper. It certainly, like most, will raise many questions.
Dr. Robert M. Mentzer, Jr. (Lexington, Kentucky): I compliment Dr. Mangi on a fine presentation and commend the authors on an important and timely paper. The purpose of this study was to identify patients in jeopardy of developing gastrointestinal complications following cardiac surgery. Among the 8709 patients studied, only 46, or 0.53%, of them developed complications that required surgical consultation. Mortality, however, was quite high.
Of particular interest are the findings that 1) mesenteric ischemia accounted for 67% of the complications, 2) almost half of these patients died within 3 days of the heart operation, and 3) the overall mortality with this diagnosis approached 70%. Thus, the prognosis for patients with gastrointestinal complications after heart surgery, especially mesenteric ischemia, is extremely bleak.
The low incidence of complications and the high mortality rate have been consistent findings for the last 10 to 15 years. Since 1994, various groups have reported the incidence of complications to range between 0.5% and 1.5%. What appears to have changed, however, is the nature of those complications. Ten years ago, the most frequently encountered complication (accounting for more than 60% of the cases) was GI bleeding. In Dr. Mangi's study, only 2 of 46, or 4%, of the patients experienced this complication.
One possible explanation for this could be the venous or arterial thrombosis that occurs as a result of heparin-induced thrombocytopenia, or HIT. HIT is thought to be caused by heparin-dependent, platelet-activating antibodies that recognize a “self” protein-platelet factor 4 bound to heparin. Typically in HIT, the platelet count fall begins 5 to 10 days after starting heparin. A rapid fall in the platelet count can occur, however, in a patient who has antibodies from recent heparin exposure.
Historically, this has not been a major problem because heparin-induced immunization in the preoperative setting has been fairly uncommon. Also, thrombocytopenia after heart surgery is usually explained by hemodilution and platelet consumption related to the use of cardiopulmonary bypass. Increasingly, however, cardiologists and other physicians are prescribing the use of heparin for acute coronary syndrome, congestive heart failure, and atrial fibrillation prior to the patient undergoing cardiac surgery. Since even low-dose subcutaneous heparin has been reported to cause HIT, it is likely that the incidence of HIT will increase.
In this regard, I have several questions. First, would you comment on whether there really has been a change in the spectrum of GI complications? Is this a real phenomenon?
Second, have you or your colleagues witnessed any increase in the incidence of HIT at your institution? What, if any, are the merits of testing for HIT antibodies in patients who need urgent but not emergent surgery and have received heparin?
Finally, a number of promising new anticoagulants are underdevelopment or in clinical trial that may be important alternatives to heparin. What, if any, is your experience with these agents?
Dr. William G. Cioffi, Jr. (Providence, Rhode Island): Succinctly stated, the conclusions from the author are: things are better, but older, sicker patients with bad hearts are still at risk for GI complications, predominately mesenteric ischemia, and, when it occurs, mortality risk is great.
Like the previous discussants, I question the incidence. The literature over the past 20 years ranges from 0.8% to 5.5% of GI complications following cardiac surgery, and the authors report only 0.5%. Does the current series represent all complications, at least those requiring intervention? I would think not. What happened to the patients with cholecystitis, peptic ulcer, GI hemorrhage, etc? Nonetheless, the series is still remarkable.
Although univariate analysis suggests a large number of variables that may predict an increased risk of complications, only peripheral vascular disease and the need for intra-aortic balloon were significant on multivariate analysis. The type of operation, other patient attributes, whether the operation was elective or not, were not identified as predictors. And this is distinctly different from the literature.
It is disappointing that the avoidance of bypass was not beneficial, at least in terms of GI complications. Why do you suppose this is true? Given your preponderance of ischemic complications, have you changed your practice other than screening for HIT? These patients fall into 2 categories, low flow versus embolic events. Do you routinely use aortic ultrasound prior to bypass? Do you routinely use transesophageal electrocardiography as you come off bypass to better treat your patients? Do you use mesenteric vasodilators? You mentioned that you screen for HIT. What has been the incidence?
The reduction of GI complications is laudatory. Like the other discussants, what has changed in your practice over the last 20 years that might be responsible for your findings? As the percentage of the population over 65 continues to increase and the population requiring cardiac surgery continues to change, revascularization is becoming less common and complex reconstruction more common. Can you provide us with other strategies that may further decrease the incidence of this complication?
The current report has set a new benchmark for these complications, and I congratulate the authors for their results.
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): I would like to focus on the 4 patients with peptic ulcer disease. Do all of your patients receive postoperative antacid prophylaxis? If so, do these 4 patients represent failure of that therapy? Or if they don't receive it, does this represent the baseline incidence of acute stress ulcer disease following operation in your patient cohort?
Dr. Abeel A. Mangi (New York, New York): I am going to address Dr. Lillemoe and Dr. Cioffi's questions together. The small sample size is due to our methodology in that we were unable to identify every single general surgical consultation or every single patient that was seen by a general surgeon or gastroenterologist. Relying on the cardiac surgery morbidity and mortality database necessarily narrows the net, if you will. So we were really only seeing patients that truly suffered consequences of a GI event. I think that speaks to the observations that you made in that the patients are certainly sicker when they are seen and that most of the problems that they wind up having are those that ultimately require intervention.
One example of this phenomenon is the pancreatitis issue, for example. The MGH did publish in the past, as Dr. Lillemoe pointed out, the relationship of pancreatitis with cardiopulmonary bypass. What we have come to notice since then is a heightened awareness of the fact that a certain percentage of patients undergoing cardiopulmonary bypass are going to suffer a chemical pancreatitis but not necessarily a clinical pancreatitis. Therefore, a general surgeon won't get involved with a patient with chemical pancreatitis unless they became symptomatic.
The issue regarding mesenteric ischemia and the fact that these patients, despite a relatively early complaint of pain, will still die is truly a vexing one and not one that I am sure any one of us has a satisfactory answer to, except perhaps to reemphasize that these patients may benefit from minimal sedation and early extubation, therefore allowing them to make the complaint of abdominal pain in timely fashion.
Patients at the MGH cardiac surgical unit come out of the operating room on fentanyl and versed infusion until they have warmed up. They are then allowed to emerge from their sedation, a process that may take up to 18 hours. Therefore, if they have developed an embolic event that presumably occurred in the operating room, the only clues that point us in that direction are if patients demonstrate a persistent unexplained acidosis or patients whose course deviates from the expected norm.
To answer a couple of Dr. Cioffi's questions more specifically, I am not convinced that the avoidance of cardiopulmonary bypass, for example, the off-pump CABG, helps one avoid GI complication. There have been 2 retrospective reviews looking at off-pump CABG and GI complication. And although the total incidence of complication appears to be about the same, closer inspection of that data suggests that the type of GI complication is different. Patients done off pump tend to have a lower incidence of mesenteric ischemia, but they tend to make up for that with higher incidence of GI bleeding, peptic ulcer disease, and diverticulitis.
The practice at the MGH traditionally has not included epiaortic ultrasonography. Of the 46 patients in our study, only 4 underwent epiaortic ultrasound. That is something that we feel should probably change. Transesophageal echocardiography has come into the mix a little more recently. It has been used routinely for probably about 4 or 5 years. But given the historical spread of this study, only 40 of 46 patients in this study actually underwent TEE.
Dr. Mentzer's point about heparin-induced thrombocytopenia is a very important one. We did not look at platelet counts in this study for the confounding reasons that he outlined. Cardiopulmonary bypass, in and of itself, is a confounder. The MGH has seen a tremendous increase in the incidence of heparin-induced thrombocytopenia. In terms of new anticoagulants, the MGH is responsible for consuming 9% of the nation's argatroban, which is, as you know, one of the newer and novel anticoagulants.
So there is certainly very acute awareness of this problem at our hospital, not only in the cardiac surgical patients but also in the peripheral vascular patients, for example. And the biggest offender in generating this pool of patients appears to be the cardiac cath lab. So our patients are now all routinely screened for HIT preoperation.
We haven't really done very many cardiopulmonary bypass runs on argatroban just given the difficulties of managing its pharmacokinetics. But the MGH has published a series using a combination of iloprost and dipyridamole in the preoperative period to try and minimize the reactivity of the platelets. These patients then undergo their heart operation with a full dose of IV heparin and seem to do actually quite well.
Finally, to address Dr. Pruitt's point regarding peptic ulcer disease: all patients coming out of the operating room are treated with proton pump inhibitors. The 4 patients in this study all had a documented history of peptic ulcer disease.
Footnotes
Reprints: David L. Berger, MD, Division of General Surgery, Massachusetts General Hospital, 15 Parkman St., WAC465, Boston, MA 02114. E-mail: dberger@partners.org.
REFERENCES
- 1.Ott MJ, Buchman TG, Baumgartner WA. Postoperative abdominal complications in cardiopulmonary bypass patients: a case-controlled study. Ann Thorac Surg. 1995;59:1210–1213. [DOI] [PubMed] [Google Scholar]
- 2.Recht MH, Smith JM, Woods SE, et al. Predictors and outcomes of gastrointestinal complications in patients undergoing coronary artery bypass surgery: a prospective, nested case-control study. J Am Coll Surg. 2004;198:742–747. [DOI] [PubMed] [Google Scholar]
- 3.Perugini RA, Orr RK, Porter D, et al. Gastrointestinal complications after cardiopulmonary following cardiac surgery: an analysis of 1477 cardiac surgery patients. Arch Surg. 1997;132:352–357. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz AT, Arslan M, Demirkile U, et al. Gastrointestinal complications after cardiac surgery. Eur J Cardiothorac Surg. 1996;10:763–767. [DOI] [PubMed] [Google Scholar]
- 5.Tsiotos GG, Mullany CJ, Zietlow S, et al. Abdominal complications following cardiac surgery. Am J Surg. 1994;167:553–557. [DOI] [PubMed] [Google Scholar]
- 6.Mercado PD, Farid H, O'Connell T, et al. Gastrointestinal complication associated with cardiopulmonary bypass procedures. Am J Surg. 1994;60:789–792. [PubMed] [Google Scholar]
- 7.Huddy SPJ, Joyce WP, Pepper JR. Gastrointestinal complications in 4,473 patients who underwent cardiopulmonary bypass surgery. Br J Surg. 1991;73:293–296. [DOI] [PubMed] [Google Scholar]
- 8.Johnston G, Vitikainem K, Knight R, et al. Changing perspective on gastrointestinal complications in patients undergoing cardiac surgery. Am J Surg. 1992;163:529–536. [DOI] [PubMed] [Google Scholar]
- 9.Lawthorne TW, Davis JL, Smith GW. General surgical complications after cardiac surgery. Am J Surg. 1976;126:254–256. [DOI] [PubMed] [Google Scholar]
- 10.Hanks JB, Curtis SE, Hanks BB, et al. Gastrointestinal complications after cardiopulmonary bypass. Surgery. 1982;92:394–399. [PubMed] [Google Scholar]
- 11.Krasna MJ, Flancbaum L, Trooskin SZ, et al. Gastrointestinal complications after cardiac surgery. Surgery. 1988;104:773–780. [PubMed] [Google Scholar]
- 12.Welling RE, Rath R, Albers JE, et al. Gastrointestinal complications after cardiac surgery. Arch Surg. 1986;121:1178–1181. [DOI] [PubMed] [Google Scholar]
- 13.Ohri SK, Desai JB, Gaer JAR, et al. Intraabdominal complications after cardiopulmonary bypass. Ann Thorac Surg. 1991;52:826–831. [DOI] [PubMed] [Google Scholar]
- 14.Leitman IM, Paull DE, Barie PS, et al. Intra-abdominal complications of cardiopulmonary bypass operations. Surg Gynecol Obstet. 1987;165:251–254. [PubMed] [Google Scholar]
- 15.Pison CW, Albert RE. General surgical complications after cardiopulmonary bypass surgery. Am J Surg. 1983;146:133–137. [DOI] [PubMed] [Google Scholar]
- 16.Wallwork J, Davidson KG. The acute abdomen following cardiopulmonary bypass surgery. Br J Surg. 1980;67:410–412. [DOI] [PubMed] [Google Scholar]
- 17.Christenson JT, Schmuziger M, Maurice J, et al. Gastrointestinal complications after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1994;108:899–906. [PubMed] [Google Scholar]
- 18.Moneta GL, Misbach GA, Ivey TD. Hypoperfusion as a possible factor in the development of gastrointestinal complications after heart surgery. Am J Surg. 1985;149:648–650. [DOI] [PubMed] [Google Scholar]
- 19.Andersson B, Nilsson J, Brandt J, et al. Gastrointestinal complications after cardiac surgery. Br J Surg. 2005;92:326–333. [DOI] [PubMed] [Google Scholar]
- 20.Sakorafus GH, Tsiotos GG. Intra-abdominal complications after cardiac surgery. Eur J Surg. 1999;165:820–827. [DOI] [PubMed] [Google Scholar]
- 21.Ohri SK, Becket J, Brannan J, et al. Effects of cardiopulmonary bypass on gut blood flow, oxygen utilization, and intramucosal pH. Ann Thorac Surg. 1994;57:1193–1199. [DOI] [PubMed] [Google Scholar]
- 22.Tao W, Zwischenberger JB, Nguyen TT, et al. Gut mucosal ischemia during normothermic cardiopulmonary bypass results from blood flow redistribution and increased oxygen demand. J Thorac Cardiovasc Surg. 1995;110:819–828. [DOI] [PubMed] [Google Scholar]
- 23.Doguet F, Litzler PY, Tamion F, et al. Changes in mesenteric vascular reactivity and inflammatory response after cardiopulmonary bypass in a rat model. Ann Thorac Surg. 2004;77:2130–2137. [DOI] [PubMed] [Google Scholar]
- 24.Puskas JD, Williams WH, Duke PG, et al. Off pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements and length of stay: a prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:797–808. [DOI] [PubMed] [Google Scholar]
- 25.Musleh GS, Patel NC, Grayson AD, et al. Off-pump coronary artery bypass surgery does not reduce gastrointestinal complications. Eur J Cardiothorac Surg. 2003;23:170–174. [DOI] [PubMed] [Google Scholar]
- 26.Sanisoglu I, Guden M, Bayramoglu Z, et al. Does off-pump CABG reduce gastrointestinal complications? Ann Thorac Surg. 2004;77:619–625. [DOI] [PubMed] [Google Scholar]
- 27.Antoniou T, Kapetanakis EI, Theodoraki K, et al. Cardiac surgery in patients with heparin-induced thrombocytopenia using preoperatively determined dosages of iloprost. Heart Surg Forum. 2002;5:354–357. [PubMed] [Google Scholar]
- 28.Brodman R, Robinson G. Internal mammary-saphenous vein composite conduit: an alternative for the proximal coronary anastomosis. Ann Thorac Surg. 1981;31:370–372. [DOI] [PubMed] [Google Scholar]
- 29.Dewey TM, Crumrine K, Herbert MA, et al. First-year outcomes of beating heart coronary artery bypass grafting using proximal mechanical connectors. Ann Thorac Surg. 2004;77:1542–1549. [DOI] [PubMed] [Google Scholar]
- 30.Sack FU, Reidenbach B, Schledt A, et al. Dopexamine attenuates microvascular perfusion injury of the small bowel in pigs induced by extracorporeal circulation. Brg J Anaesth. 2002;88:841–847. [DOI] [PubMed] [Google Scholar]
- 31.Temmesfeld-Wollbruck B, Szalay A, Mayer K, et al. Abnormalities of gastric mucosal oxygenation in septic shock: partial responsiveness to dopexamine. Am J Respir Crit Care Med. 1998;157:1586–1592. [DOI] [PubMed] [Google Scholar]
- 32.Meier-Hellmann A, Bredle DL, Specht M, et al. Dopexamine increases splanchnic blood flow but decreases gastric mucosal pH in severe septic patients treated with dobutamine. Crit Care Med. 1999;27:2166–2171. [DOI] [PubMed] [Google Scholar]
- 33.Lisbon A. Dopexamine, dobutamine, and dopamine increase splanchnic blood flow: what is the evidence? Chest. 2003;123(suppl 5):460–463. [DOI] [PubMed] [Google Scholar]
- 34.Winter DC, O'Sullivan GC, Harvey BJ, et al. Direct effects of dopamine on colonic mucosal pH: implications for tonometry. J Surg Res. 1999;83:62–68. [DOI] [PubMed] [Google Scholar]
- 35.Clavien PA, Muller C, Harder F. Treatment of mesenteric infarction. Br J Surg. 1987;74:500–503. [DOI] [PubMed] [Google Scholar]
- 36.Flynn M, Reddy S, Shepherd W, et al. Fast-tracking revisited: routine cardiac surgical patients need minimal intensive care. Eur J Cardiothorac Surg. 2004;25:116–122. [DOI] [PubMed] [Google Scholar]
- 37.Fiddian-Green RG, Baker S. Predictive value of the stomach wall pH for complications after cardiac operations: comparison with other monitoring. Crit Care Med. 1987;15:153–156. [DOI] [PubMed] [Google Scholar]


