Abstract
Purpose:
Pheochromocytomas are relatively uncommon tumors whose operative resection has clear medical and technical challenges. While the safety and efficacy of laparoscopic adrenalectomy are relatively well documented, few studies with extended follow-up have been conducted to measure the success of the procedure for the most challenging of the adrenal tumors. In addition, several reports question the applicability of a minimally invasive approach for sizeable pheochromocytomas. The purpose of our investigation was to assess the outcomes of laparoscopic adrenalectomy for pheochromocytomas in the largest study to date when performed by experienced laparoscopic surgeons.
Methods:
All pheochromocytomas removed by the authors from January 1995 to October 2004 were reviewed under an Institutional Review Board approved protocol. Eighty-five percent were documented in a prospective fashion.
Results:
Eighty consecutive patients underwent laparoscopic resection of 81 pheochromocytomas. Seventy-nine were found in the adrenal (42 left, 35 right, 1 bilateral); 2 were extra-adrenal paragangliomas. Eight patients had multiple endocrine neoplasia syndrome. Two lesions were malignant. There were 48 females and 32 males with a mean age of 45 years (range, 15–79 years). Mean tumor size was 5.0 cm (range, 2–12.1 cm); 41 of these lesions were 5 cm in size or larger. Average operative time and blood loss were 169 minutes (range, 69–375 minutes) and 97 mL (range, 20–500 mL), respectively. Intraoperative hypertension (systolic blood pressure, >170 mm Hg) was reported in 53% of patients and hypotension (systolic blood pressure, <90 mm Hg) in 28% of patients. There were no conversions to open surgery. Mean length of stay was 2.3 days (range, 1–10 days). There were 6 perioperative morbidities (7.5%) and no mortalities. No patient required a blood transfusion. No recurrence of endocrinopathy has been documented at a mean follow-up of 21.4 months.
Conclusion:
Laparoscopic resection of pheochromocytomas, including large lesions, can be accomplished safely by experienced surgeons. A short hospital stay with minimal operative morbidity and eradication of endocrinopathy support the minimally invasive approach for adrenalectomy in the setting of pheochromocytoma.
Resection of pheochromocytomas remains a surgical and physiologic challenge. The purpose of our investigation was to assess the short- and long-term outcomes of laparoscopic adrenalectomy for pheochromocytomas in the largest study to date.
Since it was first performed in 1992,1 laparoscopy has clearly become the procedure of choice for the removal of most functioning and nonfunctioning adrenal tumors. Compared with those who underwent a standard open approach, patients undergoing a laparoscopic adrenalectomy have demonstrated decreased perioperative morbidity, shorter hospitalization, and faster functional recovery.2–5 Modern indications for a minimally invasive approach have been expanded to large tumors, bilateral pathology, and metastatic malignancies.6–10
Despite early concerns about perioperative cardiovascular complications related to pneumoperitoneum, organ manipulation, and dissection, several isolated reports and small series have shown the laparoscopic approach to be safe.11–13 The feasibility of laparoscopic adrenalectomy for pheochromocytoma has been established with careful attention to proper preoperative alpha-adrenergic blockade and intraoperative management. However, when compared with other adrenal pathologies, a minimally invasive adrenalectomy for pheochromocytoma, even in experienced hands, may be associated with longer operative times, increased complications, and longer hospitalization.14 Our study presents a large multicenter experience with laparoscopic adrenalectomy for pheochromocytoma.
METHODS
After Institutional Review Board approval, an analysis of consecutive laparoscopic adrenalectomies for pheochromocytoma from January 1995 to October 2004 performed by the attending surgeons in this study was completed. Eighty-five percent of the cases were prospectively documented. Patient demographics, clinical presentation, imaging and biochemical evaluation, operative findings and intervention, intraoperative hemodynamic parameters, outcomes, and final pathology were recorded. All operations were performed in tertiary care hospitals by experienced laparoscopic surgeons. Data are expressed as mean ± SD unless otherwise specified. Student t test was used for normally distributed data. Not normally distributed data were analyzed using a Kruskal-Wallis and Wilcoxon rank sum tests. Spearman correlation was used for the analysis of continuous variables between the groups. P ≤ 0.05 was considered significant.
Preoperative Care
In all patients with preoperative signs and symptoms of catecholamine excess, alpha-adrenergic blockade was initiated at least 10 days prior to surgery. For patients with a coexisting tachycardia, beta-blockade was added. Patients with alpha blockade-induced orthostatic hypotension were treated with oral and/or intravenous volume loading during the 24 to 48 hours prior to surgery. Patients were infused with 1 to 2 L of crystalloid solution for intravascular volume expansion in the preoperative holding area. All patients had an arterial line and 2 large-bore peripheral intravenous lines or a central venous line placed prior to the induction of general anesthesia.
Surgical Technique
The adrenalectomies were performed laparoscopically through a lateral decubitus transperitoneal approach as previously described.6,36 Briefly, a diagnostic laparoscopy was performed at the beginning of each procedure to rule out local tumor invasion or diffuse metastatic spread. Three subcostal ports (5–12 mm) allowed for the introduction of a 30° or 45° laparoscope and 2 working instruments. During right adrenalectomies, a fourth 5-mm port was placed in a subxyphoid position for liver retraction. Occasionally during left adrenalectomies, a fourth port was added below the tip of the left twelfth rib to provide blunt retraction of the kidney and/or adrenal gland. This technique was particularly useful for larger tumors, which often encroached upon the vascular hilum of the kidney, making exposure of the adrenal vein difficult. Early ligation and division of the adrenal vein was carried out prior to gland manipulation and dissection when possible.
For right adrenalectomies, the right hepatic lobe was completely mobilized to provide adequate visualization and safe access to the vena cava and adrenal vein. The triangular ligament was incised to the level of the diaphragm. The retroperitoneum was then opened longitudinally along the medial aspect of the adrenal gland, and immediately adjacent to the lateral edge of the liver, until the vena cava was clearly identified. Development of the plane between the inferior vena cava and the medial margin of the gland was performed to expose the right adrenal vein. Early dissection and mobilization of the inferior retroperitoneal attachments to the tumor increased gland mobility and made venous control considerably safer.
On the left, the splenic flexure was mobilized to allow access to the splenorenal ligament. The retroperitoneal plane superficial to Gerota fascia was developed to the level of the diaphragm, allowing for medial rotation of the spleen and the pancreatic tail. A complete medial rotation of adjacent structures was critical to provide adequate exposure of the adrenal gland and vein. Gerota fascia was incised medial to the superior pole of the kidney to provide access to the left adrenal vein and the adrenal gland. The vein was then ligated and divided at its confluence with the left renal vein.
On either side, the borders of the adrenal gland were first identified and then dissected away from the retroperitoneum, using periadrenal fat as a “handle.” The larger glands, especially those greater than 5 cm, were most often resected with periadrenal fat, exposing the psoas muscle from the renal hilum cephalad to the diaphragm. The gland was never grasped to avoid hemodynamic liability, troublesome bleeding, or tumor disruption. Large adrenal veins, typically those greater than 7 mm in width, were divided with an endovascular stapler. Specimens were placed into an impervious extraction bag prior to morcellation (if necessary) and removal. The peritoneum and fascia at the trocar sites were closed endoscopically. Drains were not used.
Postoperative Care
Immediate postoperative monitoring was performed in the recovery room and then on a surgical ward or in the intensive care unit at the attending surgeon's discretion. Postoperative hypotension was treated with crystalloid fluid challenge. Nasogastric tubes were not used routinely. Clear liquids were ordered on the night of surgery. Diets were liberalized the following morning. Intramuscular narcotics or patient-controlled narcotic pumps were used for analgesia in the immediate postoperative period. Patients were discharged home after ambulating, tolerating a regular diet, and adequate pain control was established. Follow-up consisted of an office visit at 7 to 10 days and another at 3 to 4 weeks postoperatively, and subsequently as needed. Long-term follow-up included frequent blood pressure monitoring for the first year, then yearly thereafter. Urinary metanephrine levels are followed annually for a period of 5 years. Follow-up abdominal imaging was obtained only in those patients with recurrent hypertension, elevated metanephrines, malignant lesions, or paragangliomas.
RESULTS
Diagnosis
The preoperative diagnosis of pheochromocytoma was based upon clinical, biochemical, and radiographic evaluation. Sixty-seven of 80 patients presented with hypertension. The presence of a pheochromocytoma was documented with a CT scan or MRI and elevated urinary catecholamine or metanephrine levels in each of those patients. An MRI, MIBG scan, and/or PET scan was performed to further document the presence or absence of local spread or distant metastasis.
Two patients had paragangliomas in the organ of Zuckercandl. Interestingly, one had complete situs inversus. Both presented with hypertension. Their radiographic workup revealed no evidence of malignancy. Prior to surgical consultation or complete biochemical workup, 3 patients underwent percutaneous CT-guided needle biopsy of their adrenal tumor. Biopsy demonstrated a pheochromocytoma. None of the patients experienced a hypertensive crisis. Another patient presented with a large adrenal mass in association with an ipsilateral renal cell carcinoma. Workup of the adrenal lesion revealed a pheochromocytoma in the setting of a von Hippel-Lindau syndrome. Both organs were resected en bloc with a hand-assisted technique.
Eight patients were diagnosed with multiple endocrine neoplasia (MEN). One 15-year-old patient had a family history of MEN and had a positive urinary biochemical screen. Abdominal CT scan revealed bilateral adrenal masses. A second patient with known MEN had a right adrenal pheochromocytoma resected 28 years earlier. During routine testing, his urinary metanephrines were mildly elevated. An MRI revealed a 2.3-cm left adrenal mass. Two patients initially presented with medullary thyroid cancer. In each, a preoperative urinary biochemical analysis revealed the presence of pheochromocytoma. Both patients underwent an uneventful laparoscopic adrenalectomy prior to thyroidectomy. Four hypertensive patients were diagnosed with a pheochromocytoma and sporadic MEN.
In 2 cases, adrenalectomy was performed for incidentally discovered adrenal masses in normotensive patients with normal preoperative biochemical screening. In both cases, intraoperative hypertension was noted and controlled with an intravenous alpha-blockade. The hypertension and operative findings of a large hypervascular adrenal tumor led to a presumptive clinical diagnosis of pheochromocytoma. A subsequent pathologic evaluation confirmed the diagnosis of pheochromocytoma in both cases.
Outcomes
From January 1995 to October 2004, 80 consecutive patients underwent laparoscopic transperitoneal resection of 81 pheochromocytomas; 79 were adrenal tumors and 2 were extra-adrenal paragangliomas. Forty-two lesions were located on the left, 35 were on the right, and bilateral tumors were found in 1 patient. The paragangliomas were para-aortic in location. There were 48 females and 32 males. The average age was 45 years (range, 15–79 years), and the mean body mass index was 27.5 kg/m2 (range, 20.4–56.5 kg/m2). The average size of the adrenal masses was 5.0 cm (range, 2.0–12.1 cm). Histologic evaluation revealed a pheochromocytoma in all cases. Two lesions were confirmed as malignant on permanent pathology; one had extracapsular invasion, and the second had vascular invasion. In both operations, a complete resection of the gland and the periadrenal fat from the kidney to the diaphragm was performed.
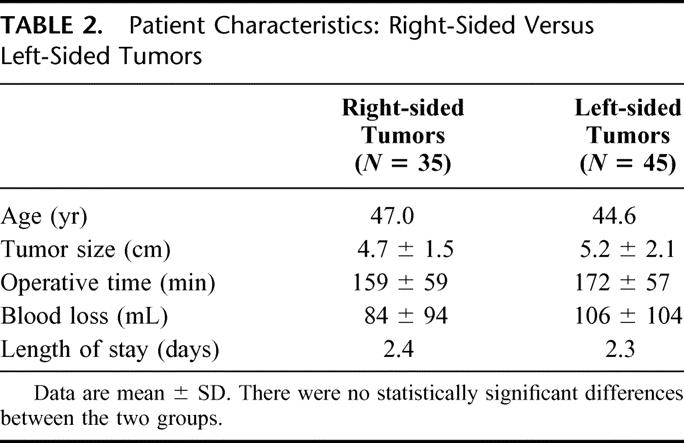
The average operating time was 169 minutes (range, 70–375 minutes). Larger tumors required significantly longer operative times (Table 1). The one bilateral adrenalectomy in this series required 346 minutes for surgery. The average blood loss was 97 mL (range, 20–500 mL). There were no intraoperative or postoperative blood transfusions, and no patient required conversion to open or hand-assisted surgery. When analyzed separately, right and left adrenalectomies resulted in similar outcomes (Table 2). In addition, patients’ age or body mass index had no affect on any of the perioperative outcomes.
TABLE 1. Patient Characteristics: Tumor Size ≤6 cm Versus >6 cm
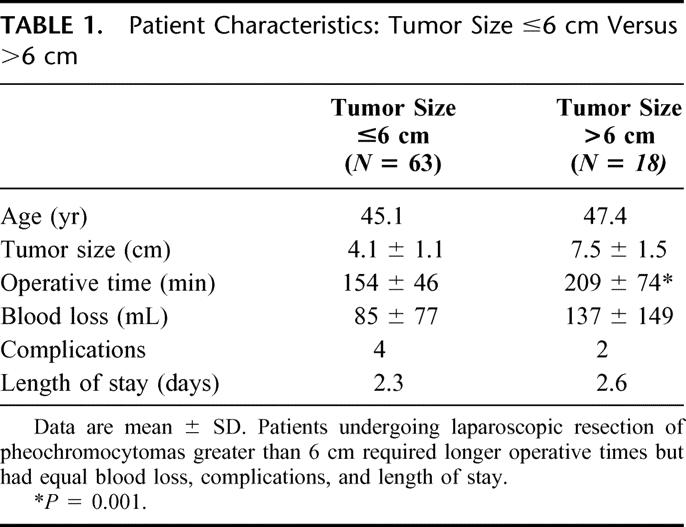
TABLE 2. Patient Characteristics: Right-Sided Versus Left-Sided Tumors
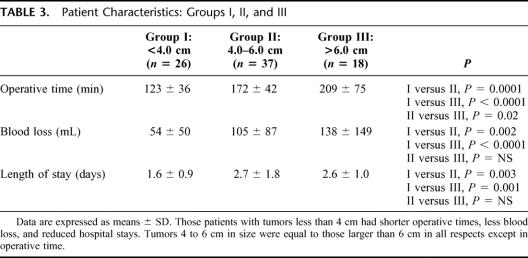
Univariate analysis demonstrated a positive correlation between tumor sizes and operative time and intraoperative blood loss (P < 0.0001 and P < 0.001, respectively; Spearman's correlation). Subgroup analysis for tumors smaller or larger than 6 cm is summarized in Table 1. Although operative times were significantly longer for the larger tumors, intraoperative blood loss, hemodynamic variability, and perioperative morbidity were similar between the 2 groups. Interestingly, if the lesion size were further divided, it appears that the break point for differences in operating room time, blood loss, and length of stay was 4 cm (Table 3). Those patients with lesions less than 4 cm had an advantageous outcome when considering perioperative outcomes. Patients with tumors between 4 and 6 cm and those with tumors greater than 6 cm were equal except in operative time.
TABLE 3. Patient Characteristics: Groups I, II, and III
Intraoperative hypertension (systolic blood pressure SBP greater than 170 mm Hg) occurred at some point in the operation in 42 (53%) patients. All were controlled with intravenous vasodilators and transient cessation of tumor manipulation. Intraoperative hypotension (systolic blood pressure less than 90 mm Hg) occurred in 22 (28%) patients. Each of these patients responded to volume infusion and/or infusion of vasopressor medication. After an uneventful adrenalectomy, 2 patients underwent concomitant laparoscopic procedures, a cholecystectomy and small ventral hernia repair.
In the postoperative period, the majority of patients were observed in the recovery room and then transferred to the floor or the an intensive care setting for observation and monitoring. The average length of hospital stay was 2.3 days (range, 1–10 days). Sixty-eight percent of the patients were discharged to home by 48 hours postoperatively (Fig. 1).
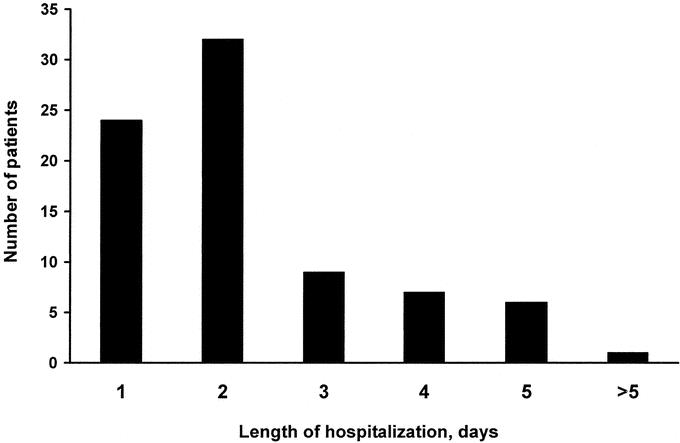
FIGURE 1. Length of hospital stay. Fifty-five (68%) patients were discharged home within the first two postoperative days.
Complications
There were no major intraoperative complications and no conversions to open adrenalectomy. There were 6 (7.5%) postoperative complications. One patient each developed wound cellulitis and a urinary tract infection, both of which responded to oral antibiotics. One patient developed a significant ileus requiring a nasogastric tube. Another had postoperative hypotension that responded to aggressive volume expansion; the patient's hemoglobin remained stable, and a bedside ultrasound revealed no sign of bleeding. One patient's recovery was complicated by a rapid and severe onset of pseudomembranous colitis necessitating oral antibiotic therapy and a prolonged hospital stay of 10 days, which was the longest in the series. There was one readmission within 30 days of surgery for a patient who presented 8 days postoperatively with a lower extremity deep venous thrombosis and micropulmonary emboli. There were no blood transfusions and no perioperative mortalities.
Serum and/or urinary metanephrine levels were followed annually. At a mean follow-up of 21.4 months (range, 1–77 months), there have been no recurrences of endocrinopathy. Nine patients, including those with paragangliomas (n= 2), malignant pheochromocytomas (n = 2), and renal cell carcinoma (n = 1), have undergone reimaging with MRI, CT scan, or an MIBG scan. To date, all biochemical evaluation and repeat imaging studies of the abdomen have been negative for tumor recurrence.
DISCUSSION
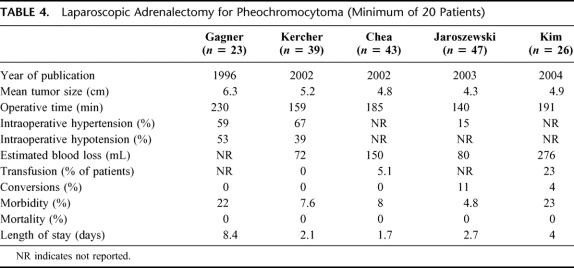
The safety and efficacy of the laparoscopic adrernalectomy are well established.2,15,16 Because of the rarity of pheochromocytomas and the relative youth of laparoscopic adrenalectomy, few large series with long-term follow-up have been conducted to measure the success of this minimally invasive procedure (Table 4). In this report, we present the largest series to date of consecutive laparoscopic adrenalectomies for pheochromocytoma. Our findings of minimal perioperative morbidity, no mortalities, and no recurrent endocrinopathies combined with the established benefits of minimal access surgery appear to validate laparoscopy as the procedure of choice for pheochromocytomas.
TABLE 4. Laparoscopic Adrenalectomy for Pheochromocytoma (Minimum of 20 Patients)
Surgical resection offers the only cure for pheochromocytomas, a disease that has challenged surgeons since the beginning of the 20th century. In 1926, Charles Mayo and Cesar Roux were the first to report a successful surgical resection of pheochromocytoma.17 Initially, perioperative mortality rates were as high as 50%.18 For decades, despite the improvements in perioperative medical management, anesthesia, and surgical techniques, adrenalectomy for pheochromocytomas carried morbidity rates as high as 40% and perioperative mortality rates of 2% to 4%.19 In addition to the catecholamine-producing nature of these tumors, these typical difficulties were likely confounded by the large midline or thoracoabdominal incisions used to resect the adrenal gland.20 As a result, with the advancement of minimally invasive techniques, laparoscopic adrenalectomy, with all of the potential benefits of minimal access techniques, evolved as the next viable surgical approach to pheochromocytomas.
The feasibility of laparoscopic adrenalectomy for pheochromocytomas was established in several small series, which emerged shortly after laparoscopic adrenalectomy was described in 1992.1,21–24 Yet fears of cardiovascular instability due of excessive catecholamine release caused by the pneumoperitoneum and/or laparoscopic dissection have fueled concerns over the role of laparoscopy in pheochromocytoma resections. In 1996, Gagner et al14 reported their experience with 23 laparoscopic adrenalectomies for pheochromocytoma. Intraoperative hypertension (SBP > 200 mm Hg) occurred in 58% of patients, with hypotension (SBP < 80 mm Hg) occurring in 53% of cases. Although seemingly high, those rates compared favorably with a 75% rate of hypertensive episodes in historical open controls.25 Similarly, when measured directly, catecholamine levels are significantly higher during open resections as well.13,26 Using slightly more stringent criteria for hemodynamic variability (SBP > 170 mm Hg or <90 mm Hg), our group has previously reported transient intraoperative hypertension and hypotension rates of 67% and 39%, respectively.27 Since our initial report, we have noted a slight decrease in the incidence of intraoperative hypertension and hypotension, with the current series demonstrating a low rate of intraoperative hemodynamic fluctuations without any adverse effects.
Despite appropriate preoperative medical management, intraoperative hypertensive is common and remains a valid concern.28 Continuous invasive monitoring and pharmacologic intervention by an experienced anesthesia team are necessary to avoid substantial cardiovascular instability. At the same time, the surgeon must avoid excessive tumor manipulation, which can result in catecholamine surges. Indeed, tumor manipulation has been shown to be the most significant intraoperative stimulus for catecholamine release during both open and laparoscopic adrenal resections.26,29–31 Clinically, sudden increases in plasma catecholamine levels can result in episodes of intraoperative hemodynamic instability.26,29,31,32 Fernandez-Cruz et al13 demonstrated that mean plasma norepinephrine and epinephrine increased 13.7- and 34.2-fold during open tumor manipulation. On the other hand, laparoscopic tumor manipulation was associated with a significantly diminished increase in plasma catecholamine levels (norepinephrine, 8.6-fold; epinephrine, 17.4-fold). Interestingly, Rocha et al26 also reported that such hormonal release occurs despite an early adrenal vein ligation, likely due to the extensive vascularity of pheochromocytomas. Although we did not measure intraoperative serum catecholamine levels, we think that the strategy of early venous control is important in decreasing hormonal surges. Moreover, careful adrenal dissection, using periadrenal fat as a handle, with minimization of direct manipulation or compression of the gland itself, is critical to avoid catecholamine-induced cardiovascular instability.
Intra-abdominal insufflation during laparoscopic pheochromocytoma excision may alone cause an increase in serum catecholamines.26,33 This stimulus may be via either a direct tumor compression or a change in tumor perfusion. In addition, pneumoperitoneum with CO2 may lead to hypercapnia and acidosis, which, in turn, are known stimuli of catecholamine secretion and hypertension.26,34 Rocha et al found a more than 10-fold elevation in catecholamines during abdominal insufflation to 12 mm Hg with CO2, with about 50% of patients experiencing hypertensive episodes.26 As a result, helium has been suggested as an alternate insufflation agent to eliminate the deleterious effects of CO2 during laparoscopic adrenalectomies for pheochromocytoma. In a prospective evaluation of 11 patients undergoing helium insufflation during laparoscopic pheochromocytoma resection, the authors demonstrated that its use avoided significant intraoperative hypercarbia or acidosis and provided greater intraoperative hemodynamic stability.29 Interestingly, though, there were no differences between the CO2 and the helium insufflation groups in either serum catecholamine surges or overall surgical outcomes.29 Based upon their data, the authors proposed helium as the insufflation agent of choice for laparoscopic adrenalectomy for pheochromocytoma patients.29 In our series, helium was not used due to its inferior overall safety profile when compared with CO2. Despite this, our patients did not experience any major intraoperative hemodynamic difficulties, likely due to careful preoperative medical management, skillful intraoperative anesthesia, and minimal adrenal gland manipulation.
In addition to the problems associated with catecholamine liberation, increased size and prominent vascularity add to the potential difficulty of pheochromocytoma removal. Similar to open resections, the laparoscopic approach to these tumors has traditionally been associated with higher complication rates than adrenalectomy for other indications.35 When compared with other indications for adrenalectomy, laparoscopic resection of pheochromocytomas results in longer operative times, higher complication rates, and longer hospitalization. Gagner et al reported in their series of 100 laparoscopic adrenal surgeries that 87% of intraoperative complications occurred in 23 pheochromocytoma patients.36 Recently, several experienced investigators have reported intraoperative complication rates ranging from 7% to 22%.11,15,27,35,37 With growing experience using advanced laparoscopic techniques, conversion rates have decreased from 22% to 0%–4%.11,27,35 Kim et al35 reported perioperative blood transfusions rates of 23%. Our results compare favorably to those series with an overall complication rate of 7.5%, no perioperative transfusions, and no conversions to laparotomy.
As has been often considered, the “learning curve” may play a significant role in improving the efficiency and safety of advanced laparoscopic procedures. That may be especially true for laparoscopic pheochromocytoma resections. The challenges of this procedure mandate the need for a sufficient experience in advanced laparoscopy in general and laparoscopic adrenalectomy, in particular, before embarking on laparoscopic adrenalectomy for pheochromocytoma. The learning curve for this operation would correspond with understanding the appropriate number and configuration of trocars, sequence of dissection, avoiding violation of unnecessary planes, preventing and recovery from bleeding, and recognition of the true target anatomy. Our low complication and conversion rates in this series are likely reflective of the authors’ experience with advanced laparoscopic surgery, laparoscopic adrenalectomy, and management of patients with pheochromocytoma.
Pheochromocytomas have a malignant potential and are frequently larger in size than other functional adrenal tumors. Both issues have also been proposed as a potential contraindication to laparoscopic resection. However, the true malignant potential of pheochromocytomas is very difficult to predict.38 Common histologic features, such as capsular invasion, vascular and lymphatic penetration, nuclear atypia, and mitotic activity, which indicate malignancy in other tumors, do not, however, necessarily indicate malignancy in pheochromocytomas,18,39 nor does the lack of these pathologic features dictate that a tumor is benign. Thus, microscopic features have a limited value in predicting the future biologic behavior of these lesions. Based on 48 years of clinical experience with pheochromocytomas, Goldstein et al39 reported that a benign-appearing lesion will ultimately prove to be malignant in 8% to 9% of cases. Similarly, recurrence rates following open resections have been reported to be about 6% to 8%.39,40 Of note, long disease-free intervals are typical of pheochromocytoma with recurrences often presenting several years to decades after initial surgery.39 It thus appears that individual tumor biology rather than the surgical approach may determine the chances of cure. Nonetheless, extreme care must be exercised to avoid intraoperative capsular disruptions and possible iatrogenic pheochromocytomatosis. Li et al41 reported 3 cases of pheochromocytoma recurrence 3 to 4 years after initial laparoscopic resection and possible tumor spillage. Two of the 3 cases were performed in community hospitals, and in each case the operation was marked by excessive tumor manipulation and/or disruption. We have observed no cases of recurrent endocrinopathy, at a mean follow-up of 21 months. Regardless of the early success of the laparoscopic approach, the known risk of pheochromocytoma recurrence remains, and lifetime follow-up is an important aspect of the postoperative care of the pheochromocytoma patient.
Large pheochromocytomas may have a slightly higher malignant potential.42 In addition, larger tumors may be more difficult to safely resect laparoscopically. As a result, many investigators have suggested that laparoscopy be avoided for pheochromocytomas larger that 7 to 8 cm.11,28,43 Our study indicates that there are no absolute contraindications based upon tumor size; indeed, 12 patients had tumors measuring at least 7 cm with the largest being 12.1 cm. What has been traditionally thought of as large tumors, lesions greater than 6 cm, were associated with longer operative times, but intraoperative blood loss, perioperative morbidity, and length of stay were not significantly different. When lesion size was further divided, it does appear that the break point for differences in operating room time, blood loss, and hospital stay was 4 cm. We have not identified a higher incidence of intraoperative hypertension with larger tumors, although surgical manipulation of these lesions, whether open or laparoscopic, would appear to be inherently more difficult. Equally importantly are anatomic considerations; larger tumors will frequently encroach upon the renal vessels. These structures must be carefully protected to avoid inadvertent injury during dissection. Given the authors’ extensive experience with nephrectomy, including operations for renal cell carcinoma, the need to operate around the renal hilum or to perform an en bloc resection of the periadrenal fat does not mandate a conversion to laparotomy. Conversion to an open procedure is warranted, however, when laparoscopic dissection cannot be performed safely or a complete resection cannot be performed without undue trauma to the gland. In the setting of pheochromocytoma, this determination must be based upon intraoperative findings of tumor invasion into adjacent structures as preoperative radiographic evaluation and histologic information, including intraoperative frozen section examination, are unreliable predictors of malignancy.
One of the greatest concerns surrounding laparoscopic adrenalectomy for pheochromocytoma is not whether the procedure should be done, but by whom should it be performed. Given the relatively low number of operations performed, the obvious potential for morbidity, and the recurrence risks if the gland is inappropriately handled, should laparoscopic adrenalectomy for pheochromocytoma be performed in low-volume centers or by surgeons with limited experience? Specific criteria that might be used to answer to this question have yet to be developed. However, a surgeon who is very proficient laparoscopically and significantly knowledgeable about adrenal anatomy may be able to perform this operation in a hospital that offers an appropriate level of anesthesia and ICU care. Those surgeons with nominal familiarity with either the surgical approach to laparoscopic adrenalectomy or the disease process should probably refer the rare patient with a pheochromocytoma to a regional tertiary center for definitive surgical therapy.
CONCLUSION
Although it is known that laparoscopic resection of pheochromocytomas can be performed safely with a short hospital stay and few complications, minimally invasive adrenalectomy for large pheochromocytoma has historically been controversial. This study demonstrates that sizeable lesions can be reliably removed by experienced surgeons. Lesions larger than 6 cm are associated with longer operative times than smaller lesions, but they are not associated with greater blood loss, higher rates of intraoperative hemodynamic instability, or longer hospital stay. Minimal perioperative morbidity and long-term eradication of endocrinopathy support a minimally invasive approach for adrenalectomy in the setting of pheochromocytoma.
Discussions
Dr. Kelly M. McMasters (Louisville, Kentucky): We have certainly come a long way from the old adage that when operating on a patient with pheochromocytoma, “one should dissect the patient away from the tumor.” The authors of this study demonstrate very elegantly that resection of pheochromocytomas can be performed safely and efficaciously using a transabdominal laparoscopic technique. It is impressive that pheochromocytomas in 80 consecutive patients could be resected using this laparoscopic technique and that tumor size ranged up to 12 cm. The morbidity was minimal, and there was no mortality. The authors are to be commended for these outstanding results.
I have a few comments and questions. First, Dr. Heniford, you alluded to a patient who had the procedure performed with the hand-assisted technique. When, if ever, is this helpful?
As you point out, there have been several studies demonstrating that larger pheochromocytomas are more likely to be malignant than smaller lesions. This concern becomes more heightened when tumor diameter exceeds 5 cm. Should the laparoscopic technique be changed for these larger lesions? Should a “wider” resection that includes surrounding adipose tissue be employed?
You mention that 2 tumors were confirmed to be malignant on permanent pathology, based on extracapsular invasion and vascular invasion. However, as you also state, Dr. Scott (now deceased) and Dr. Goldstein, both members of this Association, have written about failure of these local features to predict malignancy. Would you comment on the assertion of malignancy for these neoplasms?
Lastly, you are a very experienced laparoscopic surgeon. Laparoscopic adrenalectomy is generally considered an advanced laparoscopic case; resecting pheochromocytomas larger than 5 to 6 cm may be even more daunting. Should laparoscopic surgeons with average skill tackle pheochromocytomas? Or should they only tackle the smaller ones? When should these patients be referred to tertiary centers?
A real danger of this procedure is in tumor spillage. A surgeon trying too hard to resect a large pheochromocytoma laparoscopically and causing capsular disruption leading to peritoneal seeding with pheochromocytoma has not done any great service to his or her patient.
Clearly, however, in the hands of surgeons with particular expertise, laparoscopic adrenalectomy is likely to be as safe or safer than open techniques and results in much less morbidity.
Dr. Jeffrey S. Young (Charlottesville, Virginia): This is an excellent presentation. I just have 2 questions for the authors.
What characteristics either found preoperatively or intraoperatively would entertain the suggestion of opening the case? You had excellent experience here in not having to open any of these. But through that experience, hopefully you have been able to find some characteristics that would help others look at these cases and say perhaps either a quick conversion to an open procedure, or during the case what factors with anatomy would warrant an open procedure.
The second question is: in the 80 cases over 10 years in 4 centers, what was the greatest experience by any of the surgeons involved in the number of cases they did over that time? What was the least experience? And was there any difference in the operative time, blood loss, or length of stay between those surgeons that have performed the most cases and those that performed the least?
Dr. Martin J. Heslin (Birmingham, Alabama): I enjoyed your paper. It was an impressive series. I was wondering, as you developed prognostic factors for the laparoscopic approach, if there were cases that were not done laparoscopically at all these institutions to develop a true denominator of cases? This might further evaluate the true prognostic factors for a laparoscopic case versus an open case.
Dr. Gerard M. Doherty (Ann Arbor, Michigan): I have a similar question, but about the preoperative imaging. This series has just 2 of 80 patients who have malignancy. In the experience of most of us, the rate is somewhere between 10% and 20%, although this may be affected by referral bias. Clearly, there have been some selections. What was the preoperative imaging regimen for these patients; in particular, were the patients with larger tumors studied with MIBG or PET scans to exclude them from this study?
Dr. B. Todd Heniford (Charlotte, North Carolina): Thank you very much for the questions and the comments.
Dr. McMasters, you asked about a hand-assisted technique. There was only one case in this group that was performed hand-assisted. This patient had a concomitant renal cell carcinoma. We have a large series of nephrectomies for malignancy, and, in fact, the pheochromocytoma was found incidentally in a patient with blood in her urine. We perform the majority of these cases hand-assisted. Other lesions that might benefit from a hand-assisted technique might indeed be large lesions, such as the one that was 12 centimeters in our series, or in patients who are morbidly obese. In these situations, you might find placing your hand inside the abdomen helpful. Additionally, if you begin to have bleeding, placing your hand inside to control that or even pack it away while you proceed with other areas of dissection might be helpful.
You mentioned laparoscopic resection of lesions 5 centimeters in size or larger and if we have changed our technique. Indeed we have. We do not perform target organ resection for these lesions. We usually begin at the kidney and renal vein and roll Gerota’s facia and fat up toward the diagram, as you saw in the operative description. We do this both on the left side and the right side. I think our experience performing nephrectomies is extremely helpful. All of us have performed nephrectomy, and the majority of surgeons in this group perform transplant nephrectomy, so we know the anatomy and dissection techniques very well.
I was asked to comment on the assertion of malignancy and pheochromocytomas. The gross description or gross look at pheochromocytomas can be misleading; benign lesions can penetrate the capsule and can have vascular invasion and still absolutely be benign. Malignant lesions can have no penetration of the capsule and no vascular invasion and be malignant with metastases at the time of surgery or discovered later. Only confirmation of malignancy, such as local invasion, involvement of lymph nodes, or distant metastasis, can truly delineate whether a lesion is a cancerous one.
Should surgeons of average skill tackle pheochromocytomas? Indeed, I think that question is an excellent one. There have been no specific criteria developed to answer this question. However, surgeons that are proficient laparoscopically, work with two hands, are significantly knowledgeable about adrenal anatomy, and have ICU and appropriate Anesthesia skill available should be able to perform the operation. Those surgeons who are not familiar with adrenal anatomy who have limited access to performing adrenal surgery in general should probably refer pheochromocytomas to a tertiary center.
You mentioned tumor spillage. I think tumor spillage is a great concern in any endocrine surgery. Three years ago Dr. Norton’s group reported a small series of patients with pheochromocytomatosis in the operative field. Interestingly, the original surgeons’ operative notes describe their having had a difficult time resecting these lesions and subsequently had tumor spillage. If a surgeon is concerned that he or she is going to have tumor spillage, that should be an indication to convert a patient to open.
Concerning the questions regarding the preoperative and intraoperative characteristics which would lead one to do the case open and the possible selection bias that we may have experienced, I have answered a portion of these questions previously. I can not confirm that we had patients pre-selected for us by referring doctors, but there may have been some selection bias. Many of these patients came with imaging in hand when they were sent to our clinics. So indeed, perhaps the lesions that were malignant were not actually sent to us and may have been sent to our surgical oncology colleagues. There was a question concerning the greatest experience in the series. And that would have been mine. There was no difference in my outcomes compared to the other authors.
Concerning preoperative imaging, most of these patients had a CAT scan prior to having an MRI. MIBG scanning was used much less frequently. PET scanning was used in many of the larger lesions, but I do not have those numbers. Thank you for the opportunity to present to such a prestigious group.
Footnotes
Reprints: B. Todd Heniford, MD, Department of Surgery, Carolinas Medical Center, P.O. Box 32861, Charlotte, NC 28232. E-mail: Theniford@carolinas.org.
REFERENCES
- 1.Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med. 1992;327:1033. [DOI] [PubMed] [Google Scholar]
- 2.Brunt LM. The positive impact of laparoscopic adrenalectomy on complications of adrenal surgery. Surg Endosc. 2002;16:252–257. [DOI] [PubMed] [Google Scholar]
- 3.Brunt LM, Doherty GM, Norton JA, et al. Laparoscopic adrenalectomy compared to open adrenalectomy for benign adrenal neoplasms. J Am Coll Surg. 1996;183:1–10. [PubMed] [Google Scholar]
- 4.Imai T, Kikumori T, Ohiwa M, et al. A case-controlled study of laparoscopic compared with open lateral adrenalectomy. Am J Surg. 1999;178:50–53; discussion 54. [DOI] [PubMed]
- 5.Jacobs JK, Goldstein RE, Geer RJ. Laparoscopic adrenalectomy: a new standard of care. Ann Surg. 1997;225:495–501; discussion 501–502. [DOI] [PMC free article] [PubMed]
- 6.Heniford BT, Arca MJ, Walsh RM, Gill IS. Laparoscopic adrenalectomy for cancer. Semin Surg Oncol. 1999;16:293–306. [DOI] [PubMed] [Google Scholar]
- 7.Kebebew E, Siperstein AE, Clark OH, et al. Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal neoplasms. Arch Surg. 2002;137:948–951; discussion 952–953. [DOI] [PubMed]
- 8.Novitsky YW, Czerniach DR, Kercher KW, et al. Feasibility of laparoscopic adrenalectomy for large adrenal masses. Surg Laparosc Endosc Percutan Tech. 2003;13:106–110. [DOI] [PubMed] [Google Scholar]
- 9.Hasan R, Harold KL, Matthews BD, et al. Outcomes for laparoscopic bilateral adrenalectomy. J Laparoendosc Adv Surg Tech A. 2002;12:233–236. [DOI] [PubMed] [Google Scholar]
- 10.Imai T, Kikumori T, Shibata A, et al. Laparoscopic adrenalectomy for incidentaloma and bilateral adrenal disease. Asian J Surg. 2003;26:64–70. [DOI] [PubMed] [Google Scholar]
- 11.Cheah WK, Clark OH, Horn JK, et al. Laparoscopic adrenalectomy for pheochromocytoma. World J Surg. 2002;26:1048–1051. [DOI] [PubMed] [Google Scholar]
- 12.Mobius E, Nies C, Rothmund M. Surgical treatment of pheochromocytomas: laparoscopic or conventional? Surg Endosc. 1999;13:35–39. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Cruz L, Taura P, Saenz A, et al. Laparoscopic approach to pheochromocytoma: hemodynamic changes and catecholamine secretion. World J Surg. 1996;20:762–768; discussion 768. [DOI] [PubMed]
- 14.Gagner M, Breton G, Pharand D, et al. Is laparoscopic adrenalectomy indicated for pheochromocytomas? Surgery. 1996;120:1076–1079; discussion 1079–1080. [DOI] [PubMed]
- 15.Gonzalez R, Smith CD, McClusky DA 3rd, et al. Laparoscopic approach reduces likelihood of perioperative complications in patients undergoing adrenalectomy. Am Surg. 2004;70:668–674. [PubMed] [Google Scholar]
- 16.Smith CD, Weber CJ, Amerson JR. Laparoscopic adrenalectomy: new gold standard. World J Surg. 1999;23:389–396. [DOI] [PubMed] [Google Scholar]
- 17.Welbourn RB. Early surgical history of phaeochromocytoma. Br J Surg. 1987;74:594–596. [DOI] [PubMed] [Google Scholar]
- 18.Niemann U, Hiller W, Behrend M. 25 years experience of the surgical treatment of phaeochromocytoma. Eur J Surg. 2002;168:716–719. [DOI] [PubMed] [Google Scholar]
- 19.Werbel SS, Ober KP. Pheochromocytoma: update on diagnosis, localization, and management. Med Clin North Am. 1995;79:131–153. [DOI] [PubMed] [Google Scholar]
- 20.Scott HW Jr, Dean RH, Oates JA, et al. Surgical management of pheochromocytoma. Am Surg. 1981;47:8–13. [PubMed] [Google Scholar]
- 21.Stoker ME, Patwardhan N, Maini BS. Laparoscopic adrenal surgery. Surg Endosc. 1995;9:387–390; discussion 391. [DOI] [PubMed]
- 22.Suzuki K, Kageyama S, Ueda D, et al. Laparoscopic adrenalectomy: clinical experience with 12 cases. J Urol. 1993;150:1099–1102. [DOI] [PubMed] [Google Scholar]
- 23.Meurisse M, Joris J, Hamoir E, et al. Laparoscopic adrenalectomy in pheochromocytoma and Cushing's syndrome: reflections about two case reports. Acta Chir Belg. 1994;94:301–306. [PubMed] [Google Scholar]
- 24.Prinz RA. Laparoscopic adrenalectomy. J Am Coll Surg. 1996;183:71–73. [PubMed] [Google Scholar]
- 25.van Heerden JA, Sheps SG, Hamberger B, et al. Pheochromocytoma: current status and changing trends. Surgery. 1982;91:367–373. [PubMed] [Google Scholar]
- 26.Flavio Rocha M, Faramarzi-Roques R, Tauzin-Fin P, et al. Laparoscopic surgery for pheochromocytoma. Eur Urol. 2004;45:226–232. [DOI] [PubMed] [Google Scholar]
- 27.Kercher KW, Park A, Matthews BD, et al. Laparoscopic adrenalectomy for pheochromocytoma. Surg Endosc. 2002;16:100–102. [DOI] [PubMed] [Google Scholar]
- 28.Inabnet WB, Pitre J, Bernard D, et al. Comparison of the hemodynamic parameters of open and laparoscopic adrenalectomy for pheochromocytoma. World J Surg. 2000;24:574–578. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Cruz L, Saenz A, Taura P, et al. Helium and carbon dioxide pneumoperitoneum in patients with pheochromocytoma undergoing laparoscopic adrenalectomy. World J Surg. 1998;22:1250–1255. [DOI] [PubMed] [Google Scholar]
- 30.Feldman JM, Blalock JA, Fagraeus L, et al. Alterations in plasma norepinephrine concentration during surgical resection of pheochromocytoma. Ann Surg. 1978;188:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marty J, Desmonts JM, Chalaux G, et al. Hypertensive responses during operation for phaeochromocytoma: a study of plasma catecholamine and haemodynamic changes. Eur J Anaesthesiol. 1985;2:257–264. [PubMed] [Google Scholar]
- 32.Newell KA, Prinz RA, Brooks MH, et al. Plasma catecholamine changes during excision of pheochromocytoma. Surgery. 1988;104:1064–1073. [PubMed] [Google Scholar]
- 33.de La Chapelle A, Deghmani M, Dureuil B. Peritoneal insufflation can be a critical moment in the laparoscopic surgery of pheochromocytoma. Ann Fr Anesth Reanim. 1998;17:1184–1185. [DOI] [PubMed] [Google Scholar]
- 34.Rose CE Jr, Althaus JA, Kaiser DL, et al. Acute hypoxemia and hypercapnia: increase in plasma catecholamines in conscious dogs. Am J Physiol. 1983;245:H924–H929. [DOI] [PubMed] [Google Scholar]
- 35.Kim AW, Quiros RM, Maxhimer JB, et al. Outcome of laparoscopic adrenalectomy for pheochromocytomas vs aldosteronomas. Arch Surg. 2004;139:526–529; discussion 529–531. [DOI] [PubMed]
- 36.Gagner M, Pomp A, Heniford BT, et al. Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg. 1997;226:238–246; discussion 246–247. [DOI] [PMC free article] [PubMed]
- 37.Jaroszewski DE, Tessier DJ, Schlinkert RT, et al. Laparoscopic adrenalectomy for pheochromocytoma. Mayo Clin Proc. 2003;78:1501–1504. [DOI] [PubMed] [Google Scholar]
- 38.John H, Ziegler WH, Hauri D, et al. Pheochromocytomas: can malignant potential be predicted? Urology. 1999;53:679–683. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein RE, O'Neill JA Jr, Holcomb GW 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229:755–764; discussion 764–766. [DOI] [PMC free article] [PubMed]
- 40.van Heerden JA, Roland CF, Carney JA, et al. Long-term evaluation following resection of apparently benign pheochromocytoma(s)/paraganglioma(s). World J Surg. 1990;14:325–329. [DOI] [PubMed] [Google Scholar]
- 41.Li ML, Fitzgerald PA, Price DC, et al. Iatrogenic pheochromocytomatosis: a previously unreported result of laparoscopic adrenalectomy. Surgery. 2001;130:1072–1077. [DOI] [PubMed] [Google Scholar]
- 42.Scott HW Jr, Halter SA. Oncologic aspects of pheochromocytoma: the importance of follow-up. Surgery. 1984;96:1061–1066. [PubMed] [Google Scholar]
- 43.Staren ED, Prinz RA. Adrenalectomy in the era of laparoscopy. Surgery. 1996;120:706–709; discussion 710–711. [DOI] [PubMed]