Abstract
Objective:
To study the magnitude of complications associated with the nonoperative management of peripancreatic fluid collections and pseudocysts and to assess the surgical management of these complications. These are compared with complications associated with operative management.
Summary Background Data:
Pancreatic pseudocysts and peripancreatic fluid collections associated with acute pancreatitis have been managed with success using nonoperative techniques for more than a decade. When successful, these techniques have clear advantages compared with operative management. There has, however, been little focus on the magnitude and outcomes after complications sustained by nonoperative management. Our report focuses on these complications and pseudocysts and on the surgical management. We have been struck by the high percentage of patients who sustain significant and at times life-threatening complications related to the nonoperative management of fluid collections. We further define an association between the main pancreatic ductal anatomy and the likelihood of major complications after nonoperative management.
Methods:
Between 1992 and 2003, all patients admitted to our service with peripancreatic fluid collections or pseudocysts were monitored. We evaluated complications patients managed with percutaneous (PD) or endoscopic drainage (E). Data were collected regarding patient characteristics, need for intensive care unit (ICU) stays, hemorrhage, hypotension, renal failure, and ventilator support. We further focused on the duration of fistula drainage from patients who have had a percutaneous drainage, and we assessed the necessity for urgent or emergent operation. By protocol, all patients had pancreatic ductal anatomy evaluated by means of endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP). Patients with complications of E and PD were compared with 100 consecutive patients who underwent operative management of pseudocyst and fluid collections as their sole mode of intervention.
Results:
A total of 79 patients with complications of PD, E, or both were studied. There were 41 males and 38 females in the group of patients who sustained complications (mean age 49 years). Sixty-six of the 79 subsequently required operation to manage their peripancreatic fluid collection, 37 urgent or emergent. The mean elapsed time from diagnosis to nonoperative intervention was 18.1 days. This group of 79 patients had mean 3.1 ± 0.7 hospitalization (range, 1–7) and length-of-stay 42.7 ± 4.1 days. ICU stays were required in 36 of the 79 (46%). A defined episode of clinical sepsis was identified in 72 of 79 (91%) and was by far the most common complication. Hemorrhage requiring transfusion was identified in 16 of the 79 (20%), clinical shock 51 of the 79 (65%), renal failure 16 of the 79 (20%), ventilator support for longer than 24 hours 19 of the 79 (24%). A persistent pancreatic fistula occurred in 66 of the 79 patients (84%); mean duration was 61.4 ± 9.6 days. Sixty-three of the 79 patients with complications of E or PD had ductal anatomy (ERCP/MRCP) which predicted failure because of significant disruption or stenosis of the main pancreatic duct. Among the 100 operated patients, 69 complications occurred in 6 of the 100 (6%). Operation was initiated electively a mean interval of 42.7 days after diagnosis of pseudocyst. Hemorrhage, hypotension, renal failure, sepsis, persistent fistula, or urgent operation all were not seen in the complications associated with operated patients. CT imaging obtained at least 6 months after intervention documented complete resolution after surgery alone in 91 and 9 with cystic structures less than 2 cm. In patients with operation after failed nonoperative therapy, 6 patients had persistent cystic lesions less than 2 cm in diameter.
Conclusion:
These data support the premise that a choice between operative and nonoperative management for peripancreatic fluid collections and pseudocysts should be made with careful assessment of the pancreatic ductal anatomy, with a clear recognition of the magnitude of complications which are likely to occur should nonoperative measures be used in patients most likely to sustain complications. It is vital to recognize the magnitude and severity of complications of nonoperative measures as one chooses a modality. Ductal anatomy predicts patients who will have complications or failure of management of their peripancreatic fluid collection.
Seventy-nine patients sustaining complications after nonoperative measures to treat pancreatic pseudocyst were compared with 100 patients who underwent operative drainage of pancreatic pseudocysts.
A careful reading of the current literature regarding the operative and nonoperative management of pancreatic pseudocysts provides few discrete measures by which to choose one modality over another. Operative management of pseudocyst has a high level of success; however, morbidity rates have ranged from 4% to 30%.1–4 In any event, it is clear that a nonoperative measure would be more desirable should there be no significant loss in effectiveness of therapy. Although numbers vary widely, the success rates for both endoscopic and percutaneous management of pseudocyst ranges from 60%–90%, whereas the success rates for surgical drainage is on the order of 94%–99%. Unfortunately, the majority of reports in radiologic and endoscopic series provide data on technically successful drainage rather than success in permanently resolving the pseudocyst. Very few have long-term follow-up, and we have found none which employed cross-sectional imaging more than 6 months after intervention. Variables which must be considered when addressing nonoperative measures include the length of time that the drainage procedures require for complete resolution of pseudocyst, the length of follow-up to confirm that pseudocyst have in fact completely resolved, and the complications that may be associated with nonoperative management.
A generally observed surgical precept is that pseudocysts which persist beyond 4–6 weeks are then candidates for intervention, although there is a literature suggesting that some pseudocysts may be safely followed which are asymptomatic.5,6 Expectant management is typically abandoned when a pseudocyst exceeds 7 cm in diameter. A careful evaluation of the literature regarding percutaneous or endoscopic management of pseudocysts fails to reveal any such formula for delay before intervention. It is thus common to see a series of patients managed with nonoperative measures whose cyst or fluid collections have been instrumented less than 4 weeks after initial diagnosis.
The current report is unique in that it focuses on the surgical management of complications of nonoperative measures, with specific focus on the magnitude of these complications, the success of surgical management, the correlation with pancreatic ductal abnormalities, and the timing of the nonoperative measures and the role this variable may play in outcome.
Although patients can often be managed at home with percutaneous drain the need for urgent or emergent rehospitalization for episodes of sepsis is well documented.7 This fact assumes somewhat greater significance when a patient has had a sterile peripancreatic fluid collection prior to instrumentation. There is generally a lack of definition regarding the details and magnitude of complications.7–10
The earliest reports on endoscopic management of peripancreatic fluid collections employed transmural stent placement. Thus, these techniques were at that time limited to pseudocysts near the stomach.11,12 More recently, endoscopic ultrasound has provided a more precise approach to peripancreatic fluid collections.13 More recently, transpapillary stents have been employed. Kozarek et al14 have achieved successes even when high-grade ductal injury have been identified.15 The concept behind the transpapillary stents is the reduction of pressure in the ductal system facilitating drainage of the pseudocyst through the duct and not into the cyst. The success of this modality is consistent with our assumption that successes and failures reflect the underlying ductal anatomy. Multiple endoscopic procedures are often required before success is achieved.
The literature for operative management of pseudocyst spans at least 4 decades. Early reports describing external drainage confirmed a failure rate of 20%–30%. It is striking that the failure of 20%–30% parallels the failure rates of both endoscopic and percutaneous management of pseudocysts and resembles the frequency of moderate/severe acute pancreatitis. Internal drainage via cystgastrostomy or cyst-jejunostomy has been well established, and permanent resolution of pseudocyst is confirmed in between 91%–97% of patients.16–19 High success rates are counterbalanced somewhat by the risks of an operative procedure and with the possible complications of the procedure. Postoperative morbidity rates in reports in the 1970s and 1980s occurred at a frequency of approximately 30%.7,19 The most common significant complication in these patients was hemorrhage. Fortunately, more recent reports on the operative management of pseudocyst have documented a much lower morbidity rate, typically less than 10%.1,2,7,10,16,17
The recognition that pancreatic ductal abnormalities play a role in complicated acute pancreatitis can be traced to a report by Rutledge and Warshaw20 in 1988. In 1989, we proposed the value of ERCP to direct choices of therapy in pancreatic pseudocyst.21 Kozarek et al14 confirmed a relationship with their pioneering work on transpapillary stents for pseudocysts in 1991, and Neoptolemos et al22 added further definition of the role ductal changes played in acute pancreatit is in 2001 after previously exploring the role in 1993.23 We have recently documented the importance of evaluating main pancreatic ductal anatomy as modalities are chosen for pseudocyst management.17 In another report, we have further evaluated patients with moderate to severe acute pancreatitis associated with gallstones who developed fluid collections, and the patients whose pseudocysts failed to resolve and who subsequently required a cholecystectomy combined with drainage of the pseudocyst had documented ductal injuries possibly explaining the persistence of the cyst.18 Howard et al24 from Indiana have recently documented the high percentage of patients who have survived an episode of necrotizing pancreatitis and have recurrent episodes of acute pancreatitis, afterward having associated main pancreatic ductal abnormalities. We recently documented success in resolving pseudocysts associated with chronic pancreatitis by operative duct drainage alone.25 All of these data suggest that the management of pancreatic pseudocyst is based most appropriately on an evaluation of the pancreatic ductal anatomy. Itis thus the purpose to of this report to evaluate the magnitude of complications associated with nonoperative measures in pancreatic pseudocyst and to establish a correlation between these complications and the presence of significant pancreatic ductal abnormalities. The recognition of the magnitude of these complications, combined with the fact that ductal anatomy may well predict which patients will have these complications, strongly suggests that some logic may be applied in choosing a modality to treat peripancreatic fluid collections and pseudocysts.
METHODS
All patients admitted to our service with a diagnosis of pancreatic pseudocyst are enlisted into our pancreaticobiliary service, and clinical information is entered into our data collection for this entity. Written consents regarding participation in this study are obtained. Between 1992 and 2003, we have evaluated patients who had initial treatment of their peripancreatic fluid collection or pseudocyst with either percutaneous drainage or endoscopic drainage. Patients who had complications from their initial management with one of these 2 nonoperative measures are the focus of this report. Most of these patients were referred from outside institutions after developing the complications sustained by these measures, while some sustained these complications after initial treatment at our institution. This group of patients was compared with 100 consecutive patients treated over this period of time with operative management of pancreatic pseudocyst. Complications were compared in the 2 groups. All patients were evaluated for demographics, as well as for the mechanism or etiology of the acute pancreatitis, the severity of the episode of acute pancreatitis, and a number of important variables in their clinical course. These variables included the incidence of sepsis, hemorrhage, shock defined as systolic blood pressure less than 90 mm Hg, renal failure and a need for ventilator support. Further, these patients were evaluated for a need to be managed in a critical care setting or intensive care unit. Patients who had percutaneous drains were evaluated for persistence of pancreatic fistula. Patients with complications of nonoperative management were evaluated for the need to undergo urgent or emergent operation.
Using cross-sectional imaging, patients were evaluated for the size of peripancreatic fluid collection or pseudocyst. Patients were evaluated for the location of the pseudocyst in the head, body, or tail of the pancreas and were evaluated for the presence or absence of infected pseudocyst before and after interventions. Patients were evaluated for the number of hospitalizations required for their care. Specific note was made of the time which elapsed from the original diagnosis of acute pancreatitis to an intervention in the nonoperative patients and in the patients who had operation as their only modality for care.
We prospectively examined main pancreatic ductal anatomy by means of MRCP or ERCP in all pseudocyst patients. By policy, the evaluation of ductal anatomy is delayed until a decision is made to intervene. Some patients managed with transpapillary endoscopic drainage procedures had ductal anatomy evaluated prior to coming to our care. Otherwise, MRCP or ERCP was performed in close proximity to either operation or to a nonoperative measure being instituted. This protocol was instituted to avoid contamination of pseudocyst. With the increased use of MRCP and the consequent absence of any risk of contamination, some imaging of the ductal anatomy was performed somewhat remote from the final intervention instituted for the patient. Defining the ductal anatomy, in particular by ERCP, permitted evaluation of possible ductal disruption or ductal stenosis and delineated the location in the pancreatic parenchyma or in the pancreatic duct where ductal injuries were located. The ductal imaging particularly that obtained during ERCP delineated the presence of radiographically demonstrable communication between the main pancreatic duct and the pseudocyst or fluid collection. All of these anatomic evaluations were correlated with the severity of the original episode of acute pancreatitis. In broad terms, patients were categorized as either mild interstitial or moderate/severe pancreatitis.
Our analysis has used the terminology obtained during the international symposium on acute pancreatitis which was held in 1993 in Atlanta, Georgia.26 This symposium defined 3 categories of peripancreatic collections. According to their terminology, pseudocysts do not exist until 4 weeks have elapsed. “Peripancreatic fluid collections” lack a surrounding wall of fibrotic tissue and are seen between 0 and 4 weeks after the initial episode of acute pancreatitis. “Postnecrotic fluid collections” represent necrotic pancreas and/or necrotic peripancreatic tissues and debris. Both of these entities, if they persist beyond 4 weeks and develop a fibrous wall, are termed pseudocyst. Our analysis applies this terminology to the fluid collections which are under consideration in this report. This fact raises the possibility that certain interventions may have been initiated at a time when the fluid collections were either postnecrotic or simple peripancreatic fluid collections. During the period of analysis, however, these eventually were categorized as pseudocysts. For that reason, the focus of this paper is in the management of pancreatic pseudocyst, in spite of the fact that a portion of these patients were managed before their fluid collections had reached the state of evolution to be considered true pseudocysts according to Atlanta terminology.
Attention was directed towards those patients with nonoperative management who subsequently required operation. Specific note was made of the timing in the course of the peripancreatic fluid collections at which the nonoperative measures were instituted, the presence of infected pancreatic fluid collections, and the microorganisms found in the peripancreatic fluid collections. Nutritional status of these patients was evaluated by means of serum albumin and estimated percent body weight lost during the disease process. Indications for operation in this subset of patients were the presence of sepsis, hemorrhage, or persistence of fistula drainage. The outcomes of operation in patients who underwent surgery after failure of nonoperative measures were evaluated, including perioperative complications, resolution of pseudocyst, and mortality.
We have categorized previously the variety of ductal abnormalities that may be seen in patients with pseudocyst (Fig. 1). We have used this system to evaluate the ductal abnormalities seen in the patients included in this report. Factors such as persistence of pancreatic fistula after percutaneous drainage were correlated with the level of ductal abnormalities found in our analysis.
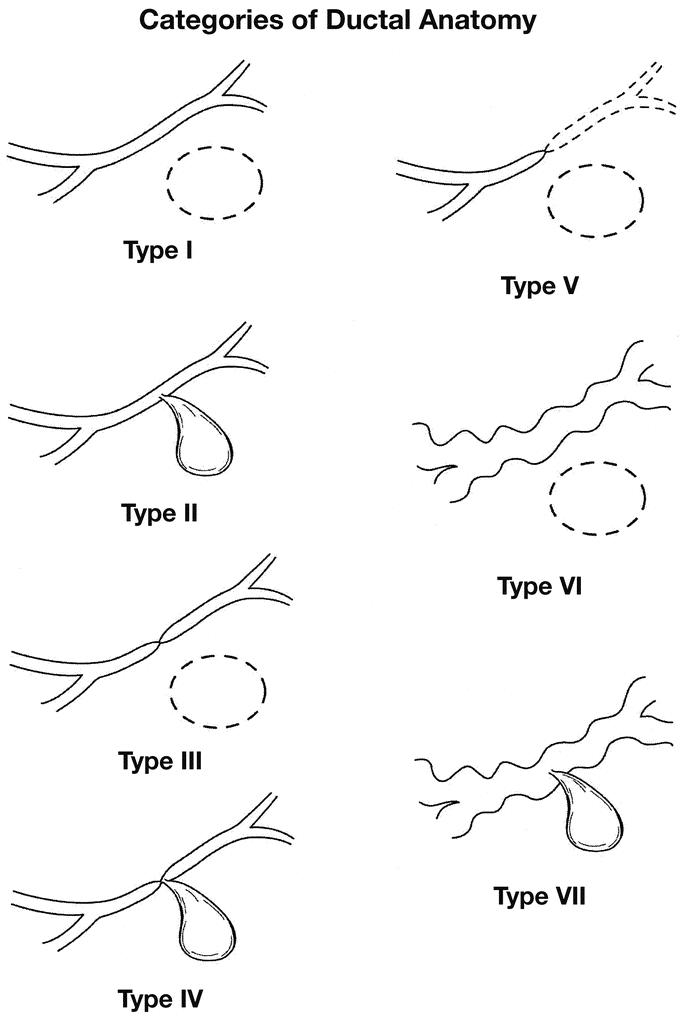
FIGURE 1. System to categorize ductal disruptions or ductal abnormalities seen in patients with pancreatic pseudocysts. The main pancreatic duct is represented in all types. Type 1 is a normal pancreatic duct with a noncommunicating pseudocyst represented by the dotted elliptical mass. Type 2 represents a normal duct with communication with the cyst. Type 5 represents the so-called isolated pancreatic segment. Types 6 and 7 represent chronic pancreatitis.
Finally, there was a subset of patients who had resolution of their pseudocyst after nonoperative measures. This group was separately evaluated for all of the categories previously mentioned. For all patients, the success of abolishing the pancreatic pseudocyst was confirmed by postoperative or postprocedural CT scan imaging or, at times, MRI imaging. The protocol dictated imaging to be obtained at least 6 months after the final designation of successful nonoperative management or successful operative management. Success was defined as no fluid collection detected. Differences in percentages were calculated using χ2 analysis and Fisher exact test where appropriate. P values less than 0.05 were considered significant.
RESULTS
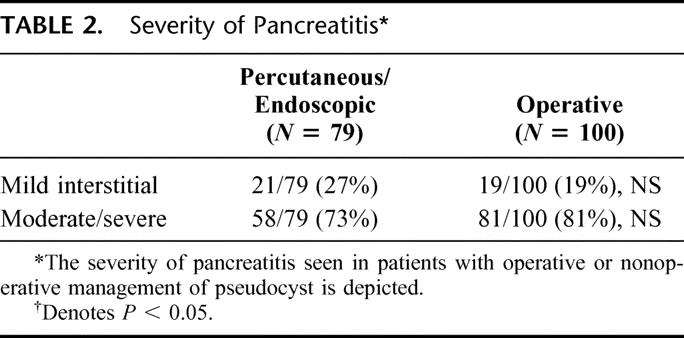
A total of 79 patients with complications of percutaneous drainage or endoscopic drainage or both have been evaluated during the study period. One hundred consecutive patients undergoing operative management of pseudocyst were compared. Sixty-six of the 79 patients treated initially with nonoperative measures subsequently required operation. Demographic features of the patients who underwent operation alone and the patients who underwent initial nonoperative measures are listed in Table 1. Among the 66 patients who required operation after failure of nonoperative measures, 37 of those operations were performed in an urgent fashion. Differences were not significant. Table 2 delineates the severity of pancreatitis in patients who underwent nonoperative measures initially versus those patients who had operative intervention as their only form of intervention for pseudocyst. In both groups, moderate/severe pancreatitis was the most common predecessor to the formation of peripancreatic fluid collections and subsequent pseudocysts. Differences were not significant.
TABLE 1. Demographic and Clinical Data of Patients
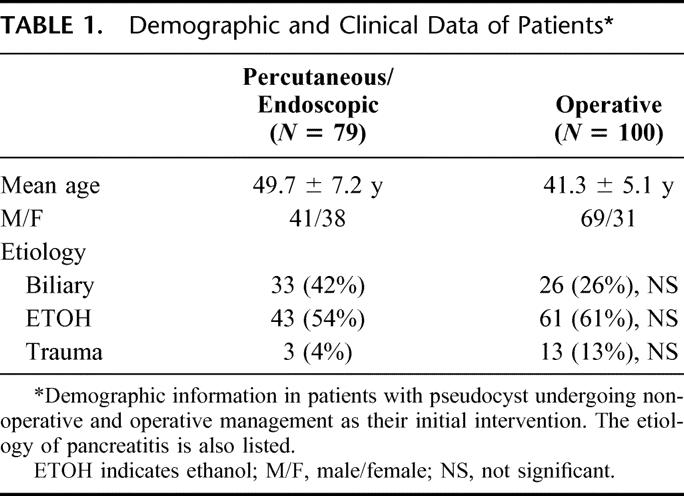
TABLE 2. Severity of Pancreatitis
Using CT scan or MRI imaging, the mean diameter of the fluid collections or pseudocysts is listed in Table 3. The mean diameter in the patients who underwent operation was slightly larger, although this difference was not significant. Table 3 further defines the location of the cyst in the head, body, or tail of the pancreas. There is a comparable distribution of cysts in the head and the body. Cysts in the tail were less common. The presence of ductal abnormalities is also listed in Table 3. Referring to Figure 1, MPD defects type 3–7 are those ductal abnormalities which we believe are poorly suited to nonoperative drainage procedures.
TABLE 3. Peripancreatic Fluid Collections
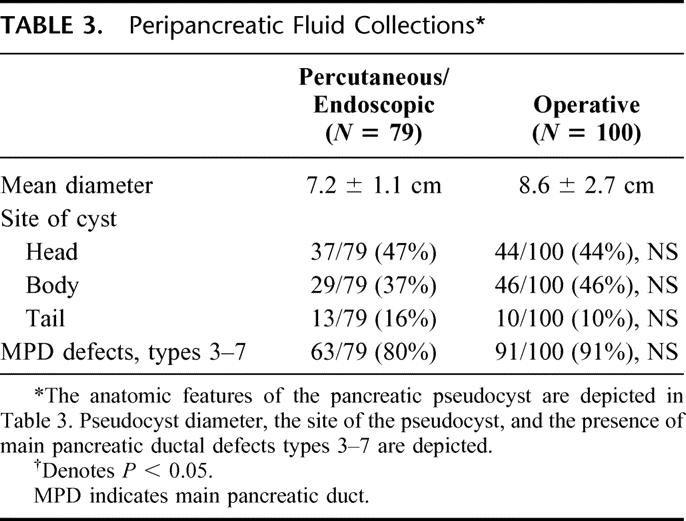
Table 4 lists the various modalities employed in the patients who had nonoperative measures. Of the 79 patients, approximately two thirds of the patients were initially managed with percutaneous drainage. Both endoscopic and percutaneous techniques were combined in 22% of patients. Among the patients who underwent endoscopic management, transmural stents were used in 14 of the 34 patients and transpapillary drainage was used in 20. Nine of the 13 patients who finally had resolution of their pseudocyst using nonoperative measures were treated with endoscopic drainage alone; 7 of these 9 were treated with transpapillary stents and the remainder with transmural stents. Two of the 7 transpapillary stents had subsequent percutaneous drains placed because of a sepsis picture after insertion of the transpapillary stents.
TABLE 4. Patients Managed by Percutaneous or Endoscopic Management
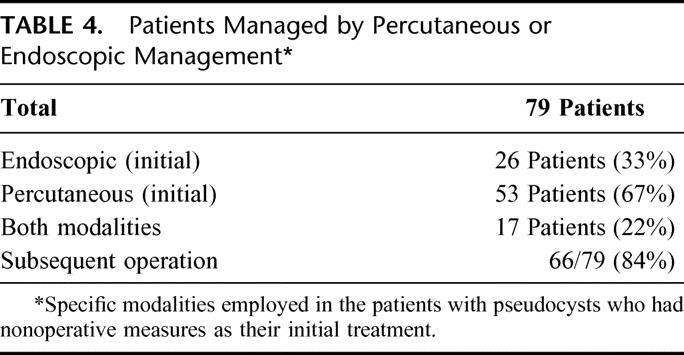
Table 5 delineates the indications for operation in patients who had complications of percutaneous or endoscopic management. Sepsis was an indication for operation in 26 of the 66, or 39%. Each of the 66 patients who finally required operation had percutaneous drainage as their only form of management or had percutaneous drainage combined with endoscopy. As a consequence of this fact, the persistence of a pancreatic fistula from the percutaneous drain was present in all patients who subsequently required operation. Consistent with this finding, significant main pancreatic ductal injuries were seen in 57 of the 66 patients who subsequently required operation (84%). Thirty-two of these 66 patients had type 5 ductal disruptions an entity, which has been coined isolated pancreatic segment.
TABLE 5. Indication for Operation in Patients With Complications of Percutaneous or Endoscopic Management
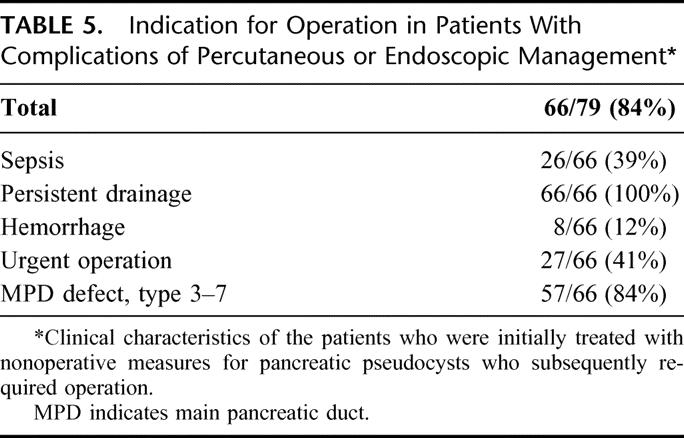
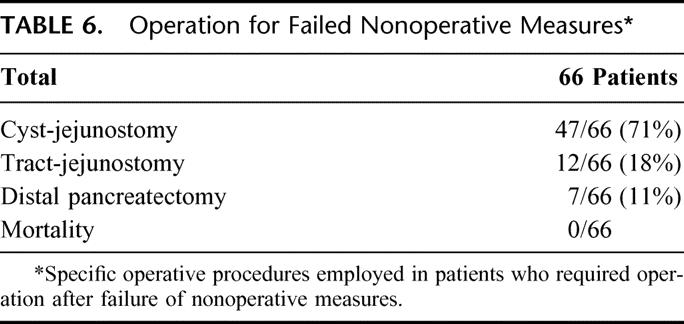
Table 6 lists the operative procedures in patients who required operation after failure of nonoperative measures. By far the most common operative procedure employed was cyst-jejunostomy in 71% of patients. Of note is that 12 of the 66, or 18% of patients, had complete collapse and essentially disappearance of the cyst by the time operative interventions were undertaken. Distal pancreatectomy was employed in a small percentage, and this procedure was primarily employed in patients who had a type 5 ductal injury with an isolated pancreatic segment. There were no mortalities.
TABLE 6. Operation for Failed Nonoperative Measures
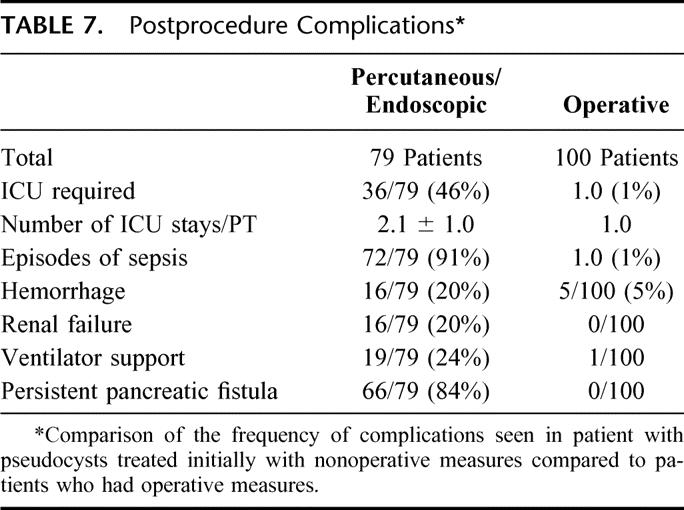
In Table 7, we depict the complications which were encountered in patients who had nonoperative measures instituted, and these were compared with the patients who had undergone operation. We wish to be clear that these 2 groups are not properly comparable because we are not able to provide the denominator of the number of patients who underwent percutaneous or endoscopic drainages with good outcome. Among the 79 patients, 72, or 91%, had at least 1 episode of sepsis during the management period. This was by far the most common significant complication noted and was viewed to be a direct result of contaminating a collection that was sterile before instrumentation or by cultures obtained and the time of drainage in 74 of 79 patients. Persistent pancreatic fistula associated with percutaneous drains was recognized in 66 out of 79, or 84%. The magnitude of these complications is reflected in Table 7. Critical care support, episodes of shock, episodes of renal failure, and a need for ventilator support are all documented at high rates. All of these complications were considerably more common in the patients with complications of percutaneous and endoscopic management compared with complications of operation. The only significant complication in the operated patients was hemorrhage, which was seen in 5 patients. No reoperations were required. In each of these patients, a ductal drainage procedure was employed for chronic pancreatitis, and parenchymal bleeding resolved spontaneously in 3 and required embolization in 2.
TABLE 7. Postprocedure Complications
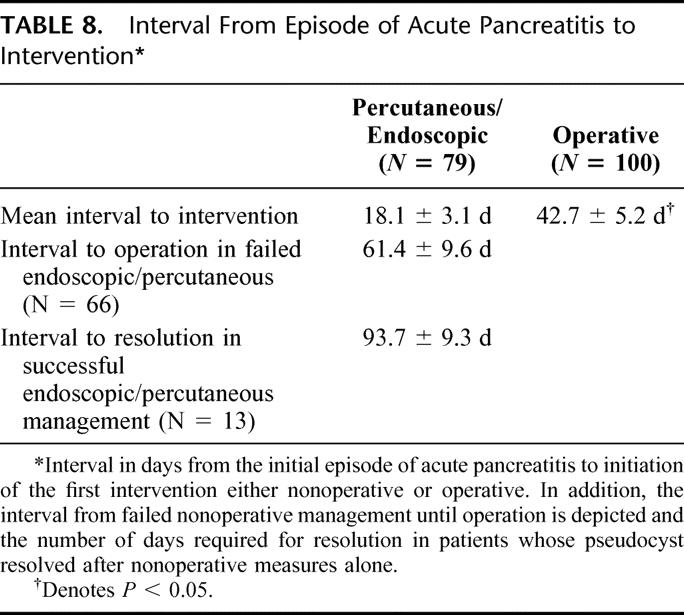
Because a large percentage of patients had interventions performed before they reached our institution, the indication for these procedures is not clear. The mean interval to intervention in patients who had nonoperative interventions was 18.1 days compared with 42.7 days in patients who had operative intervention. Nearly two thirds of patients had interventions before the fluid collection could technically be termed pseudocyst. In nonoperated patients, the mean interval from the initial episode of acute pancreatitis until operation for failed endoscopic or percutaneous management was 61.4 days. Thirteen of the patients had eventual resolution of their pseudocyst by nonoperative measures, and the mean interval from the initial episode of acute pancreatitis to resolution in this subset of patients was 93.7 days. The potential risks in these early interventions is reflected in Table 9, where patients are segregated to interventions performed less than 28 days after the diagnosis of acute pancreatitis, and these are compared with patients whose interventions were initiated more than 28 days after diagnosis (Table 8). Fifty-one of the 79, or 65%, had their intervention performed prior to 28 days. Fifty of those 51 patients, or 98%, had episodes of sepsis, and nearly half required urgent operation (Table 9). In contrast, 35% of patients had their interventions performed more than 28 days after a diagnosis of acute pancreatitis. Although sepsis occurred at a high rate in this subset, the need for urgent operation was far less common. It was required in only 28% of these patients.
TABLE 9. Impact of Early Complications and Outcomes in Endoscopic/Percutaneous Drainage Intervention
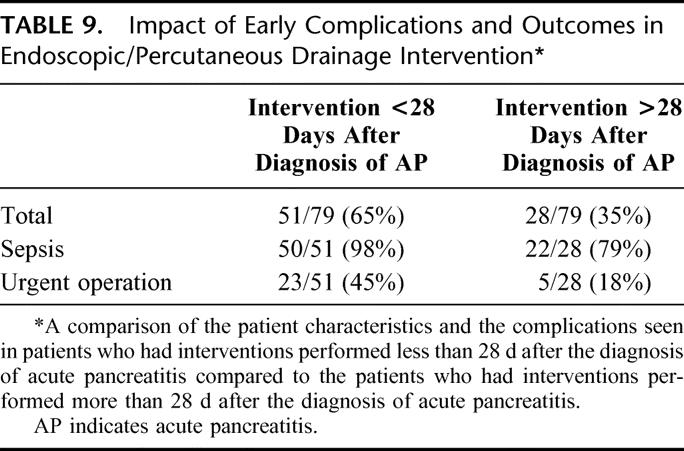
TABLE 8. Interval From Episode of Acute Pancreatitis to Intervention
Postprocedure CT scan or MRCP was performed a mean of 8.2 months after operation or after resolution of pseudocyst. Four patients of the 13 who had resolution after nonoperative measures had pseudocysts measuring 3 and 4 cm in diameter. Among the 66 operated patients who initially had percutaneous or endoscopic management, there were 6 patients in whom imaging documented cystic structures less than 2 cm. Among the 100 patients undergoing operative drainage or resections alone, 9 patients had cystic structures less than 2 cm seen on follow-up imaging. Otherwise, all other imaging reflected a complete resolution of pseudocyst.
A total of 61 out of the 79 patients included in the nonoperative group were transferred from outside hospitals to our institution. Fifty-six of these 61 patients, or 92%, demonstrated signs of sepsis at the time of their transfer. Thirty-three of the 61, or 54%, required further nonoperative measures after they reached our facility. These were often because the size of the drain placed either endoscopically or percutaneously was too small. Thirty-one of the 79 patients treated nonoperatively had necrotic debris present within their cysts. Nutritional status in the 61 transferred patients was considered poor, with mean percentage weight loss of 12.3% and mean serum albumin of 2.1 ± 1.1. The patients who underwent nonoperative management had a mean 3.1 ± 0.7 hospitalizations (range, 1–7). The mean length of stay was 42.7 ± 4.1 days. Fifty-two of the 79 patients treated nonoperatively were sent home with drains in place during their management prior to operation, and 28 of these 52 required urgent rehospitalization because of drain occlusion and sepsis picture. The average length of stay was 42.7 ± 4.1 days for the patients treated with nonoperative management. By contrast, the mean number of hospitalizations for the operated patients was 1.7 days ±0.6. The average length of stay was 6.1 ± 1.1 days. Only 3 of these 100 patients required continuous hospitalization from the episode of acute pancreatitis until operative intervention of their pseudocyst because they could not eat. The majority were followed with serial CT scans after being discharged home. Some of the 100 operated patients were referred to our institution with a pseudocyst identified at an outside institution. These therefore had a single hospitalization at out institution for their operative procedure after having been evaluated in our outpatient clinic. There were no complications sustained from the ERCP or MRCP procedures which were performed to establish the ductal anatomy.
Although the majority of patients with episodes of sepsis had cultures which were consistent with enteric organisms (most commonly E coli and Enterobacter), there were 31 patients among the 72 patients treated nonoperatively who had infections with cutaneous organisms, particularly, methicillin-resistant Staphylococcus aureus (22/31 such patients). Although not confirmatory, these findings suggest the possibility that infection of the fluid collections were the direct result of establishing communication between cyst skin flora. Preprocedural infected collections were documented in 4 patients in the operated group and in 7 patients in the nonoperative group.
DISCUSSION
Although there are ample data to support the use of nonoperative measures in the management of pancreatic pseudocysts, we believe there ought to be concerns regarding the patients who fail these measures. A careful review of the endoscopic and interventional literature provides few details about patients who fail nonoperative measures. The incidence of failure appears to range from 10%–40%.8,15 There is an interesting parallel between these data and those reported decades earlier in the surgical literature, where operative external drainage was associated with 20%–30% recurrence of pseudocyst.1–4 The specific issue regarding patients who fail nonoperative measures that is addressed by our report is the magnitude of the complications sustained. Based upon our data, it is conceivable that the majority of patients in whom these measures fail do not return to the individuals who performed the nonoperative procedures but rather are referred to another institution and often to the management of a surgeon. Thus, reports in the literature of nonoperative measures may lack details on their major complications.
Peripancreatic fluid collections are rare in simple interstitial pancreatitis. Kourtesis et al27 reported that 37% of patients in a group of 128 patients with acute pancreatitis developed pancreatic fluid collection, the majority of which resolved spontaneously. Fifteen patients (12%) developed chronic pseudocysts. In view of the fact that the Atlanta Symposium of 1993 designates fluid collections accumulating within the first 4 weeks after an episode of acute pancreatitis as simple fluid collections and not pseudocysts, the literature is difficult to interpret because some reports have defined resolution of these fluid collections as spontaneous resolution of pseudocysts.26 Appropriately, resolution of pseudocyst should only be applied when the cystic structure has persisted beyond 4 weeks after the initial episode of acute pancreatitis. There exists a potential flaw in the literature on nonoperative measures because of the possibility that these have been initiated in the early weeks after the episode of acute pancreatitis at a time when fluid collections may be expected to have a high likelihood of spontaneous resolution. Warshaw and Rattner19 found no spontaneous resolution of pseudocysts which persisted for 6 weeks or more. These data are supported by observations made by Bradley et al4 in 1979 in their landmark paper regarding the long-term follow up of pseudocysts, where they found persistence of pseudocyst to be the norm in patients whose cyst persisted longer than 6 weeks, and they further found that the likelihood of major complications in pseudocysts was far more common in those patients who had persistence of pseudocyst. Significantly, when Bradley et a4 lassessed independently the group whose cysts persisted beyond 13 weeks, they found 75% who developed major complications.
In 1989, we identified the usefulness of identifying the pancreatic ductal anatomy in evaluating pancreatic pseudocyst.21 In 1993, Neoptolemos et al23 evaluated main pancreatic ductal anatomy in patients with acute pancreatitis with necrosis. They made the important observation that only 10% of patients with less than 25% of the pancreas sustaining necrosis developed pseudocyst. In contrast, in patients with greater than 25% necrosis of the pancreas, 65% developed pseudocyst. Further clues may be drawn from analyses by Vitas and Sarr,5 Maringhini et al,28 and Warshaw and Rattner,19 each of whom confirmed that complications in pseudocysts, which were simply followed, occurred between 10% and 23% of the time. Interestingly, this frequency parallels the frequency of severe pancreatitis in most large series. Further clarity to this issue is provided by Lau et al,29 who in 2001 found that main pancreatic ductal disruption was seen in 37% of 144 patients with severe acute pancreatitis. In 2002, we published an evaluation of failures of percutaneous drainage of pancreatic pseudocysts and found ductal abnormalities were responsible for failure in the majority of these patients.17 Our conclusion from this report was that ductal anatomy should be used as the measure by which each separate modality in the treatment of pseudocyst is chosen.
The current report comes as a result of these studies and focuses on patients with pseudocysts referred to our care either as a transfer from an outside institution or from our own institution with complications associated with nonoperative measures. An inherent flaw in this study is that we do not have a denominator for the total number of nonoperative measures employed, and therefore we cannot suggest the percentage of patients who sustained these complications among all patients so treated. Looking at the patients managed at our institution, there appears to be a minimum frequency of 40% for episodes of drain occlusion and sepsis. We believe that our data in this report add further to this premise that ductal anatomy predicts outcomes by correlating pancreatic ductal anatomy with complications and failures of nonoperative management. Based upon our data, strong concerns should be raised regarding nonoperative measures being employed weeks before any operative procedures would ever be considered in the management of pancreatic fluid collections or pseudocysts. We believe that this raises a concern not only because of the complications incurred by these procedures but also by the possibility that a percentage of these fluid collections would likely have resolved spontaneously had no interventions been performed at such an early stage.
A further flaw in our study is that the operated patients are not strictly comparable to the patients who are treated with nonoperative measures who had complications. Many historical reports of the surgical management of pseudocysts have defined complication of rates as high as 30% and 40%.1–4 More recent complication rates for operative management of pseudocysts have been in the range of 5%–15%.1,2,7,10,16,17 We do believe that operative management has fewer complications of the magnitude seen in the group of patients under analysis in this report.
Nearly half of the patients who were transferred from outside facilities after nonoperative measures were admitted with a septic picture and required immediate transfer to our intensive care unit. One may argue that the patient's developing sepsis at the outside institutions result from poor management rather than failure of the nonoperative techniques. Our data suggest that patients managed after transfer to our institution and patients who had their nonoperative measures instituted at our institution each had a very high rate of sepsis. It would appear that these data are consistent with data seen in prior studies looking at nonoperative interventions. Early reports on these techniques reported short catheter placements and high recurrence rates.11 As a result of this failure rate, the catheters were then left in place for a longer period of time to prevent recurrence. The apparent tradeoff for this policy change has been a higher rate of infection of fluid collection and in many cases a tradeoff for episodes of critical sepsis.7,11,17
Sixty-three of the 79 patients treated for complications of nonoperative measures had significant ductal disruptions sustained as a result of their episode of acute pancreatitis. This correlates with the frequency of episodes of moderate to severe acute pancreatitis in this group. As mentioned previously, the literature suggests that persistence of cysts is more common after moderate to severe acute pancreatitis and that ductal anatomic abnormalities are far more common after moderate to severe acute pancreatitis. One may infer on the basis of this information that nonoperative measures should be reconsidered in patients who have sustained an episode of moderate to severe acute pancreatitis. The easy availability of MRCP makes evaluations of ductal anatomy easier, without the threat and invasion and contamination resulting from ERCP. Unfortunately, the lack of clarity of MRCP for subtle changes prevents important observations from being made. Specifically, it is rare to define easily a tract between the main pancreatic duct and the pseudocyst cyst using MRCP. Although major ductal injuries should be apparent with MRCP, we have been impressed that complete ductal disruption toward the tail of the pancreas may be incorrectly interpreted as normal pancreatic duct, failing to recognize the short isolated segment of pancreatic duct. The tapering and normal appearance of the visualized pancreatic duct is misinterpreted as a normal pancreatogram.
ERCP in our hands has not resulted in complication for these patients. We do carry a policy of performing this procedure in close proximity to some intervention to prevent episodes of sepsis, should the ERCP result in contamination of the peripancreatic fluid collection or pseudocyst. We generally do not consider delineating ductal anatomy until the decision is made that some intervention should be employed. Distinction should be made between severe necrotizing pancreatitis and the needs for necrosectomy in this group of patients. Although some of these patients had had nonoperative interventions early in the course of their disease after episodes of moderate to severe acute pancreatitis, none of these patients were candidates for necrosectomy. Much of the necrotic debris found at the time of operative drainage was autodigested fat and digested blood clot. We address patients who are candidates for necrosectomy separately in our management of acute inflammatory diseases of the pancreas. It is, however, clear that patients with necrotizing pancreatitis with necrosis followed over time may develop pseudocysts containing necrotic debris. The distinction between these 2 groups may be a challenge. Some peripancreatic fluid collections contain clear fluid without necrotic debris. These typically resolve; however, those that persist are also categorized as pseudocyst. Any intervention initiated prior to this arbitrary 4-week boundary cannot be technically defined as pseudocyst. The reasoning in our report for including these patients under the title of pseudocyst is the fact that they evolved into distinct pseudocysts with a fibrous wall formed as confirmed by CT scanning. Unfortunately, one must recognize the distinctions among simple fluid collections, pancreatic necrosis, and patients with postnecrotic debris, all of whom may progress to mature pseudocyst containing either fluid alone or containing debris. We describe events in our report that took place before the patients could formally be described as having pseudocysts. We suspect that these interventions were undertaken in the flawed assumption that they were treating pseudocysts.
We recommend the following algorithm in the management of peripancreatic fluid collections and pseudocysts. Initially, without evidence of complications, simple observation for a minimum of 6 weeks is employed. Infected pseudocyst should be managed with percutaneous drainage until the patient is stabilized. Severe nutritional deficits, at times an indication for percutaneous drainage, should be addressed because this measure may permit the patient to achieve nutritional repletion. Both of these often serve as a bridge to surgery. Once the pseudocyst is established as persistent, we will observe truly asymptomatic patients with small cysts. It is our experience that the absence of symptoms is not common. We recommend intervention in all pseudocysts greater that 6 cm and in all symptomatic patients. We use ductal anatomy to guide choice of modality. Types V, VI, and VII ductal injuries are all managed operatively. Types I and II are always managed nonoperatively. Types III and IV are still under debate.
There are a number of issues which we have discovered regarding the operative management in patients who have had a failure of nonoperative measures. Perhaps most important has been our belief that the patient should have a period of stabilization prior to operation, if this is possible. This is the reason that we have often used our own nonoperative measures after transfer or after recognized failure of these measures to reverse episodes of sepsis and to improve nutritional status prior to intervention. Philosophically, we believe there is a very discrete place for interventions that are nonoperative in the management of peripancreatic fluid collections. Providing a bridge to permit resolution of sepsis or to permit restoration of nutritional stability is an additional example. Although the majority of patients underwent their interventions less than 28 days after the original episode of acute pancreatitis, the majority of patients were transferred to our care a mean of 5 weeks after their initial episode of acute pancreatitis. It is this delay in transfer which often results in nutritional deficits and permits the episode of sepsis to have reached significant magnitude before transfer to surgical care. We believe that this management principal of stabilizing the patient and restoring nutritional status plays a role in our high success rate in managing patients who have had complications of nonoperative measures. One technically significant challenge in managing patients with failure of nonoperative measures is the complete abolition of the prior cystic structure once it has been decompressed and the walls have fused. Dissection is considerably more challenging than the dissection involved in simply defining a pseudocyst and draining it. Avoiding this technically challenging aspect of surgical care may serve as yet another reason to evaluate pancreatic ductal anatomy to identify those patients prone to failure.
Discussions
Dr. David B. Adams (Charleston, South Carolina): I would like to commend Dr. Nealon for this excellent clinical review on the modern management of pancreatic pseudocysts. The manuscript, which I reviewed with great interest, has a wealth of radiologic data and, more importantly, it contains clinical correlative data from Dr. Nealon's bedside and operating room experience. Experience is the key concept evident in this manuscript. I have long been puzzled why intelligent men disagree on pancreatic pseudocyst management. It was a lawyer who helped me solve this puzzle. The great American jurist Oliver Wendell Holmes wrote: “The life of the law has not been logic, it has been experience.” When Holmes was on the Supreme Court he would invite his fellow justices to name any legal principle they liked and he would use it to decide the case under consideration either way. The management of pancreatic pseudocysts is similar. Experience, not prospective clinical studies or animal studies, has been the method of study which keeps this topic very much in the realm of 19th-century medicine and surgery and is why many of us like it so much. Unlike inguinal hernias or gallstones, pancreatic pseudocysts remain relatively infrequent, and there is not a usable experiment to take to the lab. In regards to pancreatic pseudocyst management, we decide, and then we deduce.
The deductions Dr. Nealon makes are clear, and there are many with which I agree and many with which I disagree. He is forthright in stating that pseudocyst nomenclature is always problematic in clinical reviews. He states clearly that the focus of the paper is the management of pancreatic pseudocyst, in spite of the fact that a portion of these patients were managed before their fluid collections reached the state of evolution to be considered true pseudocysts. This is not a problem. This is clinical medicine. He clearly affirms that the nonoperative and the operative group are not comparable. It is not a problem that he still compares them. This is experience at work. His deduction is different than mine in regards to percutaneous drainage of pseudocysts. I feel percutaneous drainage remains useful as a temporizing measure in sick patients prior to operation; he feels that percutaneous drainage makes a person sicker prior to operation. What we agree on is that ERCP is essential in managing patients with pancreatic pseudocysts in order to clearly define ductal anatomy prior to undertaking operation.
I have a few questions for Dr. Nealon. When would you use percutaneous drainage or endoscopic drainage in the management of pancreatic pseudocyst or a pancreatic fluid collection?
Would you comment on the 18% of patients mentioned in the manuscript who had complete disappearance of the cyst by the time operation was undertaken?
Is MRCP ever sufficient alone as a preoperative study, or is ERCP needed in every patient who requires operative management?
Do you have an opinion on any difference in safety and efficacy of endoscopic versus percutaneous drainage?
Finally, would you comment on the patients who were transferred to you for care. Which patients with pancreatic pseudocysts require management at a specialized surgical center?
Dr. Charles J. Yeo (Baltimore, Maryland): I congratulate Drs. Nealon and Walser for keeping pancreatic inflammatory disease on the map at meetings such as this. I am not going to recapitulate the data, but I do want to ask several questions and get you to focus on some of the controversies.
First, Dr. Nealon, you have made it quite clear that head-to-head (or mano-a-mano) comparisons between your group of 79 patients who were percutaneously or endoscopically treated and the 100 that you operated on are really unfair. It is a very biased selection, a biased comparison. But I would be interested in your sense of the numbers of percutaneous or endoscopically drained patients that actually thrive, that never see a surgeon, that never get transferred to us, and that are able to avoid an operation. Do you think it is 10% of the patients that undergo endoscopic or percutaneous drainage, or is it 90% that have trouble? Just a sense from your experience of what that percentage is.
Second, you have made it very clear, and I agree, that before we intervene in patients with complicated pseudocysts, we need to know the ductal anatomy. I am curious: do you think that a modern 2005 MRCP done on a great scanner can give you the same information as an ERCP? Certainly the old-fashioned 2000 MRCPs are terrible, and I think most of us would be hesitant to use them for any specific ductal anatomic visualization.
Thirdly, I was struck by the disconnect between the percentage of patients that were treated endoscopically with transpapillary stents initially (that was only 23%) versus the percent of patients who were thusly treated with transpapillary stents successfully nonoperatively, and that is 54%. Do you think that these data add support to the rationale for transpapillary stenting in the proper anatomic setting?
Fourthly, a very important corollary to your study which you allude to in the manuscript is—and here is the question—what are the indications for intervention in patients with acute fluid collections (that is, less than 4 weeks old) or pseudocysts (that is, greater than 4 weeks old) following an episode of acute pancreatitis? You allude to it in your conclusion. Does the presence of an asymptomatic 6-cm acute fluid collection require treatment? How about if it is 10 cm 6 weeks into the course? How about if it is 15 cm 8 weeks into the course? What is the proper algorithm? And when should treatment or intervention be recommended?
Lastly, in looking at your data in the 66 patients with complications who underwent operation, all of those patients (the “sine qua non” in every one of those patients from your manuscript), had “persistent drainage,” I suspect meaning an ongoing amylase-rich fluid leak via percutaneous drain. The question is, which tricks or treatments did you use to avoid surgery in those 66 patients? By that I mean octreotide, drain removal or advancement outwards, transpapillary drainage?
Again, my congratulations to you and your team for keeping pancreatic inflammatory disease on the literature map, and thanks for the opportunity to discuss these data.
Dr. Thomas R. Gadacz (Augusta, Georgia): Did you look at the comorbidities on these patients who had either percutaneous or endoscopic drainage? Was this a factor that put them in a higher category and contributed to their poor outcomes?
Dr. Stephen B. Vogel (Gainesville, Florida): As a follow-up to Dr. Yeo's question, I would like to ask how you deal with patients who have had percutaneously placed drains for symptomatic postoperative pancreatic fluid collections. There are many of these placed at our institution, and they most often are sent to the surgical clinics for follow-up. We don't disagree with this approach since it has been our policy to treat very symptomatic postoperative pancreatic fluid collections by percutaneous drainage if this will allow them to begin eating and be discharged from the hospital. We also use the same approach to very symptomatic postpancreatitis fluid collections when the pressure of the fluid collection on the stomach leads to symptoms that delay discharge.
On the other hand, few if any of our internists or gastroenterologists have ever followed a patient with a percutaneous catheter, [much less] removed it in clinic. I would like to ask how you handle these catheters. I would also like to add that our experience is perhaps similar to yours. Although our intent was to have the patient discharged from the hospital, the ultimate outcome of most of these patients is either a recurrent fluid collection when the catheter is discontinued or a chronic draining pancreatic fistula through the catheter in spite of extensive use of somatostatin. At our institution, most of these patients will undergo future internal drainage if the fluid collections recur or undergo “capping” of the fistula tract by a roux-Y at the time of future surgery. I would like to know how you handle percutaneous catheters where the drainage is fairly constant in spite of Sandostatin therapy. Thank you.
Dr. Kenneth W. Sharp (Nashville, Tennessee): Dr. Nealon, you have achieved your purpose. You have stimulated a lot of discussion. I have 2 quick questions.
With all of these percutaneous patients getting so many episodes of sepsis, how many of these patients had suspicion of infection or sepsis as the reason for their percutaneous intervention? I think this is somewhat of a self-chosen group. I think the patients are infected and they stick something in them; if they are not infected, they get infected.
Secondly, those patients who had endoscopic management, how many of them had actually an ERCP with that procedure? Could you really tell what ductal anatomy was, or did they just go in and stuff an endoscopic stent in the papilla?
Dr. William H. Nealon (Galveston, Texas): I guess I first need to make sure that everyone in the audience understands that I see a very clear role for nonoperative measures in pseudocyst. The message of this report is not to say never use these modalities. The message is to try to use a bit of a protocol when to institute them and to try to understand that there are ways to evaluate the anatomy and to decide whether there is a likelihood of failure or not. And the reason, aside from saying you would like something not to fail, is when they fail they can also cause a lot of trouble.
In my manuscript I mention, and I guess Dr. Adams didn't see them, many of these patients actually have subsequent often percutaneous management after they reach my care. And I use those modalities just as he said, as a bridge for patients who come sick. Typically, these patients come not the immediate moment after they have had their intervention but after they have sat at an outside hospital, becoming both septic and nutritionally unsound, until they come in a position where the risk factor for operation, in my opinion, would be excessively high. And for that reason, I will often use these measures as a bridge in this type of patient who is sent. So that is how I would use them. I would also say that patients who have a pancreatic pseudocyst and I assess ductal anatomy and the ductal anatomy seems favorable, then I use these nonoperative measures all the time.
Dr. Adams mentioned the subset of patients who had complete disappearance of their cyst. And this is a point that I make in the manuscript that I thought there wasn't time to mention here. But another issue for us surgeons is that many times when you have used percutaneous drainage for a long period of time, the cyst has actually collapsed and has no more cavity at all. And the difference in the technical challenge of that operative procedure, you actually have to dissect your way down to the base of the tract to find the communication with the pancreas and make an anastomosis, there is, in my opinion, much harder than one where you simply have to find the cyst and sew it. So you actually convert the operation which is typically pretty easy to an operation that can be very challenging once you have had the percutaneous management and the collapse of the pseudocyst. Those are the patients who have persistent fistula.
Yes, I think MRCP will finally replace ERCP. I think we all know that there are nuances right now that you can't see with the MRCP, but I am absolutely confident that it will replace ERCP and we will be able to use that in a much safer fashion without worrying about contaminating the pseudocyst when we do that procedure.
Dr. Adams asked how many of these patients need to be transferred. I think that the aspect of transferring a patient is an important one. It is hard for me to say which ones need to be transferred, with the exception of saying if you have evaluated the anatomy and they look like they have a really high likelihood of success, then there is no need to transfer them to an inside institution.
But I do worry that the stated complication rate is 5% to 20%. And that is among people who are really good at this. Most of us who manage these diseases know there are people in the general population of interventional radiologists and GI medicine, people not in major medical centers, who may not have anywhere near the same skill, even use necessarily the right size stents and things like that. So I wonder if it is not safe to guess that the complication rates are considerably higher than the ones stated in the literature when they are being referred in from many different outside institutions.
Dr. Yeo asked about how many of these patients do well. And again I would say the majority do well. If we go by the literature, 5% to 20%; if you go by my suspicions, it may be 5% to 20% have trouble; it may be that 30% or even 40% have trouble if you look at everybody all over institutions rather than just those who are reported in major series.
The denominator, if you imagine a 10% incidence, the denominator we would need of operative to be comparable would be if 80 complications are here, then that would be 800 patients to look at in an operative group to be comparable. If I wanted to say a 20% incidence in complications, then 400 patients with operation would be necessary. If I am guessing a little higher percentage rate, then it starts to get closer to the number of operatives. Clearly, my denominator is not right with 100 operated patients. But everything depends upon what accepted likely rate of complication we think we are going to see in nonoperative measures.
The transpapillary stents I think have a great place, and they really support my theory that ductal anatomy dictates the behavior of pseudocysts. So using transpapillary stents among the ways that we know to nonoperatively treat really fits my bias. It is clear that some ductal anatomy would suggest that is not a reasonable thing. And one of—I guess Dr. Sharp asked whether we saw ductal anatomy. All of the stents that were placed were placed during ERCP, and they did see ductal anatomy, including complete disruption of the duct, and still replace the stents in those patients.
The indication for intervention was less than 4 weeks. I guess Dr. Vogel mentions, and certainly I have had times when the patients are primarily under my care, to drain patients because they can't eat in order to get them out of the hospital and get their nutrition back. Because of the current state and the risks I believe they have of operation, I will use interventions earlier.
The asymptomatic patient, Dr. Yeo knows better than anyone, is a controversial area. His data in his paper from back in the early '90s suggested that patients with a cyst size greater than 7 cm had an extremely low likelihood of resolution and an extremely high likelihood of complication. So we will operate on some asymptomatic patients with very large pseudocysts. However, my experience has been the truly asymptomatic patients are relatively unusual.
We did use—for the patients with persistent drainage—we did use octreotide. Most of the drains were already in place; we had some patients on TPN with octreotide, things like that.
We did look carefully at the comorbidity. Dr. Gadacz asked about that. In fact, the demographics were very similar in the groups. The patients were often quite young, as a matter of fact. Coming in with severe underlying lung disease, heart disease, renal disease, was not seen.
I think the follow-up question is key. And I really do worry that we are unique, this group in this room, for the degree to which we take ownership in the care of a patient the minute we meet them, and we do that preoperatively, intraoperatively, postoperatively, and maybe even years down the line. And I really do worry that interventional radiology will perform a procedure—and I am overstating because I am sure there are some very careful practitioners, but too often the follow-up of those patients is not maintained by those individuals. In a similar fashion, I worry that there are times when GI medicine manages these things that they don't necessarily follow these patients as their own patients in long-term follow-up. I think this is a safer part of surgery. I really hope that we never lose it. And it may be that that is a message that we could take to the rest of our colleagues.
Dr. Sharp asked how many of them were infected preoperative. Only 6 of the 79, from what we can gather, actually were treated for infected pseudocysts. I think too often people see a bunch of fluid and they sort of get focused like a pit bull on that and they better get that fluid out of there without real understanding of what the purpose is in doing that.
Footnotes
Reprints: William H. Nealon, MD, University of Texas Medical Branch, Department of Surgery, 301 University Boulevard, Galveston, TX 77555-0544. E-mail: wnealon@utmb.edu.
REFERENCES
- 1.Cooperman AM. The pancreas revisited, I: diagnosis, chronic pancreatitis: surgical treatment of pancreatic pseudocyst. Surg Clin North Am. 2001;81:2001. [DOI] [PubMed] [Google Scholar]
- 2.Kohler H, Schafmayer A, Ludtke FE, et al. Surgical treatment of pancreatic pseudocysts. Br J Surg. 1987;74:813–815. [DOI] [PubMed] [Google Scholar]
- 3.Ephgrave K, Hunt JL. Presentation of pancreatic pseudocyst: implications for timing of surgical intervention. Am J Surg. 1986;151:749–753. [DOI] [PubMed] [Google Scholar]
- 4.Bradley EL III, Clements JL JR, Gonzalez AC. The natural history of pancreatic pseudocyst: a unified concept of management. Am J Surg. 1979;137:135–141. [DOI] [PubMed] [Google Scholar]
- 5.Vitas GJ, Sarr MG. Selected Management of pancreatic pseudocysts: operative versus expectant management. Surgery. 1992;111:123–130. [PubMed] [Google Scholar]
- 6.Yeo CJ, Bastides JA, Lynch-Nyhan A, et al. The natural history of pancreatic pseudocyst documented by computed tomography. Surg Gynecol Obstet. 1990;170:411–417. [PubMed] [Google Scholar]
- 7.Grace PA, Williamson RCN. Modern management of pancreatic pseudocysts. Br J Surg. 1993;80:573–581. [DOI] [PubMed] [Google Scholar]
- 8.D'Agostino HB, Von Sonnenberg E, Sanchez RB, et al. Treatment of pancreatic pseudocyst with percutaneous drainage and octreotide. Radiology. 1993;187:685–688. [DOI] [PubMed] [Google Scholar]
- 9.Header R, Meyer AA, Jelanko GA, et al. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg. 1999;6:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams DB, Anderson MC. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg. 1992;215:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baillie J. Pancreatic pseudocyst: part I. Gastroentest Endosc. 2004;59:873–879. [DOI] [PubMed] [Google Scholar]
- 12.Barthet M, et al. Management of cyst and pseudocysts complicating chronic pancreatitis. Gastroenterology. 1993;17:270–276. [PubMed] [Google Scholar]
- 13.Chak A. Recent advances in endoscopic ultrasound. endosonographic-guided therapy of pancreatic pseudocysts. Gastrointest Endosc. 2000;52:S23–S27. [DOI] [PubMed] [Google Scholar]
- 14.Kozarek RA, Call TJ, Patterson DJ, et al. Endoscopic transpapillary therapy for disrupted pancreatic duct ans peripancreatic fluid collections. Gastroenterology. 1991;100:1362–1370. [PubMed] [Google Scholar]
- 15.Schultz SM, Leung JWC. Pancreatic endo-therapy for pseudocysts and fluid collections. Gastroentest Endosc. 2002;56. [DOI] [PubMed] [Google Scholar]
- 16.Nealon WH, Matin S. Analysis of surgical success in preventing recurrent acute exacerbations in chronic pancreatitis. Ann Surg. 2001;233:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann Surg. 2002;235:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nealon WH, Bawduniak J, Walser E. Appropriate timing of cholecystectomy in patients who present with moderate to severe gallstone-associated acute pancreatitis with peripancreatic fluid collection. Ann Surg. 2004;239:741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warshaw Al, Rattner DW. Timing of surgical drainage of pancreatic pseudocyst. clinical and chemical criteria. Ann Surg. 1985;202:720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutledge PL, Warshaw AL. Persistent acute pancreatitis: a variant treated by pancreatoduodenectomy. Arch Surg. 1988;123:597–600. [DOI] [PubMed] [Google Scholar]
- 21.Nealon WH, Townsend CM Jr, Thompson JC. Preoperative endoscopic retrograde cholangiopancreatography (ERCP) in patients with pancreatic pseudocyst associated with resolving acute and chronic pancreatitis. Ann Surg. 1989;209:532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neoptolemos JP, London NJ, Carr-Locke DL. Assessment of main pancreatic duct integrity by endoscopic retrograde pancreatography in patients with acute pancreatitis. Br J Surg. 2001;181:411–415. [DOI] [PubMed] [Google Scholar]
- 23.Howard TJ, Moore SA, Saxena R, et al. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery. 2004;136:909–916. [DOI] [PubMed] [Google Scholar]
- 24.Nealon WH, Walser E. Duct drainage alone is sufficient in the operative management of pancreatic pseudocyst in patients with chronic pancreatitis. Ann Surg. 2003;235:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley EL III. A clinically based classification system for acute pancreatitis. Arch Surg. 1993;128:586–590. [DOI] [PubMed] [Google Scholar]
- 26.Maringhuni A, Uomo G, Patti R, et al. Pseudocysts in acute nonalcoholic pancreatitis: incidence and natural history. Dig Dis Sci. 1999;44:1669–1679. [DOI] [PubMed] [Google Scholar]
- 27.Kourtesis G, Wilson SE, Williams RA. The clinical significance of fluid collections in acute pancreatitis. Ann Surg. 1990;56:796–799. [PubMed] [Google Scholar]
- 28.Lau ST, Simchuk EJ, Kozarek RA, et al. A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg. 2001;181:411–415. [DOI] [PubMed] [Google Scholar]
- 29.Neoptolemos JP, London NJ, Carr-Locke DL. Assessment of main pancreatic duct integrity by endoscopic retrograde pancreatography in patients with acute pancreatitis. Br J Surg. 1993;80:94–99. [DOI] [PubMed] [Google Scholar]


