Abstract
Immunoglobulin G1 (IgG1), IgG2a, and IgG2b switch variants were derived from an IgG3 monoclonal antibody directed against the VP3 envelope glycoprotein of lactate dehydrogenase-elevating virus (LDV). Among the four antibodies, IgG2a delayed the onset and progression of LDV-induced polioencephalomyelitis more than did the other subclasses. This suggests that the IgG2a predominance observed in many IgG antibody responses elicited by live viruses could, at least under some circumstances, correspond to the selection of the best protection for the infected host.
Mouse infection with various viruses triggers an antiviral antibody response that is largely restricted to the immunoglobulin G2a (IgG2a) isotype (5). The infectious process itself, rather than some molecular characteristics of viral antigens, is responsible for this isotypic bias of antiviral responses, possibly through the production of gamma interferon (19). Because of the functional properties of the IgG2a subclass, such as complement activation (16), binding to Fc receptors (12), and mediation of antibody-dependent cell-mediated cytotoxicity (15), it is possible that this selection of a particular isotype corresponds to the most effective response to the virus. However, relatively few studies have compared the protective abilities of the different antiviral antibody IgG subclasses. Whereas some authors have shown that, in their models, the protection did not depend upon the IgG subclass of the antiviral antibodies (1, 9, 17, 24), others, using either polyclonal antibodies or unrelated monoclonal antibodies, have observed that IgG2a displayed a stronger effect (2, 13, 18, 21, 28). However, differences in specificity and/or affinity could account for variations in neutralizing ability. To avoid this pitfall, switch mutants can be derived from a given monoclonal antibody. This approach has shown that IgG2a antibodies directed against herpes simplex virus (14) and yellow fever virus (27) were the most protective in vivo. However, with anti-Sindbis virus antibodies, no evidence that the IgG subclass is important for in vitro clearance of the virus was found but the IgG2a isotype was not included in the study (30).
Lactate dehydrogenase-elevating virus (LDV) induces life-long viremia in infected mice despite the production of neutralizing antibodies (26). Anti-LDV monoclonal antibodies reacting with the VP3 viral protein and derived from both infected mice and animals immunized with inactivated virions have been shown to partly neutralize the virus in vitro (7, 10). Although IgG2a is the predominant isotype of the anti-LDV response elicited by infection (6), this partial in vitro neutralization has been reported with monoclonal antibodies of all four IgG subclasses, with slightly better efficacy of IgG3 (7, 10). However, heterogeneity in LDV populations that, in most cases, contain some antibody-resistant quasispecies may explain the rapid emergence in vivo of these nonneutralizable virions in the presence of a normal antibody response and therefore the persistence of viremia in immunocompetent animals (3, 4, 22). In contrast, both polyclonal and monoclonal anti-LDV antibodies can suppress a lethal polioencephalomyelitis that develops in some mice, like C58 and AKR animals, after infection with antibody-sensitive neurotropic LDV quasispecies (11, 23). It is not known whether the subclass of anti-LDV IgG antibodies determines their ability to protect mice against this polioencephalomyelitis.
To assess the role of the isotype in the antiviral efficacy of anti-LDV antibodies, switch mutants were derived from C3904H12, a neutralizing IgG3 anti-VP3 monoclonal antibody originally obtained from an infected BALB/c mouse (6, 7). The spontaneous isotype switch variants secreting IgG1, IgG2b, and IgG2a were sequentially isolated by the following procedure (adapted from references 8 and 29). The IgG3 parental hybridoma was first treated by two exposures to rat anti-IgG3 antibody LO-MG3-13 (obtained from H. Bazin, Brussels, Belgium), followed by rabbit complement (Cedarlane, Hornby, Ontario, Canada) for cell lysis. Ten thousand nonlysed cells per well were then grown to confluency in 96-well flat-bottom plates in Iscove’s medium containing 10% fetal calf serum and supplemented with 0.24 mM l-asparagine, 0.55 mM l-arginine, 1.5 mM l-glutamine, and 0.05 mM 2-mercaptoethanol. All wells were screened by radioimmunoassay (RIA) for the presence of IgG1 with a locally produced goat anti-rabbit immunoglobulin polyclonal antibody, followed by a rabbit anti-mouse IgG1 antibody (gift of J. Van Snick). Cells from wells that appeared to be positive were subcultured. Four or five rounds were necessary to ensure that class switch antibodies were present. Wells found by enzyme-linked immunosorbent assay (ELISA) to contain IgG1-secreting cells were further cloned at least three times by limiting dilution. A cell line producing antibody of the IgG2b isotype was isolated from the IgG1-secreting line in an identical manner, by using locally produced rat anti-mouse IgG1 for lysis of IgG1-positive cells (D5002B5; gift of J. Van Snick) and a rat anti-mouse IgG2b monoclonal antibody for detection (LO-MG2b-1; obtained from H. Bazin). Finally, a cell line producing IgG2a was obtained from the IgG2b hybridoma with a rat anti-mouse IgG2a monoclonal antibody obtained from H. Bazin (LO-MG2a-9) without prior lysis of IgG2b-positive cells.
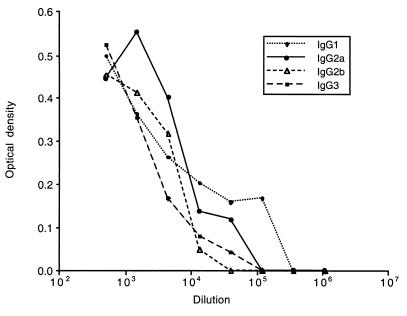
Initial detection of switch variants required the high sensitivity of the RIA, whereas after subcloning, screening by ELISA was sensitive enough. For both assays, wells were coated with a goat anti-rabbit polyclonal antibody, followed by a rabbit anti-mouse IgG1 antibody, with rat anti-mouse IgG2b, or with rat anti-mouse IgG2a monoclonal antibodies (obtained from H. Bazin). Bound immunoglobulin was revealed with I125-labeled sheep anti-mouse IgG antibodies (RIA; Amersham Belgium, Ghent, Belgium), peroxidase-conjugated donkey antibodies (ELISA; Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.), or rat anti-mouse IgG (LO-MK1; H. Bazin). The anti-LDV specificity of switch mutant antibodies was checked by ELISA on plates coated with pelleted virus (6) and revealed with peroxidase-conjugated rat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 antibodies. In addition, by using the same ELISA, inhibition of the binding to LDV of each switch variant antibody with every other isotype confirmed that switching was not associated with a change in specificity (data not shown). Switch variants were used as sterile ascitic fluids. Based on preliminary electrophoresis and ELISAs, ascitic fluids were diluted as follows to contain similar anti-LDV antibody concentrations: IgG1, 1/10; IgG2a, 1/1; IgG2b, 1/10; IgG3, 1/5. Serial dilutions of these initial ascitic fluid dilutions were then tested by ELISA on plates coated with pelleted virus by using as the revealing second antibody a peroxidase-conjugated rat monoclonal antibody reacting with mouse kappa light-chain immunoglobulin (LO-MK-1; obtained from LO/IMEX, University of Louvain, Brussels, Belgium) after it had been checked that this revealing antibody reacted similarly with all of the mouse IgG subclasses. As shown in Fig. 1, this ELISA confirmed that the chosen ascitic fluid dilutions contained very similar amounts of anti-LDV antibodies.
FIG. 1.
Anti-LDV contents of switch variant ascitic fluids. Initial dilutions of ascitic fluids (IgG1, 1/10; IgG2a, 1/1; IgG2b, 1/10; IgG3, 1/5) were serially diluted as indicated and tested by ELISA on LDV-coated wells. A rat monoclonal antibody reacting similarly with all mouse IgG subclasses was used as the revealing reagent.
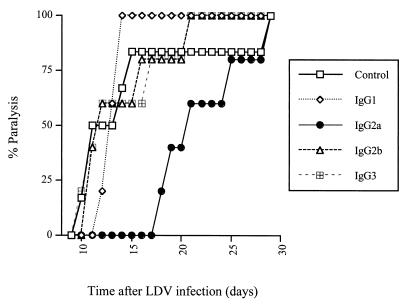
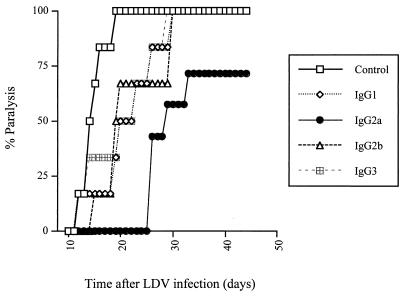
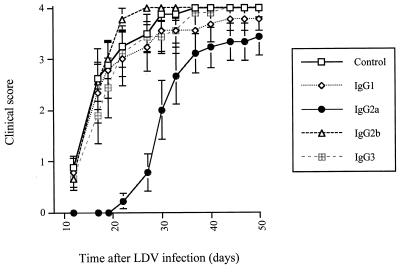
The protective effect of antibody switch variants on LDV-induced polioencephalomyelitis was tested in female C58/J mice from The Jackson Laboratory (Bar Harbor, Maine) that were maintained in microisolators with sterile food and water and used at a minimum age of 4 months. Animals were infected by intraperitoneal injection of neurovirulent LDV strain c (kindly given by E. K. Godeny; 20). This virus had been amplified once in C58/J mice, and the equivalent of 2 μl of plasma from mice infected for 1 day, diluted in 500 μl of saline, was used for infection. C58/J mice were immunosuppressed by three intraperitoneal injections of 5 mg of cyclophosphamide (Cycloblastine; Pharmacia and Upjohn) on day 2 before infection and days 5 and 12 postinfection (p.i.). Progressive and severe paralysis developed about 2 weeks p.i., leading to death of most of the animals that were not protected by anti-LDV antibodies. On days 2, 4, and 7 p.i., separate groups of mice were treated intravenously with 100 μl of ascitic fluid containing an IgG1, IgG2a, IgG2b, or IgG3 anti-VP-3 LDV switch variant that was diluted in sterile NaCl to ensure the use of equivalent antibody amounts, based on ELISA results, as shown in Fig. 1. As shown in Fig. 2 for a typical experiment, the IgG2a isotype delayed paralysis, usually by 7 to 14 days, in all treated mice, although most of the animals finally developed the disease. In contrast, the other subclasses had little or no effect on the onset of polioencephalomyelitis. To formally rule out the hypothesis that the better protection conferred by the IgG2a isotype was due to differences in antibody concentrations, the efficacy of undiluted ascitic fluid was tested. From the ELISA results, it could be estimated that in this experiment, animals received approximately 10 times more IgG1 and IgG2b and 5 times more IgG3 than IgG2a. Results shown in Fig. 3 indicate that while some protection could be observed with the IgG1, IgG2b, and IgG3 switch mutants, the efficacy of the IgG2a antibody was still much better. For instance, 3 weeks after LDV inoculation, none of the animals that received the IgG2a anti-LDV switch mutant showed signs of polioencephalomyelitis whereas all of the mice that received the virus alone and approximately half of the animals that were treated with the IgG1, IgG2b, or IgG3 isotype had started developing paralysis. In addition, a 1/5 dilution of the IgG2a switch variant had a still better protective effect than the undiluted IgG1, IgG2b, and IgG3 antibodies, although it was slightly less efficient than undiluted IgG2a (data not shown). Prolongation of the treatment after day 7 p.i. by a weekly antibody injection did not completely prevent the disease (Fig. 4). Finally, when the course of polioencephalomyelitis was assessed with clinical scores (1, paresis of one or two legs; 2, paresis of more than two legs; 3, flaccid paralysis of more than two legs; 4, total flaccid paralysis), IgG2a anti-LDV antibody consistently reduced disease severity over time while the other subclasses were either less efficacious or not protective at all (results of one experiment are shown in Fig. 4). However, IgG2a did not clearly change the final outcome, as most animals finally developed a lethal disease.
FIG. 2.
Delayed LDV-induced polioencephalomyelitis after treatment with an anti-LDV IgG2a switch variant. Onset of paralysis was monitored daily in groups of five or six immunosuppressed C58 mice infected with LDV and treated on days 2, 4, and 7 p.i. with NaCl alone or with anti-LDV switch variant-containing ascitic fluids diluted in NaCl to obtain similar antibody concentrations (IgG1, 1/10; IgG2a, 1/1; IgG2b, 1/10; IgG3, 1/5).
FIG. 3.
Prevention of LDV-induced polioencephalomyelitis by treatment with undiluted anti-LDV switch mutant-containing ascitic fluids. Onset of paralysis was monitored daily in groups of six or seven immunosuppressed C58 mice infected with LDV and treated on days 2, 4, and 7 p.i. with NaCl or with undiluted anti-LDV switch variant-containing ascitic fluids. The antibody concentration was estimated by ELISA to be approximately 10 times higher for IgG1 and IgG2b and 5 times higher for IgG3 than for IgG2a.
FIG. 4.
Clinical course of LDV-induced polioencephalomyelitis in mice treated with anti-LDV switch mutants. The clinical course of paralysis was monitored in groups of eight or nine immunosuppressed C58 mice infected with LDV and treated on days 2, 4, and 7 p.i. and then once weekly with NaCl alone or with anti-LDV switch variant-containing ascitic fluids diluted in NaCl to obtain similar antibody concentrations (IgG1, 1/10; IgG2a, 1/1; IgG2b, 1/10; IgG3, 1/5). The clinical score was determined as follows: 1, paresis of one or two legs; 2, paresis of more than two legs; 3, flaccid paralysis of more than two legs; 4, total flaccid paralysis. Mice were sacrificed at stage 4. Results are shown as the mean score ± the standard error.
These results clearly indicate that the subclass of anti-LDV IgG antibodies affects their ability to protect mice against LDV-induced polioencephalomyelitis, with greater efficacy of the IgG2a isotype. Whereas the mechanisms of this antibody protection remain unknown, it may be postulated that they involve an IgG2a-mediated function such as Fc receptor binding or complement activation. This in vivo effect clearly differs from the in vitro neutralizing activity of anti-LDV monoclonal antibodies that has been shown after incubation of LDV with IgG3 and, to a lesser extent, IgG2a anti-VP-3 antibodies (7, 10; data obtained with our switch mutant antibodies not shown) but not with IgM anti-VP-3 and IgG1 anti-VP-1 antibodies and that could be related to agglutination of viral particles, as suggested by its strong increase when a monoclonal rheumatoid factor was added to anti-LDV antibodies, or to binding of multiple antibody molecules to LDV VP3 glycoprotein, leading to disruption of the virus (25). Interestingly, other virus-induced neurological diseases, triggered by lymphocytic choriomeningitis virus and yellow fever virus, appear to be better prevented by IgG2a antibodies than by other isotypes (2, 27). Thus, our observation confirms that, under some circumstances, the selection of an antiviral IgG2a antibody response is beneficial for the infected host. To provide the best protection against viral pathogenicity, vaccination protocols should therefore take into account the isotypic distribution of antibodies.
Acknowledgments
We are indebted to J. Van Snick and P. Masson for critical reading of the manuscript, to F. Cormont for helpful discussions, and to T. Briet, M.-D. Gonzales, and A. Thonon for expert technical assistance.
This work was supported by the Fonds National de la Recherche Scientifique (FNRS), the Fonds de la Recherche Scientifique Médicale (FRSM), the Loterie Nationale, the Fonds de Développement Scientifique (UCL), the State Prime Minister’s Office—S.S.T.C. (Interuniversity Attraction Poles grant 44), and the “Actions de recherche concertées” from the Communauté française de Belgique—Direction de la Recherche scientifique (Concerted Actions grant 99/04-239), Belgium. D.M.-G. is a scientific research worker and J.-P.C. is a research director with the FNRS.
REFERENCES
- 1.Bachmann, M. F., U. Kalinke, A. Althage, G. Freer, C. Burkhart, H.-P. Roost, M. Aguet, H. Hengartner, and R. M. Zinkernagel. 1997. The role of antibody concentration and avidity in antiviral protection. Science 276:2024–2027. [DOI] [PubMed] [Google Scholar]
- 2.Baldridge, J. R., and M. J. Buchmeier. 1992. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J. Virol. 66:4252–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, Z., K. Li, and P. G. W. Plagemann. 2000. Neuropathogenicity and sensitivity to antibody neutralization of lactate dehydrogenase-elevating virus are determined by polylactosaminoglycan chains on the primary envelope glycoprotein. Virology 266:88–98. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Z., R. R. R. Rowland, G. W. Anderson, G. A. Palmer, and P. G. W. Plagemann. 1997. Coexistence in lactate dehydrogenase-elevating virus pools of variants that differ in neuropathogenicity and ability to establish a persistent infection. J. Virol. 71:2913–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutelier, J.-P., J. T. M. van der Logt, F. W. A. Heessen, G. Warnier, and J. Van Snick. 1987. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 165:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutelier, J.-P., E. Van Roost, P. Lambotte, and J. Van Snick. 1986. The murine antibody response to lactate dehydrogenase-elevating virus. J. Gen. Virol. 67:1099–1108. [DOI] [PubMed] [Google Scholar]
- 7.Coutelier, J.-P., and J. Van Snick. 1988. Neutralization and sensitization of lactate dehydrogenase-elevating virus with monoclonal antibodies. J. Gen. Virol. 69:2097–2100. [DOI] [PubMed] [Google Scholar]
- 8.Faguet, G. B., and J. F. Agee. 1993. A simple technique for the rapid enrichment of class and subclass hybridoma switch variants. A 1000-fold enrichment in half the time, for half the cost. J. Immunol. Methods 165:217–224. [DOI] [PubMed] [Google Scholar]
- 9.Fleming, J. O., R. A. Shubin, M. A. Sussman, N. Casteel, and S. A. Stohlman. 1989. Monoclonal antibodies to the matrix (E1) glycoprotein of mouse hepatitis virus protect mice from encephalitis. Virology 168:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harty, J. T., and P. G. W. Plagemann. 1988. Formalin inactivation of the lactate dehydrogenase-elevating virus reveals a major neutralizing epitope not recognized during natural infection. J. Virol. 62:3210–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harty, J. T., and P. G. W. Plagemann. 1990. Monoclonal antibody protection from age-dependent poliomyelitis: implications regarding the pathogenesis of lactate dehydrogenase-elevating virus. J. Virol. 64:6257–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heusser, C. H., C. L. Anderson, and H. M. Grey. 1977. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J. Exp. Med. 145:1316–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hocart, M. J., J. S. Mackenzie, and G. A. Stewart. 1989. The immunoglobulin G subclass responses of mice to influenza A virus: the effect of mouse strain, and the neutralizing abilities of individual protein A-purified subclass antibodies. J. Gen. Virol. 70:2439–2448. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaka, S. T., P. Piacente, J. Silva, and E. M. Mishkin. 1995. IgG subtype is correlated with efficiency of passive protection and effector function of anti-herpes simplex virus glycoprotein D monoclonal antibodies. J. Infect. Dis. 172:1108–1111. [DOI] [PubMed] [Google Scholar]
- 15.Kipps, T. J., P. Parham, J. Punt, and L. A. Herzenberg. 1985. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J. Exp. Med. 161:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klaus, G. G. B., M. B. Pepys, K. Kitajima, and B. A. Askonas. 1979. Activation of mouse complement by different classes of mouse antibody. Immunology 38:687–695. [PMC free article] [PubMed] [Google Scholar]
- 17.Kümel, G., H. C. Kaerner, M. Levine, C. H. Schröder, and J. C. Glorioso. 1985. Passive immune protection by herpes simplex virus-specific monoclonal antibodies and monoclonal antibody-resistant mutants altered in pathogenicity. J. Virol. 56:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leclerc, C., P. Martineau, S. van der Werf, E. Deriaud, P. Duplay, and M. Hofnung. 1990. Induction of virus-neutralizing antibodies by bacteria expressing the C3 poliovirus epitope in the periplasm. The route of immunization influences the isotypic distribution and the biologic activity of the antipoliovirus antibodies. J. Immunol. 144:3174–3182. [PubMed] [Google Scholar]
- 19.Markine-Goriaynoff, D., J. T. M. van der Logt, C. Truyens, T. D. Nguyen, F. W. A. Heessen, G. Bigaignon, Y. Carlier, and J.-P. Coutelier. 2000. IFN-γ-independent IgG2a production in mice infected with viruses and parasites. Int. Immunol. 12:223–230. [DOI] [PubMed] [Google Scholar]
- 20.Martinez, D., M. A. Brinton, T. G. Tachovsky, A. H. Phelps. 1980. Identification of lactate dehydrogenase-elevating virus as the etiological agent of genetically restricted, age-dependent polioencephalomyelitis of mice. Infect. Immun. 27:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKendall, R. R., and W. Woo. 1988. Murine IgG subclass responses to herpes simplex virus type 1 and polypeptides. J. Gen. Virol. 69:847–857. [DOI] [PubMed] [Google Scholar]
- 22.Monteyne, P., and J.-P. Coutelier. 1994. Difference in neutralization between lactate dehydrogenase-elevating virus isolated from acutely and chronically infected mice. J. Gen. Virol. 75:1173–1176. [DOI] [PubMed] [Google Scholar]
- 23.Murphy, W. H., J. F. Nawrocki, and L. R. Pease. 1983. Age-dependent paralytic viral infection in C58 mice: possible implications in human neurologic disease. Prog. Brain Res. 59:291–303. [DOI] [PubMed] [Google Scholar]
- 24.Palladino, G., K. Mozdzanowska, G. Washko, and W. Gerhard. 1995. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 69:2075–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plagemann, P. G. W., J. T. Harty, and C. Even. 1992. Mode of neutralization of lactate dehydrogenase-elevating virus by polyclonal and monoclonal antibodies. Arch. Virol. 123:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowson, K. E. K., B. W. J. Mahy, and M. Bendinelli. 1966. Riley virus neutralizing activity in the plasma of infected mice with persistent viraemia. Virology 28:775–778. [DOI] [PubMed] [Google Scholar]
- 27.Schlesinger, J. J., M. Foltzer, and S. Chapman. 1993. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology 192:132–141. [DOI] [PubMed] [Google Scholar]
- 28.Smucny, J. J., E. P. Kelly, P. O. Macarthy, and A. D. King. 1995. Murine immunoglobulin G subclass responses following immunization with live dengue virus or a recombinant dengue envelope protein. Am. J. Trop. Med. Hyg. 53:432–437. [DOI] [PubMed] [Google Scholar]
- 29.Spira, G., A. Bargellesi, J.-L. Teillaud, and M. D. Scharff. 1984. The identification of monoclonal class switch variants by sib selection and an ELISA assay. J. Immunol. Methods 74:307–315. [DOI] [PubMed] [Google Scholar]
- 30.Ubol, S., B. Levine, S.-H. Lee, N. S. Greenspan, and D. E. Griffin. 1995. Roles of immunoglobulin valency and the heavy-chain constant domain in antibody-mediated downregulation of Sindbis virus replication in persistently infected neurons. J. Virol. 69:1990–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]