Abstract
The capacity of recombinant adenoviruses (rAd) to induce immunization against their transgene products has been well documented. In the present study, we evaluated the vaccinal adjuvant role of rAd independently of its vector function. BALB/c mice received one subcutaneous injection of a mixture of six lipopeptides (LP6) used as a model immunogen, along with AdE1° (109 particles), a first-generation rAd empty vector. Although coinjected with a suboptimal dose of lipopeptides, AdE1° significantly improved the effectiveness of the vaccination, even in the absence of booster immunization. In contrast to mice that received LP6 alone or LP6 plus a mock adjuvant, mice injected with AdE1° plus LP6 developed both a polyspecific T-helper type 1 response and an effector CD8 T-cell response specific to at least two class I-restricted epitopes. The helper response was still observed when immunization was performed using LP6 plus a mixture of soluble capsid components released from detergent-disrupted virions. When mice were immunized with LP6 and each individual capsid component, i.e., hexon, penton base, or fiber, the results obtained suggested that hexon protein was responsible for the adjuvant effect exerted by disrupted Ad particles on the helper response to the immunogen. Our results thus have some important implications not only in vaccinology but also for gene therapy using rAd vectors.
Some viruses have the natural ability to induce vigorous inflammatory and immune responses. Among them, adenoviruses (Ad) are empirically considered good priming vaccination vectors. Preclinical and clinical studies using recombinant Ad (rAd) as vaccination vectors have shown their capacity to generate intense and prolonged cellular Th1 and humoral responses against the transgene product (11, 17, 30, 38, 44). This effect could be due, at least in part, to the strong immune response induced by Ad itself (11, 47, 48). Other studies have demonstrated the important implication of the capsid components in cellular and humoral recognition of Ad (9, 10, 15, 19, 29). Therefore, the specific recognition of individual capsomers may account for some of the immunostimulatory properties of Ad. However, the early events underlying the initial nonspecific stimulation of the immune system induced by capsid components have not yet been elucidated.
Several early steps of the virus life cycle depend on the efficiency and legitimacy of interactions of Ad structural proteins with cell components, e.g., attachment of the virus to the cell receptors involves both fiber and penton base, and penton base has been shown to be responsible for the escape of the virus from the endosome (reviewed in reference 45). These early events take place before the onset of transcription of viral genes implicated in immune evasion. It has been demonstrated that rAd induces dendritic cell activation comparable to that induced by wild-type Ad, irrespective of the presence or absence of the E1, E2A, E3, or E4 region (39). Thus, viral capsomer-cell interactions are probably critical events for the activation of innate and adaptive immunity. Indeed, it has been shown that Ad fiber is mitogenic in vitro and can trigger gamma interferon (IFN-γ) production by T cells (43). More recently, it has been reported that rAd induces the synthesis of a cascade of C-X-C and C-C chemokines within the first hours postinjection, independently of viral gene expression (34). In particular, the induction of IP-10, a chemokine implicated in Th1 responses, was related to direct stimulation of the transcription factor NF-κB by the viral capsid (1).
In the present study, we used mice to examine the possibility that rAd might function as immune adjuvants, irrespective of their gene vector function. For this purpose, we used a mixture of six HIV-1 Gag-, Nef- and Env-derived lipopeptides, constituting LP6, as a model immunogen. In contrast to proteins or peptides, this lipopeptide formulation can induce immune responses when injected without an adjuvant. Indeed, the repetitive injection of LP6 has previously been shown to induce cellular and humoral immune responses in seronegative volunteers (12). Our results demonstrated that one subcutaneous injection of whole Ad virions was sufficient to potentiate both CD4+ and CD8+ T-cell responses directed to LP6. The helper response was maintained when free capsomers released from deoxycholate-disrupted Ad particles were coinjected. Further experiments using purified Ad capsid components strongly suggested that this immunostimulatory effect was essentially due to hexon capsomers, since hexon alone was able to induce the cellular response.
MATERIALS AND METHODS
Lipopeptides and peptides.
Synthesis of the six lipopeptides constituting the LP6 mixture (BACHEM Feinchemikalien, Bubendorf, Switzerland) has been described previously (12, 22). Briefly, the three Nef and the two Gag lipopeptides were obtained, respectively, from the human immunodeficiency virus (HIV) LAI Nef and Gag protein sequences. The Env lipopeptide was derived from a V3-Env gp120 consensus sequence. The lipopeptides were formulated as mixed micelles, solubilized in 80% acetic acid, subjected to sterilizing filtration, and lyophilized. The purity profile was assessed by high-pressure liquid chromatography. The LP6 batches underwent extensive pharmacotoxicological testing and were absolutely devoid of endotoxin contamination. The six long peptides, corresponding to the lipopeptide immunogens, and six short overlapping peptides (8 to 9 amino acids) were obtained from Neosystem (Strasbourg, France). The sequences of these peptides are given in Table 1.
TABLE 1.
Lipopeptides of the immunogen, corresponding long peptides, and short H-2d epitopic peptides
| Lipopeptide sequencea | Long peptide nameb | Short peptide
|
||
|---|---|---|---|---|
| Nameb | Sequencec | Potential H2 restrictionc | ||
| VGFPVTPQVPLRPMTYKAAVDLSHFLKEKGGL K(Palm)-NH2 | Nef66-97 | Nef77-85 | RPMTYKAAV | Ld |
| TQGYFPDWQNYTPGPGVRYPLTFGWCYKLVP K(Palm)-NH2 | Nef117-147 | Nef126-133 | NYTPGPGV | Kd |
| EWRFDSRLAFHHVARELHPEYFKN K(Palm)-NH2 | Nef182-205 | Nef190-198 | AFHHVAREL | Kd |
| DLNTMLNTVGGHQAAMQMLKETINEEAAEWDR K(Palm)-NH2 | Gag183-214 | Gag197-205 | AMQMLKETI | Kd |
| NPPIPVGEIYKRWIILGLNKIVRMYSPTSILD K(Palm)-NH2 | Gag253-284 | Gag253-262 | IYKRWIIL | Kd |
| TRPNNNTRKSIHIGPGRAFYATGEIIGDIRQAH K(Palm)-NH2 | Env303-335 | Env315-323 | IGPGRAFYA | Dd |
Sequences were modified in the C-terminal position by a palmitoyl-lysylamide group, K(Palm)-NH2.
Peptide names are derived from the positions of their first and last amino acids in the HIV LAI sequence for Nef and Gag and in the HIV BX08 sequence for Env.
The short peptides were selected for in vitro studies according to their anchoring residues to the H-2d molecules (boldfaced), except for Gag197-205, which had been previously demonstrated by Mata et al. to be H2-Kd restricted (27).
Virus and capsid components.
Deletion mutant AdE1° was provided by M. Mehtali (Transgène, Strasbourg, France). It was derived from a human Ad serotype 5 genome and carried a deletion of the early regions E1 (nucleotides [nt] 459 to 3327) and E3 (nt 28592 to 30480) (24). One PFU of AdE1° measured by the standard plaque assay on 293 cells corresponded to 100 physical particles estimated by optical density measurement of the viral DNA content (28). The virus concentration was 5 × 108 particles/μl of saline buffer containing 0.005% Tween 80. For capsid disruption, AdE1° samples (109 particles) in 50 mM Tris-HCl were treated with sodium deoxycholate (DOC; final concentration, 0.5%) and heated at 56°C for 90 s. Treatment with such a mild detergent has been shown to disrupt the viral capsid and to release the nucleoprotein core, groups of nine hexon capsomers, free peripentonal hexons, penton base, and fiber capsomers (2, 3, 35).
Isolation of Ad hexon, penton base, and fiber capsomers.
Two sources of Ad capsid structural proteins were used: (i) the pool of soluble antigens recovered from Ad2-infected HeLa cell lysates (4) and (ii) individual recombinant Ad proteins expressed in baculovirus-infected Sf9 cells (20, 21, 37). The penton base and fiber used were recombinant proteins isolated from insect cells. The hexon samples were isolated from HeLa or Sf9 cells, as indicated. The three native proteins, Ad5 fiber, Ad2 hexon (88.0% homology with Ad5 hexon [7]), and Ad2 penton base (98.6% homology with Ad5 penton base [36]), were purified as previously described (4), with some modifications. Anion-exchange chromatography was performed using a high-performance liquid chromatography system (BioLogic DuoFlow; Bio-Rad) and a DEAE-Sepharose Fast Flow column (DFF-100; Sigma) equilibrated in 50 mM sodium phosphate buffer, pH 6.8 (PB-50). Samples of 2 to 3 mg of protein were applied to the column, and elution was obtained by applying a 0.0 to 0.6 M NaCl gradient in PB-50. Fiber protein was eluted at 200 mM salt, penton base was eluted at 250 mM salt, and hexon was eluted at 425 mM salt. Protein samples were then further purified and concentrated using concentrator membranes with a 100-kDa cutoff (Vivaspin-100; Vivascience Ltd., Binbrook Lincoln, United Kingdom). The final protein concentration was estimated using the Bradford assay (Bio-Rad). Protein samples were analyzed by conventional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and immunoblotting, as previously described (20, 21). The recombinant trimeric Ad2 hexon was obtained by coexpression with a recombinant Ad2 100,000-molecular-weight (100K) protein in Sf9 cells (S.-S. Hong and P. Boulanger, unpublished data). Hexon trimers (23) were separated from the 100K protein (6.2S) by ultracentrifugation in a sucrose density gradient (23) prior to the DEAE-Sepharose chromatographic step (unpublished data).
Immunization of mice and cell lines.
BALB/c mice (H-2d) were purchased from Harlan (Gannat, France). Groups of three mice were immunized subcutaneously at the tail base with LP6 (10 μg of each lipopeptide). LP6 was resuspended in an isotonic glucose-mannitol mixed solution and injected alone or mixed with either AdE1° (109 particles/mouse), DOC-disrupted AdE1° (109 particles/mouse), mock Ad adjuvant (virus storage buffer), or one of the major capsid components, hexon, penton base, and fiber (1 μg/mouse), in a final volume of 100 μl. Ten days later, mice were sacrificed and the spleens from each mouse group were removed and pooled.
Proliferation and cytokine release assays.
Triplicate cultures of spleen cells (5 × 105/well in 96-well microplates) were performed in culture medium (RPMI 1640, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 50 μM β-mercaptoethanol) supplemented with 5% BALB/c serum (Harlan Sera-Lab, Loughborough, United Kingdom). Various concentrations of the six long peptides (from 30 to 0.03 μg/ml), medium alone, or 5 μg of concanavalin A (ConA)/ml was added. Aliquots of supernatant were removed at 24, 36, and/or 48 h, respectively, for assessment of interleukin-2 (IL-2), IFN-γ, and/or IL-4 production. IL-2 secretion was assayed using the CTL-L2 bioassay, and IL-4 was assayed using the CT4-S bioassay. IFN-γ was quantified by enzyme-linked immunosorbent assay (ELISA). Standard curves were obtained using recombinant mouse cytokines (R&D Systems). After 3 days of culture, proliferation was measured by adding 1 μCi of [3H]thymidine (Amersham)/well 18 h before harvesting. Results were expressed as mean counts per minute from triplicate tests. In all experiments presented or cited in Results, levels of proliferation of the different spleen cell samples in response to ConA were similar.
Anti-Gag253-284 ELISA.
Flat-bottom 96-well plates (Maxisorp; Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μl of Gag253-284 at a concentration of 5 μg/ml in a 0.1 M Na2CO3 buffer (pH 9.6) and then blocked for 2 h at room temperature with phosphate-buffered saline (PBS) with 0.05% Tween 20 and 5% fetal calf serum (PBT buffer). After several washes (PBS-Tween), duplicate aliquots of sera diluted in PBT were incubated for 2 h at room temperature. Plates were then washed, and a rabbit anti-mouse immunoglobulin G (IgG) conjugated to alkaline phosphatase was added for 1 h. After several washes, the enzymatic activity was revealed with a fluorescent substrate solution composed of 4-methylumbelliferyl phosphate (Sigma). Results are expressed as means of fluorescent activities after subtraction of the plate background.
Cell lines and ELISPOT-IFN-γ assay.
After overnight incubation with each long peptide (at 10 μg/ml) in culture medium supplemented with 10% fetal calf serum, spleen cells were washed and resuspended at 2 × 106/ml in 24-well plates. Human recombinant IL-2 (rIL-2) (10 U/ml; Boehringer GmbH, Mannheim, Germany) was added at day 3, and cells were tested in an ELISPOT-IFN-γ assay 7 days after the restimulation.
Nitrocellulose microplates (Millipore) were coated with 5 μg of an anti-mouse IFN-γ monoclonal antibody (PharMingen)/ml. Duplicate aliquots of spleen cells (5 × 105/well for freshly isolated cells and 2 × 105/well for day-7 cell lines) were added together with the six long peptides and short peptides (10 μg/ml ex vivo and 1 μg/ml at day 7) in culture medium supplemented with 10% fetal calf serum and with (ex vivo) or without (day 7) 30 U of human rIL-2/ml. After 24 h of incubation, plates were washed in 1× PBS-0.05% Tween 20 and a biotinylated rat anti-mouse IFN-γ antibody (PharMingen) was added for 18 h at 4°C. Spots were visualized by adding, successively, alkaline-phosphatase-labeled ExtrAvidin (Sigma Chemical Co.) for 1 h and substrate (Bio-Rad) for 30 min. The number of spots, each of them representing an IFN-γ-secreting cell, were counted with a transmitted-light stereomicroscope using image-analyzing software connected to a camera (KS ELISPOT system; Carl Zeiss Vision, Hallbergmoos, Germany). Results are expressed as the number of spots per 106 cells after subtraction of the background obtained with an irrelevant peptide (background levels are specified in figure legends). IFN-γ secretion in response to ConA (5 μg/ml) was verified in parallel.
CD4 and CD8 T-cell separation.
Fresh spleen cells were separated by magnetic cell sorting using magnetic beads conjugated to monoclonal rat anti-mouse CD8a and CD4 antibodies (MACS MicroBeads; Miltenyi Biotec, Bergisch Gladbach, Germany). The purity (>95% CD8+ or CD4+ T cells in the positive fraction) was assessed by fluorescence-activated cell sorter. CD8+ and CD4+ T cells separated from the day-7 sample of restimulated spleen cells were used in an ELISPOT-IFN-γ assay at a concentration of 105 cells/well, as shown in Fig. 3B. Ex vivo separated CD8+ T cells were used in an ELISPOT-IFN-γ assay at a concentration of 2 × 105 cells/well, in parallel with nonpurified spleen cells (5 × 105 cells/well), as shown in Fig. 4. CD8− T cells (38 to 42% CD4+ T cells, versus 35 to 40% in total spleen cells) were tested in a proliferation assay at a concentration of 5 × 105 cells/well, as shown in Fig. 1. CD4+ T cells (3 × 105 cells/well) were tested in a proliferation assay, as shown in Fig. 8.
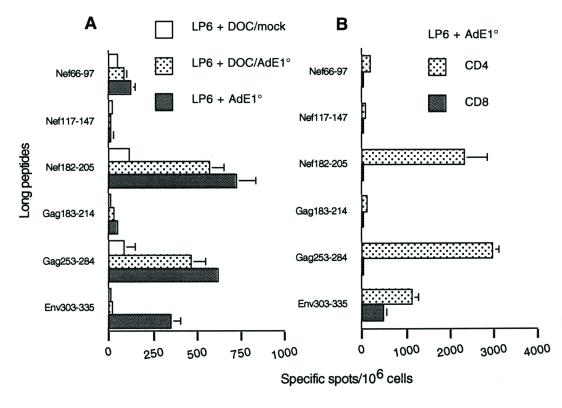
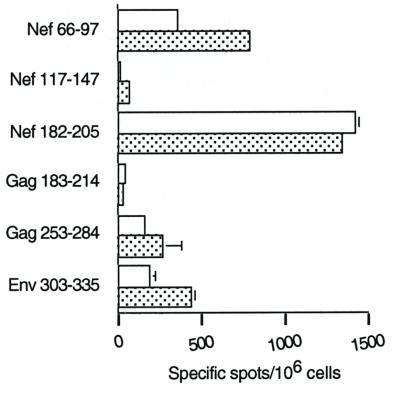
FIG. 3.
Day-7 ELISPOT-IFN-γ responses of mice immunized with LP6 plus DOC-treated AdE1°. The six long peptides (sequences given in Table 1), at a final concentration of 1 μg/ml, were incubated for 24 h with day-7-samples of spleen cells. (A) Total spleen cells from mice immunized with either LP6 plus a DOC-treated mock adjuvant, LP6 plus DOC-treated AdE1°, or LP6 plus untreated AdE1°. (B) CD4+ and CD8+ purified spleen cells from mice immunized with either LP6 alone or LP6 plus AdE1°. Background values obtained with an irrelevant peptide (<25 spots per 106 cells) were subtracted from the mean values obtained with each specific peptide, and results were expressed as specific spots per 106 cells (± standard deviations).
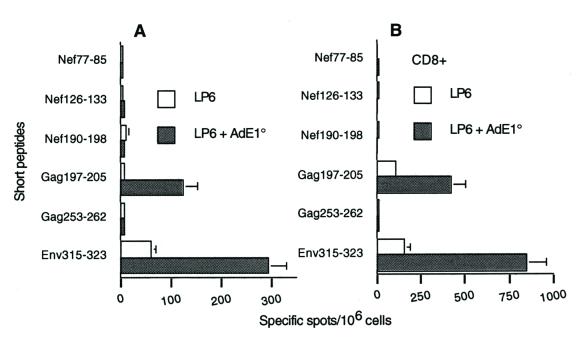
FIG. 4.
Ex vivo ELISPOT-IFN-γ responses. Six short peptides overlapping the long peptide sequences (see Table 1), at a final concentration of 10 μg/ml, and human rIL-2 at a final concentration of 30 U/ml were incubated for 24 h with freshly isolated total spleen cells (A) or CD8+ purified spleen cells (B) from mice immunized with LP6 alone or LP6 plus AdE1°. Background values obtained with an irrelevant peptide (<10 spots per 106 cells in both cases) were subtracted from the mean values from triplicate tests obtained with each specific peptide, and results wereexpressed as specific spots per 106 cells (± standard deviations).
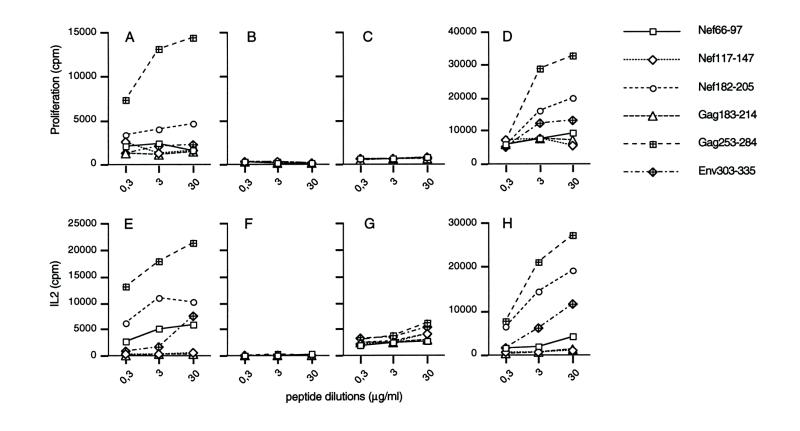
FIG. 1.
Proliferation and IL-2 secretion in response to the six long peptides. Spleen cells from mice immunized with either LP6 plus AdE1° (A and E), LP6 alone (B and F), or AdE1° alone (C and G) and spleen cells depleted of CD8+ T cells from mice immunized with LP6 plus AdE1° (D and H) were cultured with serial dilutions of the six long peptides corresponding to the LP6 immunogens. Proliferation was evaluated at day-3 by [3H]thymidine incorporation (A through D). IL-2 secretion was measured, using the CTL-L2 bioassay, in supernatants harvested at 24 h (E through H). Acid-precipitable radioactivity was determined, and results were expressed in counts per minute (mean of three separate experiments). To standardize the IL-2-dependent proliferation of CTL-L2 cells, a standard curve was obtained using 12 pg (709 cpm) to 25,000 pg (138,488 cpm) of mouse rIL-2 (PharMingen)/ml.
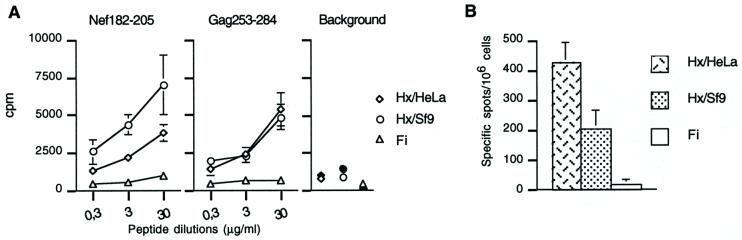
FIG. 8.
Helper response of mice immunized with LP6 plus either human cell-produced Hx or Sf9 cell-produced Hx. (A) Proliferative response. CD4+ spleen cells from mice immunized with either LP6 plus Hx/HeLa (Hx isolated from Ad-infected HeLa cells), LP6 plus Hx/Sf9 (recombinant Hx isolated from baculovirus-infected Sf9 cells), or LP6 plus Fi (recombinant Fi isolated from baculovirus-infected-Sf9 cells, used as a negative control) were cultured either in the presence of an aliquot from serial dilutions of long peptide Nef182-205 or Gag253-284 or in the presence of medium (open symbols) and 30 μg of Nef117-147/ml (solid symbols) to evaluate background. Proliferation was measured at day 3 by [3H]thymidine incorporation, and results were expressed in counts per minute (means of three separate experiments ± standard deviations). Note that some standard deviations are too small to appear on the graphs. (B) Day-7 ELISPOT-IFN-γ response. Day-7 samples of total spleen cells from mice immunized with LP6 plus Hx/HeLa, LP6 plus Hx/Sf9, or LP6 plus Fi were incubated for 24 h with 1 μg of Nef182-205/ml. Background values obtained with an irrelevant peptide (<25 spots per 106 cells) were subtracted from the mean values obtained with the specific peptide, and results were expressed as specific spots per 106 cells (± standard deviations).
RESULTS
Induction of T-helper type 1 and humoral responses by coinjection of AdE1° with a model immunogen.
We used a first-generation rAd, AdE1°, without a transgene, to avoid any interference due to the immunogenicity of the transgene product. A total of 109 particles of AdE1° were coinjected with a mixture of lipopeptides constituting LP6 (Table 1) at the tail bases of BALB/c mice. Such lipopeptides were known to elicit both CD4 and CD8 T-cell responses in mice, macaques, and humans (12, 33, 42), but higher doses and a booster injection were usually needed. The rationale for choosing LP6 as a model immunogen was twofold. First, the immune response to the injection of lipopeptides (including LP6) without an adjuvant in mice had been extensively studied (26, 42; also our unpublished data). Second, LP6 was chemically produced and therefore totally devoid of endotoxin contamination, in contrast to more commonly used, commercially available antigens (40). Ten days after subcutaneous injection, the mice were sacrificed, and spleen cell proliferation and IL-2 secretion in response to the six long peptides corresponding to the lipopeptides were analyzed. As expected for the low dose of lipopeptides injected, no response was observed in mice receiving LP6 without an adjuvant (Fig. 1B and F). In contrast, significant immunological responses to several long peptides of the immunogen were observed in mice receiving LP6 plus AdE1° (Fig. 1A and E). A response was detected in the proliferation assay with two peptides, Gag253-284 and Nef182-205. A net response was also detected with Gag253-284 and Nef182-205, and to a lesser extent with Nef66-97 and Env303-335, in the more-sensitive IL-2 secretion assay. Significant proliferation and IL-2 secretion of a population of spleen cells depleted of CD8+ T cells were observed in response to Gag253-284, Nef182-205, and Env303-335 in mice injected with AdE1° plus LP6 (Fig. 1D and H), confirming that these three peptides were involved in the T-helper response. The response to LP6 antigens obtained with 108 particles of AdE1° was significantly lower, but the response induced by incomplete Freund adjuvant (IFA) was not quantitatively higher than the response induced by 109 particles of AdE1° (data not shown). IFN-γ, but not IL-4, was detected in proliferation supernatants, suggesting that it corresponded to a Th1 response (data not shown). The hypothesis that the effect was due to the reactivation of a memory response to murine retroviral epitopes could be excluded, since no response was observed in mice injected with AdE1° alone (Fig. 1C and G). In addition, no response was observed in mice immunized with LP6 plus a mock adjuvant, which also ruled out a possible adjuvant effect of the virus storage buffer (data not shown). Note that in all proliferation experiments presented or cited in this report, levels of proliferation of the different spleen cell samples in response to ConA were similar.
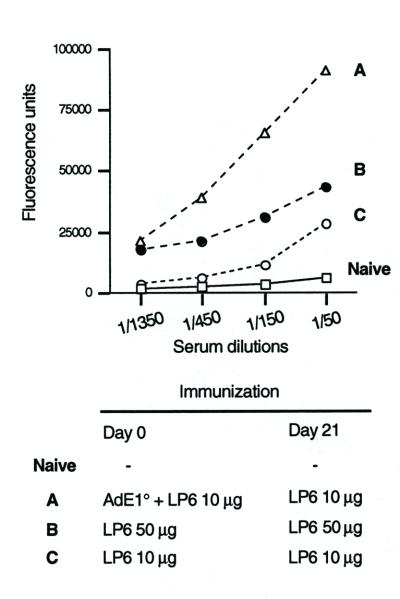
As the humoral response to the long peptides was nearly undetectable 10 days after a single injection of LP6 with or without AdE1° adjuvant, independent immunizations were performed to induce specific antibodies. Those mice that had been injected with a combination of AdE1° and LP6 received a booster injection of 10 μg of LP6 without AdE1° 21 days later. The immune response was evaluated 15 days after the booster injection and compared to the response induced by two injections (at day 0 and day 21) of 10 or 50 μg of LP6 alone. Anti-Gag253-284 IgG was detected in the sera of all three mouse groups, but at a markedly higher level in Ad-injected mice (Fig. 2). Levels of anti-Ad antibodies indicated that the mice were immunized against the virus (data not shown). Interestingly, Gag253-284 has been found to be immunodominant in the human humoral response to LP6 (12). These results point to a crucial role of Ad priming in the induction of the humoral response to LP6.
FIG. 2.
Humoral response to Gag253-284. Mice were subcutaneously injected twice with LP6 under the various conditions indicated. Serum Gag253-284-specific ELISA IgGs were monitored 2 weeks after the last injection. Naive mice were used as controls. Results are means from duplicate tests.
DOC treatment of Ad has been shown to disrupt the viral particle and release the subcapsid elements, i.e., (i) the inner core containing the viral genome associated with core protein V and histone-like protein VII, (ii) the apex and facet proteins penton base (Pb), fiber (Fi), and IIIa, and (iii) groups of nine hexons (Hx) comprised of Hx capsomers connected to Hx-associated proteins VI, VIII, and IX (3, 8, 35). BALB/c mice were immunized with LP6 and 109 particles of DOC-disrupted AdE1°. Control mice were immunized with LP6 plus a DOC-treated mock adjuvant and LP6 plus intact AdE1°. Figure 3A shows the results of a representative ELISPOT-IFN-γ assay, obtained in response to the six long peptides and performed at day 7 after one single in vitro restimulation with the six long peptides. The response to the helper immunodominant peptides, Gag253-284 and Nef182-205, in mice immunized with DOC-disrupted Ad particles was similar, qualitatively and quantitatively, to the response in mice immunized with intact Ad virions as an adjuvant. Figure 3B shows that the IFN-γ response to these two peptides is mediated by CD4 T cells only. In contrast, the response to Env303-335 is both CD4 and CD8 mediated, which implies that at least one class I Env303-335-derived peptide was generated during the assay. Therefore, the absence of response to Env303-335 in mice immunized with DOC-disrupted particles may be due to an absence of CD8 response to the Env303-335 class I epitope. Overall, these results suggested that the Th1 response was maintained when disrupted Ad particles were used as an adjuvant.
Induction of a polyepitopic CD8 response by coinjection of AdE1° with LP6.
To investigate the CD8+ T-cell response, we analyzed the amino acid sequences of the six long peptides and identified six potential epitopes according to the anchoring residues with the H-2d molecules. These are referred to as short peptides (Table 1). A cytotoxic H2-Kd-restricted epitope had been described previously by others (Gag197-205 [27]). Spleen cells from mice immunized either with AdE1° plus LP6 or with LP6 alone were then tested ex vivo in an ELISPOT-IFN-γ assay. Representative results from four independent experiments are shown in Fig. 4A. In mice immunized with AdE1° plus LP6, significant responses to the class I Gag197-205 epitope and to Env315-323 were detected. E315-323 is an H2-Dd motif and contains one H2-Ld (Env316-323) and one H2-Dd (Env315-322) motif. Control LP6 mice showed similar responses, although at significantly lower levels. Responses obtained with the CD8+ purified fraction confirmed that these two peptides corresponded to (or contained) major histocompatibility complex (MHC) class I-restricted epitopes (Fig. 4B). Because the sequence of the short peptide Env315-323 is part of the sequence of the long peptide Env303-335, the CD8 response to the former was also detected during the assay of the CD8 response to the latter (Fig. 3B). No response was detected in mice immunized with AdE1° alone, and the CD8 response in mice immunized with DOC-treated particles as an adjuvant was not significantly different from the response in mice immunized with LP6 alone (data not shown). Therefore, in spite of the low dose of lipopeptides used (10 μg each) and the absence of booster immunization, coinjection of 109 Ad particles, corresponding to 107 PFU, significantly improved the immunogenicity of our model antigen.
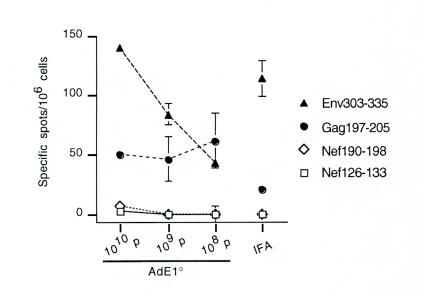
Dose-response analysis was performed to further characterize the effect of AdE1°. Mice were immunized with LP6 in combination with either 1010, 109, or 108 AdE1° particles. ELISPOT-IFN-γ responses of fresh spleen cells isolated from these mice were evaluated in comparison with the responses of mice immunized with LP6 emulsified in IFA (Fig. 5). The response to Env315-323 increased with the amount of coinjected Ad. The CD8 response induced by LP6-plus-IFA immunization was not markedly different from the mouse response to AdE1° plus LP6. Similar results were obtained with purified CD8+ T cells (data not shown).
FIG. 5.
Ex vivo ELISPOT-IFN-γ responses in mice immunized with LP6 plus various doses of AdE1° or IFA. Four short peptides overlapping the long peptides’ sequences (see Table 1) at a final concentration of 10 μg/ml and human rIL-2 at a final concentration of 30 U/ml were incubated for 24 h with freshly isolated spleen cells from mice immunized either with LP6 in combination with 1010, 109, or 108 particles of AdE1° or with LP6 emulsified in IFA. Background values obtained with an irrelevant peptide (<12 spots per 106 cells) were subtracted from the mean values from triplicate tests obtained with each specific peptide, and results were expressed as specific spots per 106 cells (± standard deviations). Note that some standard deviations are too small to appear on the graphs. Values obtained in response to ConA were between 360 and 502 spots per 106 cells.
Adjuvant effects of individual capsomers.
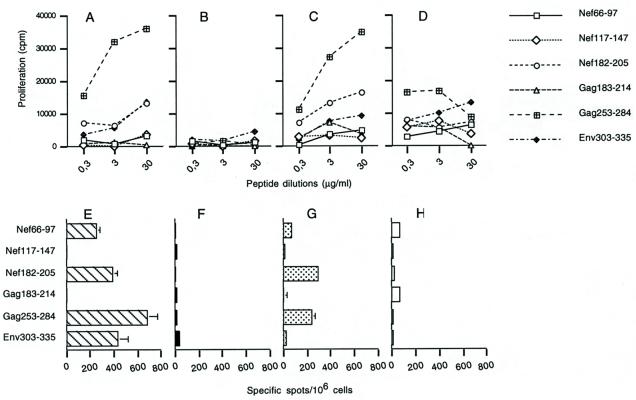
To determine the roles of the capsid components, mice were immunized with LP6 in combination with each of the three major capsid proteins, Pb, Fi, and Hx (Fig. 6). Each mouse was injected with 1 μg of capsomer, which corresponds approximately to 8 times the number of Hx molecules, 166 times the number of Pb molecules, and 275 times the number of Fi molecules contained in 109 Ad particles. A proliferative response to the two major CD4 epitopes Gag253-284 and Nef182-205 was observed in mice immunized with the Hx-plus-LP6 combination, an effect which was not detected in mice immunized with Pb plus LP6 or with Fi plus LP6 (Fig. 7B, C, and D). This response was qualitatively similar to the response induced by AdE1° plus LP6, although it did not reach similar levels (Fig. 7A). Nonspecific proliferation was observed in mice immunized with Fi plus LP6, with relatively high background signals, and hectic cellular proliferation independent of the peptide dose. We hypothesize that this effect was due to induction of B-cell proliferation by the Fi, as reported previously (13). These proliferation results were confirmed by the amounts of IL-2 found in proliferation supernatants (data not shown) and by the ELISPOT-IFN-γ assay performed in vitro with restimulated spleen cells (Fig. 7E, F, G, and H).
FIG. 6.
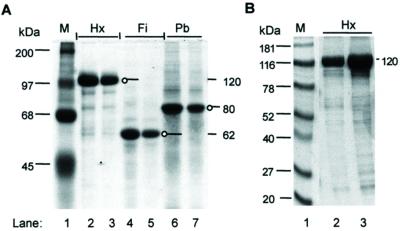
SDS-polyacrylamide gel electrophoretic analysis of Ad capsomers Hx, Pb, and Fi. Protein samples were electrophoresed in an SDS-12.5% polyacrylamide gel and stained with Coomassie blue. (A) Lane 1, prestained molecular mass markers (M) (Gibco Life Technology), with molecular masses indicated in kilodaltons on the left; lanes 2 and 3, Hx polypeptide unit from Hx capsomers isolated from Ad-infected HeLa cells (10 and 5 μg of protein, respectively); lanes 4 and 5, Fi (5 and 2.5 μg, respectively); lanes 6 and 7, Pb (6 and 3 μg, respectively). Hx, Fi, and Pb migrate with apparent molecular masses of 120, 62, and 80 kDa, respectively. The discrete band with a molecular mass 2 kDa lower than full-length Pb corresponds to a spontaneous cleavage product lacking the C-terminal 20 residues of the Pb sequence (20). (B) Lane 1, ProSieve color protein markers (M) (FMC Bioproducts); lanes 2 and 3, recombinant Hx protein isolated from baculovirus-infected Sf9 cells (6 and 15 μg of protein, respectively).
FIG. 7.
Cellular immune responses of mice immunized with LP6 plus purified capsomers to the long peptides. (A to D) Proliferative responses. Spleen cells from mice immunized with either LP6 plus AdE1° (A), LP6 plus Pb (B), LP6 plus Hx (C), or LP6 plus Fi (D) were cultured in the presence of an aliquot from a serial dilution of the six long peptides (see Table 1). Proliferation was evaluated at day 3 by [3H]thymidine incorporation, and results were expressed in counts per minute (means of three separate experiments). (E to H) Day-7 ELISPOT-IFN-γ responses. Day-7 samples of spleen cells from the same mice—i.e., mice immunized with either LP6 plus AdE1° (E), LP6 plus Pb (F), LP6 plus Hx (G), or LP6 plus Fi (H)—were incubated for 24 h with the six long peptides at a final concentration of 1 μg/ml. Background values obtained with an irrelevant peptide (<20 spots per 106 cells) were subtracted from the mean values obtained with the specific peptide, and results were expressed as specific spots per 106 cells (± standard deviations).
Recombinant Ad Pb and Fi produced in baculovirus-infected Sf9 cells have been shown to oligomerize and form capsomers indistinguishable from capsomers assembled in mammalian cells (20, 37). In contrast, recombinant Hx protein was found to be insoluble as expressed alone in Sf9 cells and failed to trimerize. Formation of trimeric Hx capsomers required the coexpression of the recombinant 100K protein, acting as a chaperone protein (32; Hong and Boulanger, unpublished). This is why the original Hx capsomer samples tested in mice were isolated from Ad-infected HeLa cells. However, to eliminate a possible effect due to the source of the Hx capsomers, we then tested the activity of Hx capsomers purified from Sf9 cells coexpressing Hx and 100K proteins. The proliferative response of CD4+ T cells to Nef182-205 and Gag253-284 epitopes from mice immunized with LP6 plus recombinant Hx protein was not significantly different from that observed in mice immunized with LP6 plus Hx protein isolated from HeLa cells (Fig. 8A). Significant responses to Nef182-205 were also detected in both groups of mice in a day-7 ELISPOT-IFN-γ assay (Fig. 8B). These data strongly suggested that the observed adjuvant effect was associated with the Hx protein.
Adjuvant effect of Ad in mice preimmunized with the Ad.
In our model, the LP6 antigens are present only in a mixture with the virus and not expressed by its genome, which implies that they cannot be affected by the presence of neutralizing antibodies against the virus. It was therefore particularly interesting to find out whether the existence of a preimmune status with regard to Ad would interfere with the Ad adjuvant effect. Mice were infected with 1010 particles (108 PFU) of AdE1°; then, 18 days later (at the peak of the humoral neutralizing response [see reference 17]), they received an injection of AdE1° plus LP6. Helper responses to Nef182-205 were particularly high and did not differ among Ad-plus-LP6-immunized mice whether they were preimmunized with Ad or not, as shown by a day-7 ELISPOT-IFN-γ (Fig. 9). However, the responses to Gag253-284, Nef66-97, and Env303-335 underwent twofold decreases in preimmunized mice (Fig. 9). These results suggest an impact of neutralizing antibodies on the Ad adjuvant effect that could be attributed to the loss of specific interactions of the Hx protein with cell receptors.
FIG. 9.
Impact of preimmunization with Ad on the day-7 ELISPOT-IFN-γ responses of mice immunized with Ad plus LP6. Mice were infected (or not) by 1010 particles (108 PFU) of AdE1° and 18 days later received an injection of AdE1° plus LP6. Spleen cells from preimmunized mice (open bars) and nonpreimmunized mice (stippled bars) were tested at day 7 in an ELISPOT-IFN-γ assay, and results are expressed as for Fig. 3.
DISCUSSION
The present results show that, during lipopeptide immunization, Ad virions can provide an adjuvant effect. The polyepitopic CD4 and CD8 responses generated by a single Ad-plus-LP6 immunization were qualitatively identical to the responses induced by LP6 alone when injected twice at a 3-week interval (our unpublished data). The CD4 component of the adjuvant effect could be obtained with DOC-disrupted virus capsids. Further analyses suggested that it was not associated with Fi or Pb and that it was carried instead by the Hx protein. However, as observed with disrupted virus, no significant improvement of the CD8 response was obtained with Hx. Several hypotheses could be put forward to explain the differences that were observed between the adjuvant activities of intact Ad on the one hand and DOC-treated Ad or isolated Hx capsomer on the other hand.
(i) The possibility that viral DNA could play a role in the adjuvant effect was unlikely, since in this case, DOC-treated Ad would have a stronger adjuvant effect than intact Ad. After DOC treatment, viral DNA is partially uncoated and only associated with core proteins V and VII, making the DNA accessible to the environment (in particular to nucleases), whereas in intact virions the viral DNA is packed with histone-like proteins to form the inner core of the particle (3, 35).
(ii) Theoretically, the detergent DOC could have altered the structure of the capsid proteins, hence modifying their interactions with host cells and diminishing their immunostimulatory activity. However, capsomers released by DOC-treatment of Ad particles have been shown to retain full immunogenicity, as well as morphological integrity, as observed under the electron microscope (2, 3, 35).
(iii) Another hypothesis is based on the multivalent status of the viral capsid, which could favor multiple interactions required for inducing immunostimulatory effects. For example, critical motifs might be exposed at the surface of the intact capsid only if two neighboring capsid components were available. Likewise, isolated Hx capsomers might not expose the same conformational epitopes as groups of nine Hx capsomers released upon DOC treatment, or a fortiori as intact capsid facets and edges. In this case, one could predict that monomeric Hx would have less immunizing capacity than assembled Hx trimers.
(iv) As an alternative possibility, the adjuvant effect could also be a complex process which requires the synergistic or additive action of two or three (or more) of the major structural proteins. Thus, the intact capsid could provide a multistep interaction mechanism, as for viral internalization, which involves successive binding of Fi and Pb to their respective receptors. The two latter hypotheses are not mutually exclusive. Experiments are now in progress to explore the adjuvant activity of monomeric Hx compared to trimeric Hx capsomer and to combinations of individual capsomers. Stepwise reconstitution of the capsid or capsid substructures will eventually be undertaken.
Our results also raise the question of how the Hx protein per se exerts its adjuvant effect. First, the specific immune response to the Hx (9, 29) could account for the secretion of a pattern of Th1 cytokines by activated B and T cells. In addition, specific anti-Hx CD8 or CD4 T cells may activate antigen presenting cells via the expression of CD40L (25). Ad can also directly stimulate dendritic cells (DC). Infection of DC by rAd results in upregulation of both MHC and costimulatory molecules (31, 39) and in enhanced capacity to elicit cytotoxic T lymphocytes (CTL) in response to the transgene product (5, 16). IFN-α/β, an important cytokine of the innate immune response which is induced by a number of viruses, could contribute to DC maturation and activation (18, 41). Morelli et al. have recently suggested that activation of the transcription factor NF-κB is involved in rAd-induced DC maturation by mechanisms independent of viral transcription (31). In agreement with their results, Hirschowitz et al. have shown that UV-psoralen-inactivated Ad, in contrast to heat-inactivated Ad and peptides containing an RGD sequence, could induce DC maturation, suggesting that this phenomenon is dependent on viral entry into the cell and independent of viral gene expression (14). Finally, the early secretion of various chemokines has been related to direct stimulation by the Ad capsid, with the induction of IP-10 implicating the NF-κB pathway (1, 34).
One could hypothesize that Hx plays a role in these mechanisms of direct stimulation. Consistent with a role of anti-Hx antibodies, the adjuvant activity of AdE1° particles was partially blocked in Ad-preprimed mice. Indeed, the murine neutralizing response essentially relies on anti-Hx antibodies (46), in contrast to the human neutralizing response, which depends on the synergistic activity of anti-Pb and anti-Fi antibodies (9). At present, no cellular receptor specific for Hx protein has been identified. Yet it seems unlikely that the principal structural protein of the adenovirus has no target on the cell. Hx is released into the cytoplasm upon endosomal disruption and could interact with cytoplasmic kinases implicated in the Raf/mitogen-activated protein kinase signaling pathway (6). Activated mitogen-activated protein kinases have been shown to activate a number of transcription factors, including NF-κB, which in turn stimulate the expression of many cytokine genes.
The identification of the molecular mechanisms underlying the adjuvant role of a viral protein should add to our understanding of the early events initiating the immune response. It will also contribute to the development of new vaccine approaches and to the improvement of strategies for rAd-based gene therapy.
Acknowledgments
This study was supported by a grant from the Institut National de la Santé et de la Recherche Médicale. V. Molinier-Frenkel was initially supported by a fellowship from the Ligue Nationale contre le Cancer and subsequently from the Association Française de Lutte contre la Mucoviscidose. F. Gaden was supported by a fellowship from the French association Vaincre la Mucoviscidose.
We are indebted to H. Gras-Masse for providing us with LP6 and to M. Mehtali for AdE1°. We also thank S. Kaveri and F. Farace for critical reading of the manuscript.
REFERENCES
- 1.Borgland, S. L., G. P. Bowen, N. C. Wong, T. A. Libermann, and D. A. Muruve. 2000. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-κB. J. Virol. 74:3941–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulanger, P., P. Lemay, G. E. Blair, and W. C. Russell. 1979. Characterization of adenovirus protein IX. J. Gen. Virol. 44:783–800. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger, P. A., C. Devaux, and M. H. Loucheux-Lefebvre. 1978. Comparative optical properties of free and assembled hexon capsomeres of human adenovirus type 2. FEBS Lett. 85:52–56. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger, P. A., and F. Puvion. 1973. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur. J. Biochem. 39:37–42. [DOI] [PubMed] [Google Scholar]
- 5.Brossart, P., A. W. Goldrath, E. A. Butz, S. Martin, and M. J. Bevan. 1997. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J. Immunol. 158:3270–3276. [PubMed] [Google Scholar]
- 6.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford-Miksza, L., and D. P. Schnurr. 1996. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 70:1836–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furcinitti, P. S., J. van Oostrum, and R. M. Burnett. 1989. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J. 8:3563–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahery-Ségard, H., F. Farace, D. Godfrin, J. Gaston, R. Lengagne, T. Turz, P. Boulanger, and J. Guillet. 1998. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 72:2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahéry-Ségard, H., V. Juillard, J. Gaston, R. Lengagne, A. Pavirani, P. Boulanger, and J.-G. Guillet. 1997. Humoral response to the capsid components of recombinant adenoviruses: routes of immunization modulate virus-induced Ig subclass shifts. Eur. J. Immunol. 27:653–659. [DOI] [PubMed] [Google Scholar]
- 11.Gahéry-Ségard, H., V. Molinier-Frenkel, C. Le Boulaire, P. Saulnier, P. Opolon, R. Lengagne, E. Gautier, A. Le Cesne, L. Zitvogel, A. Venet, C. Schatz, M. Courtney, T. Le Chevalier, T. Tursz, J. Guillet, and F. Farace. 1997. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J. Clin. Investig. 100:2218–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gahery-Segard, H., G. Pialoux, B. Charmeteau, S. Sermet, H. Poncelet, M. Raux, A. Tartar, J. P. Levy, H. Gras-Masse, and J. G. Guillet. 2000. Multiepitopic B- and T-cell responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine. J. Virol. 74:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, M., P. Tiensiwakul, and N. Khoobyarian. 1982. Adenovirus fiber protein (FP) functions as a mitogen and an adjuvant. Cell. Immunol. 73:397–403. [DOI] [PubMed] [Google Scholar]
- 14.Hirschowitz, E. A., J. D. Weaver, G. E. Hidalgo, and D. E. Doherty. 2000. Murine dendritic cells infected with adenovirus vectors show signs of activation. Gene Ther. 7:1112–1120. [DOI] [PubMed] [Google Scholar]
- 15.Jooss, K., H. Ertl, and J. M. Wilson. 1998. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J. Virol. 72:2945–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jooss, K., Y. Yang, K. J. Fisher, and J. M. Wilson. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 72:4212–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juillard, V., P. Villefroy, D. Godfrin, A. Pavirani, A. Venet, and J.-G. Guillet. 1995. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur. J. Immunol. 25:3467–3473. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kafri, T., D. Morgan, T. Krahl, N. Sarvetnick, L. Sherman, and I. Verma. 1998. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc. Natl. Acad. Sci. USA 95:11377–11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karayan, L., B. Gay, J. Gerfaux, and P. A. Boulanger. 1994. Oligomerization of recombinant penton base of adenovirus type 2 and its assembly with fiber in baculovirus-infected cells. Virology 202:782–795. [DOI] [PubMed] [Google Scholar]
- 21.Karayan, L., S. S. Hong, B. Gay, J. Tournier, A. D. d’Angeac, and P. Boulanger. 1997. Structural and functional determinants in adenovirus type 2 penton base recombinant protein. J. Virol. 71:8678–8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinguer, C., D. David, M. Kouach, J. M. Wieruszeski, A. Tartar, D. Marzin, J. P. Levy, and H. Gras-Masse. 1999. Characterization of a multi-lipopeptide mixture used as an HIV-1 vaccine candidate. Vaccine 18:259–267. [DOI] [PubMed] [Google Scholar]
- 23.Lemay, P., and P. Boulanger. 1980. Physicochemical characteristics of structural and non-structural proteins of human adenovirus 2. Ann. Virol. (Inst. Pasteur) 131E:259–275. [Google Scholar]
- 24.Lusky, M., M. Christ, K. Rittner, A. Dieterle, D. Dreyer, B. Mourot, H. Schultz, F. Stoeckel, and M. Mehtali. 1998. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J. Virol. 72:2022–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackey, M. F., R. J. Barth, Jr., and R. J. Noelle. 1998. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J. Leukoc. Biol. 63:418–428. [DOI] [PubMed] [Google Scholar]
- 26.Martinon, F., H. Gras-Masse, C. Boutillon, F. Chirat, B. Deprez, J. G. Guillet, E. Gomard, A. Tartar, and J. P. Levy. 1992. Immunization of mice with lipopeptides bypasses the prerequisite for adjuvant. Immune response of BALB/c mice to human immunodeficiency virus envelope glycoprotein. J. Immunol. 149:3416–3422. [PubMed] [Google Scholar]
- 27.Mata, M., P. J. Travers, Q. Liu, F. R. Frankel, and Y. Paterson. 1998. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 161:2985–2993. [PubMed] [Google Scholar]
- 28.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molinier-Frenkel, V., H. Gahery-Segard, M. Mehtali, C. Le Boulaire, S. Ribault, P. Boulanger, T. Tursz, J. G. Guillet, and F. Farace. 2000. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J. Virol. 74:7678–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molinier-Frenkel, V., C. Le Boulaire, F. A. Le Gal, H. Gahery-Segard, T. Tursz, J. G. Guillet, and F. Farace. 2000. Longitudinal follow-up of cellular and humoral immunity induced by recombinant adenovirus-mediated gene therapy in cancer patients. Hum. Gene Ther. 11:1911–1920. [DOI] [PubMed] [Google Scholar]
- 31.Morelli, A. E., A. T. Larregina, R. W. Ganster, A. F. Zahorchak, J. M. Plowey, T. Takayama, A. J. Logar, P. D. Robbins, L. D. Falo, and A. W. Thomson. 2000. Recombinant adenovirus induces maturation of dendritic cells via an NF-κB-dependent pathway. J. Virol. 74:9617–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morin, N., and P. Boulanger. 1986. Hexon trimerization occurring in an assembly-defective, 100K temperature-sensitive mutant of adenovirus 2. Virology 152:11–31. [DOI] [PubMed] [Google Scholar]
- 33.Mortara, L., H. Gras-Masse, C. Rommens, A. Venet, J. G. Guillet, and I. Bourgault-Villada. 1999. Type 1 CD4+ T-cell help is required for induction of antipeptide multispecific cytotoxic T lymphocytes by a lipopeptidic vaccine in rhesus macaques. J. Virol. 73:4447–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965–976. [DOI] [PubMed] [Google Scholar]
- 35.Nermut, M. V. 1984. The architecture of adenoviruses, p. 5–34. In H. S. Ginsberg (ed.), The adenoviruses. Plenum Press, New York, N.Y.
- 36.Neumann, R., J. Chroboczek, and B. Jacrot. 1988. Determination of the nucleotide sequence for the penton-base gene of human adenovirus type 5. Gene 69:153–157. [DOI] [PubMed] [Google Scholar]
- 37.Novelli, A., and P. A. Boulanger. 1991. Deletion analysis of functional domains in baculovirus-expressed adenovirus type 2 fiber. Virology 185:365–376. [DOI] [PubMed] [Google Scholar]
- 38.Prevec, L., J. B. Campbell, B. S. Christies, L. Belbeck, and F. L. Graham. 1990. A recombinant human adenovirus vaccine against rabies. J. Infect. Dis. 161:27–30. [DOI] [PubMed] [Google Scholar]
- 39.Rea, D., F. H. Schagen, R. C. Hoeben, M. Mehtali, M. J. Havenga, R. E. Toes, C. J. Melief, and R. Offringa. 1999. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J. Virol. 73:10245–10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reis e Sousa, C., and R. N. Germain. 1999. Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J. Immunol. 162:6552–6561. [PubMed] [Google Scholar]
- 41.Santini, S. M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauzet, J. P., B. Deprez, F. Martinon, J. G. Guillet, H. Gras-Masse, and E. Gomard. 1995. Long-lasting anti-viral cytotoxic T lymphocytes induced in vivo with chimeric-multirestricted lipopeptides. Vaccine 13:1339–1345. [DOI] [PubMed] [Google Scholar]
- 43.Tiensiwakul, P., and N. Khoobyarian. 1983. Adenovirus fiber protein produces synthesis of interferon in mouse spleen and macrophage cultures. Intervirology 20:52–55. [DOI] [PubMed] [Google Scholar]
- 44.Tripathy, S. K., H. B. Black, E. Goldwasser, and J. M. Leiden. 1996. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 2:545–550. [DOI] [PubMed] [Google Scholar]
- 45.Whittaker, G. R., and A. Helenius. 1998. Nuclear import and export of viruses and virus genomes. Virology 246:1–23. [DOI] [PubMed] [Google Scholar]
- 46.Wohlfart, C. 1988. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 62:2321–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, Y., F. A. Numes, K. Berencsi, E. E. Furth, E. Gönczöl, and J. M. Wilson. 1994. Cellular immunity to viral antigens limits E1 deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 91:4407–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]