Abstract
A key event in virus-induced inflammation (leukocyte extravasation through the endothelium) is the local activation of endothelial cells, as indicated by the expression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin. In order to identify triggers of inflammation in adenovirus infection, we inoculated respiratory and ocular epithelial cells with adenovirus type 37 (Ad37), a human pathogen associated with keratoconjunctivitis as well as urogenital and respiratory infections. Fluids from virus-infected epithelial cells activated ICAM-1 (and to a lesser extent, VCAM-1) expression on cultured human umbilical vein endothelial cells. Blocking studies with anticytokine antibodies implicated interleukin-1α (IL-1α) as the epithelial cell-derived factor which activated endothelial cell ICAM-1 expression. The results thus identify epithelial cell-derived IL-1α as a potentially important activator of endothelial cells in Ad37-induced inflammation.
Adenoviruses are associated with a variety of diseases in humans and other animals (37). At least 51 different serotypes of human adenoviruses cause disease, often involving the mucosal epithelial membranes of the respiratory, gastrointestinal, and urogenital tracts as well as the eye conjunctivae (7, 9). These diseases are often characterized by intense inflammatory reactions (15, 22, 26), the mechanisms of which are not completely understood.
A key event in inflammation is the expression of vascular endothelial cell adhesion molecules which facilitate leukocyte extravasation into damaged tissues, including those involved in virus infection. For example, intercellular adhesion molecule 1 (ICAM-1; also called CD54) and vascular cell adhesion molecule 1 (VCAM-1; also called CD106) are members of the immunoglobulin supergene family which function in leukocyte-endothelial cell and leukocyte-matrix adhesive interactions. ICAM-1, VCAM-1, and another endothelial cell adhesion molecule, E-selectin (CD 62E), are upregulated on the surface of the endothelium by inflammatory cytokines, cellular stress, and virus infection (24). These adhesive molecules play a critical role in leukocyte adhesion to vascular endothelium and transendothelial migration (30).
In an approach aimed at understanding the triggers involved in adenovirus-associated inflammatory responses we investigated the hypothesis that specific immunomodulatory factors are released after epithelial infection with adenoviruses and that these factors subsequently activate specific adhesion molecules on vascular endothelial cells to initiate leukocyte recruitment. For this study we used adenovirus type 37 (Ad37), a virus associated with human inflammatory keratoconjunctivitis as well as urogenital and respiratory infections (4, 5, 6, 26, 32).
To establish whether the cells used in our study were susceptible to infection with Ad37, respiratory epithelial A549 cells (19) and corneal epithelial cells (10) were inoculated with virus at a multiplicity of infection of approximately 3 PFU/cell and radiolabeled for 1 h at 24 h postinfection with [35S]methionine-[35S]cysteine (Translabel; ICN, Montreal, Quebec, Canada), and cell extracts were immunoprecipitated with rabbit Ad37 immune serum and protein A-bearing, formalin-fixed Staphylococcus aureus as previously described (11). Both cell types showed typical Ad viral protein profiles by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (2, 36) and both gave rise to similar yields of progeny virus (106 to 107 PFU/ml after 24 h) by plaque assay (35) on A549 cells (data not shown).
Activation of vascular endothelial cell ICAM-1 expression by culture supernatants from adenovirus-infected A549 cells and corneal epithelial cells.
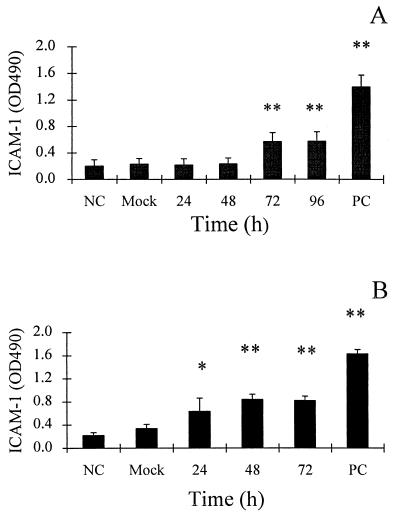
We examined the hypothesis that infected epithelial cells might secrete a factor(s) which activates vascular endothelial cells. Human umbilical vein endothelial cells (HUVEC) were isolated and cultured in gelatin-coated flasks as described by Jaffe et al. (14), with modifications reported previously (12). The expression of ICAM-1, VCAM-1, and E-selectin on endothelial cells was determined by enzyme-linked immunosorbent assay as previously described (3, 13). Briefly, endothelial cells were cultured in 96-well plates until confluent. Dilutions (1:4) of epithelial cell supernatants (from which >99.9% of virus infectivity was removed by UV irradiation [254 nm; 4,000 J/m2], as indicated by plaque assay [35] on A549 cells) were added to individual wells. (The irradiation step was desirable to minimize viral cytopathic effect [18] on endothelial cells; however, control experiments showed that infectious virus alone was not capable of upregulating adhesion molecule expression on endothelial cells [data not shown].) Following incubation of HUVEC cultures with the supernatants for 3 h (for E-selectin assay) or for 18 h (for ICAM-1 and VCAM-1 assay), ICAM-1, VCAM-1, and E-selectin were detected by enzyme-linked immunosorbent assay using anti-ICAM-1, -VCAM-1, and -E-selectin antibodies (R&D Systems, Minneapolis, Minn.) and a secondary antibody of goat anti-mouse immunoglobulin G (IgG)-peroxidase conjugate (Sigma). After washing, color was developed with orthophenylenediamine (Sigma) and optical density was determined at 490 nm. As a positive control, endothelial cells were stimulated by tumor necrosis factor alpha (Genentech, South San Francisco, Calif.; 10 U/ml). As shown in Fig. 1, culture supernatants from both adenovirus-infected A549 and corneal epithelial cells activated HUVEC ICAM-1 expression. HUVEC ICAM-1-stimulating activity was evident in culture supernatants from Ad-infected A549 cells harvested at 72 and 96 h postinfection (Fig. 1A). More strikingly, culture supernatants from Ad-infected corneal epithelial cells taken as early as 24 h postinfection stimulated HUVEC ICAM-1 expression (Fig. 1B). No significant induction of vascular endothelial cell ICAM-1 expression was observed by culture supernatant (72 h postinfection) from mock-infected epithelial cells (Fig. 1) or by UV-inactivated, purified adenovirus (data not shown).
FIG. 1.
ICAM-1 expression on vascular endothelial cells is upregulated after treatment (18 h) with culture supernatants from Ad-infected A549 cells or Ad-infected human corneal epithelial cells. (A) Endothelial ICAM-1 expression after 18-h exposure to culture fluids from Ad-infected A549 cells, mock-infected A549 cells, and medium alone (negative control; NC). (B) Endothelial ICAM-1 expression after 18-h exposure to culture fluids from Ad-infected human corneal epithelial cells, mock-infected A549 cells, and medium alone (negative control; NC). PC, positive control (vascular endothelial cells treated for 18 h with TNF-α [10 U/ml]). Data are from three to nine separate experiments with triplicate samples and are represented as the means ± standard deviations of the means. Significant differences from the mock sample are indicated by * (P < 0.05) and ** (P < 0.01).
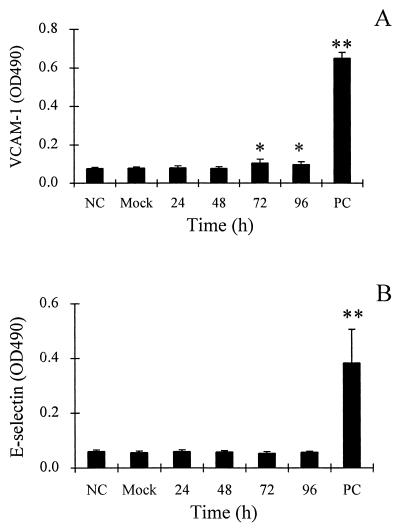
Compared to ICAM-1, endothelial cell VCAM-1 showed a lesser but significant increase in expression after treatment with Ad-infected A549 culture fluids (Fig. 2A). E-selectin was not significantly activated by Ad-infected A549 culture fluids under the conditions of the assay (Fig. 2B).
FIG. 2.
Effects of culture supernatants from Ad-infected A549 cells on endothelial cell expression of VCAM-1 and E-selectin. (A) Endothelial VCAM-1 expression after 18-h exposure to culture fluids from Ad-infected A549 cells, mock-infected A549 cells, and medium alone (negative control; NC). (B) Endothelial E-selectin expression after 3-h exposure to culture fluids from Ad-infected A549 cells, mock-infected A549 cells, and medium alone (negative control; NC). PC, positive control (vascular endothelial cells treated for 18 h with TNF-α [10 U/ml]). Data are from three separate experiments with triplicate samples and are represented as the means ± standard deviations of the means. Significant differences from the mock sample are indicated by * (P < 0.05).
Interleukin-1α (IL-1α) is the major endothelial cell-activating factor induced by adenovirus infection of epithelial cells.
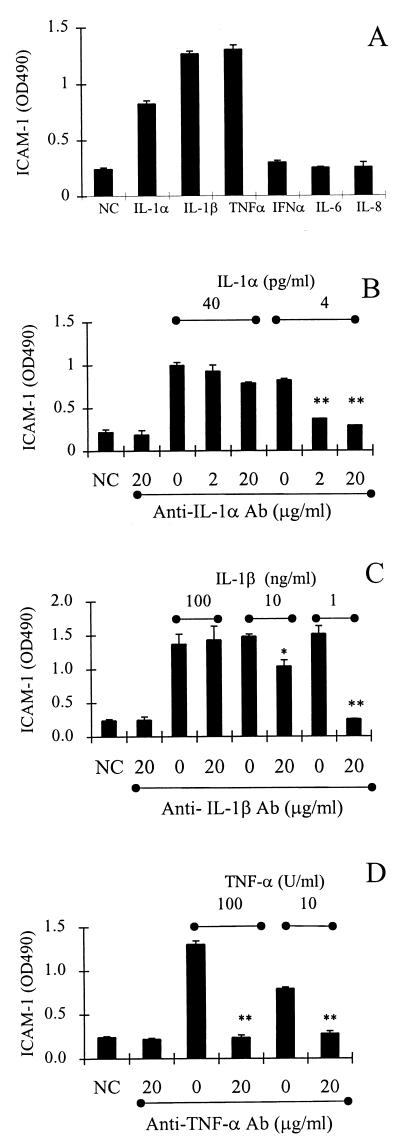
Epithelial cells secrete several cytokines (31), including IL-1α/β, TNF-α, IL-6, and IL-8, which could possibly activate vascular endothelial cells. In order to identify cytokines capable of activating endothelial cells, cultures of endothelial cells were treated with recombinant IL-1α (R&D Systems), IL-1β (Endogen, Woburn, Mass.), TNF-α (Genentech), IL-6 (PeproTech, Rocky Hill, N.J.), and IL-8 (PeproTech) for 18 h and then assayed for ICAM-1 activation. As shown in Fig. 3A, ICAM-1 was activated in vascular endothelial cells by IL-1α, IL-1β, and TNF-α but not by IL-6 or IL-8. As controls for specificity, the activation of ICAM-1 by IL-1α, IL-1β, or TNF-α was blocked by the appropriate antibodies, anti-IL-1α (Endogen; 20 μg/ml) (Fig. 3B), anti-IL-1β (Endogen; 20 μg/ml) (Fig. 3C), and anti-TNF-α (Endogen; 20 μg/ml) (Fig. 3D).
FIG. 3.
ICAM-1 expression on vascular endothelial cells is activated by recombinant cytokines. (A) ICAM-1 expression on endothelial cells incubated for 18 h with IL-1α (4 pg/ml), IL-1β (100 pg/ml), TNF-α (100 U/ml), IL-6 (1 μg/ml), and IL-8 (1 μg/ml). (B) ICAM-1 expression on endothelial cells incubated for 18 h with IL-1α in the presence or absence of anti-IL-1α antibody at the indicated concentration. (C) ICAM-1 expression on endothelial cells incubated for 18 h with IL-1β in the presence or absence of anti-IL-1β antibody at the indicated concentration. (D) ICAM-1 expression on endothelial cells incubated for 18 h with TNF-α in the presence or absence of anti-TNF-α antibody at the indicated concentration. NC, negative control (medium alone); PC, positive control (vascular endothelial cells treated for 18 h with TNF-α [10 U/ml]). Data are from three to six separate experiments with triplicate samples and are represented as the means ± standard deviations of the means. Significant differences between antibody-treated and untreated samples are indicated by * (P < 0.05) and ** (P < 0.01).
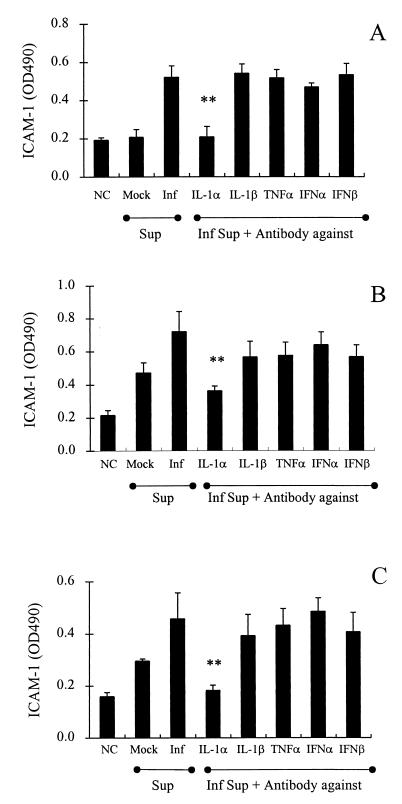
Using the above-characterized anticytokine antibodies in an approach to identify endothelial cell-activating factors, culture fluids from Ad-infected A549 cells were treated with anticytokine antibodies (20 μg/ml) and then incubated for 18 h with HUVEC monolayers in 96-well plates. As shown in Fig. 4A, endothelial ICAM-1 expression was blocked by treating Ad-infected A549 cell supernatants with anti-IL-1α antibody but was not significantly blocked by antibodies against IL-1β, TNF-α, alpha interferon, or beta interferon. When A549 cell supernatants were diluted further (10- to 20-fold), a similar pattern of blocking with anti-IL-1α antibody was achieved, thereby minimizing the possibility that the supernatants contained high levels of cytokines other than IL-1α which were not neutralized by the respective antibodies employed (data not shown). Analogous studies also demonstrated that IL-1α is the major endothelial cell-activating factor released from Ad-infected corneal epithelial cells (Fig. 4B and C).
FIG. 4.
Identification of endothelial ICAM-1-activating factor released from Ad-infected epithelial cells as IL-1α. ICAM-1 expression on vascular endothelial cells after stimulation with culture fluids from Ad-infected (72 h) A549 cells (A), Ad-infected (24 h) corneal epithelial cells (B), or Ad-infected (72 h) corneal epithelial cells (C) is blocked by anti-IL-1α antibody (20 μg/ml) but not by other anticytokine antibodies. Inf, infected cells; Sup, supernatant; NC, negative control (medium alone); PC, positive control (vascular endothelial cells treated for 18 h with TNF-α [10 U/ml]). Data are from three to five separate experiments with triplicate samples and are represented as the means ± standard deviations of the means. Significant differences between antibody-treated and untreated samples are indicated by ** (P < 0.01).
Transendothelial leukocyte migration and recruitment to sites of virus-infected tissues are essential to the inflammatory response and host defense against viral infection. Activation of vascular endothelial cell adhesion molecules is a prerequisite step for endothelial migration of leukocytes; this activation is most often mediated by specific cytokines. Although macrophages are major cytokine producers in such cases, there is evidence that front-line epithelial cells might also play a critical role (1, 23, 31, 34). Since epithelial cells are often primary targets of virus infection, epithelial cell-derived cytokine responses may precede those of macrophages and may therefore be crucial to the initial steps of inflammation. In this study, we show that Ad37 triggers vascular endothelial cell-activating factors, notably IL-1α, in both respiratory and corneal epithelial cells. Moreover, IL-1α has both autocrine and paracrine effects on cytokine expression (8) which could amplify and broaden the endothelial cell adhesion molecule response.
To our knowledge, this is the first demonstration of endothelial cell activation in adenovirus infection. Ad37 used in this study is a predominant pathogen of epidemic keratoconjunctivitis (5, 6, 27) and is also associated with respiratory tract infections (5, 26). A prominent clinical feature is intense corneal inflammation (26). The present study provides potential insights into the mechanism of Ad37-induced inflammation by identifying epithelial cell-derived IL-1α, which can activate vascular endothelial cell ICAM-1 (and to a lesser extent VCAM-1) expression, thereby initiating leukocyte recruitment. In agreement with the above observation, we find that authentic IL-1α upregulates endothelial cell ICAM-1 more effectively than VCAM-1 (data not shown).
It will be important to test and extend the conclusions of this study in future in vitro and in vivo investigations. An obvious question is whether epithelial cell-derived IL-1α and its associated effects on vascular endothelial cells may contribute to the pathology seen in clinical adenoviral conjunctivitis, particularly vascular extravasation and accumulation of lymphocytes commonly observed in the conjunctival follicles (15). In addition to the corneal epithelial cells used in this study, it will also be of interest to examine Ad infection and cytokine production in conjunctival epithelial cells, since such cells form a layer in the eye just above the vessel-rich substantia propria. Efforts are under way to obtain a pure source of conjunctival epithelial cells for this purpose (the only readily available conjunctival epithelial cell line is the Chang line, which is contaminated with HeLa cells [16]). It will also be of interest to determine whether the ability of Ad37 to activate endothelial cells via epithelial cell IL-1α is a characteristic shared by other adenoviruses.
In contrast to cytokines such as TNF-α and IL-1β, IL-1α has rarely been implicated in endothelial cell activation. IL-1α is predominantly intracellular (8) and is rarely found in circulatory or inflammatory fluids. Extracellular release of IL-1α is likely increased in cases of cell damage and is associated with certain pathological conditions of corneal epithelial cells (25, 28, 29). Moreover, the extracellular release of IL-1α may be selectively triggered by infection with strongly cytopathic viruses. For example, IL-1α is released from respiratory syncytial virus-infected A549 cells (23) but not from corneal epithelial cells infected with the less membrane-disrupting herpes simplex virus type 1 (33).
Both corneal and respiratory (A549) epithelial cells responded to Ad37 infection with the release of endothelial cell-activating factors; however, the response was earlier (24 versus 72 h postinfection) and reached higher levels in the corneal cell line. While this may reflect the preferred clinical tropism of Ad37 for epithelial cells of ocular origin (4), no obvious difference in permissiveness for Ad37 was observed between respiratory and corneal epithelial cells (data not shown). Since corneal epithelial cells secrete low levels of IL-1α constitutively (23) and since IL-1α possesses autocrine properties (20, 21), corneal epithelial cells may respond to virus infection with a particularly strong IL-1α release. This response may be an important trigger for activation of the local vascular endothelium to promote blood leukocyte adhesion and recruitment into the site of virus infection, thus mobilizing host defense mechanisms.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (grants 14299 and 42431 to R.A. and MT7684 to A.C.I.) as well as the National Science Council, Taiwan. R.A. is an Associate of the Dalhousie Medical Research Foundation.
We gratefully acknowledge the technical assistance of D. Rowter.
REFERENCES
- 1.Adler, K. B., B. M. Fischer, D. T. Wright, L. A. Cohn, and S. Becker. 1994. Interactions between respiratory epithelial cells and cytokines: relationships to lung inflammation. Ann. N. Y. Acad. Sci. 725:128–145. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, C. W., P. R. Baum, and R. F. Gesteland. 1973. Processing of adenovirus 2-induced proteins. J. Virol. 12:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, R., S. Wang, C. Osiowy, and A. C. Issekutz. 1997. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J. Virol. 71:4226–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnberg, N., Y. Mei, and G. Wadell. 1997. Fiber genes of adenoviruses with tropism for the eye and the genital tract. Virology 227:239–244. [DOI] [PubMed] [Google Scholar]
- 5.Chang, C. H., M. M. Sheu, K. H. Lin, and C. W. Chen. 2001. Hemorrhagic viral keratoconjunctivitis in Taiwan caused by adenovirus types 19 and 37: applicability of PCR-RFLP in detecting adenovirus genotypes. Cornea 20:295–300. [DOI] [PubMed] [Google Scholar]
- 6.de Jong, J. C., R. Wigand, G. Wadell, D. Keller, C. J. Muzerie, A. G. Wermenbol, and G. J. Schaap. 1981. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J. Med. Virol. 7:105–118. [DOI] [PubMed] [Google Scholar]
- 7.de Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. W. Slaterus, D. P. Wertheim-Van, G. J. Van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello, C. A. 1998. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int. Rev. Immunol. 16:457–499. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg, H. S. 1999. The life and times of adenoviruses. Adv. Virus Res. 54:1–13. [DOI] [PubMed] [Google Scholar]
- 10.Griffith, M., R. Osborne, R. Munger, X. Xiong, C. J. Doillon, N. L. Laycock, M. Hakim, Y. Song, and M. A. Watsky. 1999. Functional human corneal equivalents constructed from cell lines. Science 286:2169–2172. [DOI] [PubMed] [Google Scholar]
- 11.He, R. T., B. L. Innis, A. Nisalak, W. Usawattanakul, S. Wang, S. Kalayanarooj, and R. Anderson. 1995. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J. Med. Virol. 45:451–461. [DOI] [PubMed] [Google Scholar]
- 12.Issekutz, A. C. 1998. Adhesion molecules mediating neutrophil migration to arthritis in vivo and across endothelium and connective tissue barriers in vitro. Inflamm. Res. 47(Suppl. 3):S123–S132. [DOI] [PubMed] [Google Scholar]
- 13.Issekutz, A. C., and N. Lopes. 1993. Endotoxin activation of endothelium for polymorphonuclear leucocyte transendothelial migration and modulation by interferon-gamma. Immunology 79:600–607. [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi, T. K., S. Sato, K. Tsubota, and E. Takamura. 1991. Cytological evaluation of adenoviral follicular conjunctivitis by cytobrush. Ophthalmologica 202:156–160. [DOI] [PubMed] [Google Scholar]
- 16.Lavappa, K. S., M. L. Macy, and J. E. Shannon. 1976. Examination of ATCC stocks for HeLa marker chromosomes in human cell lines. Nature 259:211–213. [DOI] [PubMed] [Google Scholar]
- 17.Leland, B. J., and J. P. Metcalf. 1999. Type-specific induction of interleukin-8 by adenovirus. Am. J. Respir. Cell Mol. Biol. 21:521–527. [DOI] [PubMed] [Google Scholar]
- 18.Lewis, A. M., and J. L. Cook. 1979. Association of tumor induction by ultraviolet light-inactivated adenovirus 2-simian virus 40 recombinants with a specific segment of simian virus 40 DNA. JNCI 63:695–705. [DOI] [PubMed] [Google Scholar]
- 19.Lieber, M., B. Smith, A. Szakal, W. Nelson-Rees, and G. Todaro. 1976. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 17:62–70. [DOI] [PubMed] [Google Scholar]
- 20.Maier, J. A., M. Statuto, and G. Ragnotti. 1994. Endogenous interleukin 1 alpha must be transported to the nucleus to exert its activity in human endothelial cells. Mol. Cell. Biol. 14:1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier, J. A., P. Voulalas, D. Roeder, and T. Maciag. 1990. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science 249:1570–1574. [DOI] [PubMed] [Google Scholar]
- 22.McCoy, R. D., B. L. Davidson, B. J. Roessler, G. B. Huffnagle, S. L. Janich, T. J. Laing, and R. H. Simon. 1995. Pulmonary inflammation induced by incomplete or inactivated adenoviral particles. Hum. Gene Ther. 6:1553–1560. [DOI] [PubMed] [Google Scholar]
- 23.Patel, J. A., M. Kunimoto, T. C. Sim, R. Garofalo, T. Eliott, S. Baron, O. Ruuskanen, T. Chonmaitree, P. L. Ogra, and F. Schmalstieg. 1995. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 13:602–609. [DOI] [PubMed] [Google Scholar]
- 24.Roebuck, K. A., and A. Finnegan. 1999. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 66:876–888. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto, S., and K. Inada. 1992. Human corneal epithelial, stromal and endothelial cells produce interleukin-6. Nippon Ganka Gakkai Zasshi 96:702–709. [PubMed] [Google Scholar]
- 26.Schaap, G. J., J. C. de Jong, O. P. van Bijsterveld, and W. H. Beekhuis. 1979. A new intermediate adenovirus type causing conjunctivitis. Arch. Ophthalmol. 97:2337–2338. [DOI] [PubMed] [Google Scholar]
- 27.Sheu, M. M., K. H. Lin, W. L. Huang, and C. W. Chen. 1988. Adenovirus types 19 and 37 isolated from viral conjunctivitis in the Kaohsiung area during 1983–1984: molecular epidemiological study by DNA endonuclease cleavage analysis. Kao-Hsiung I Hsueh K’o Hsueh Tsa Chih 4:72–80. [PubMed] [Google Scholar]
- 28.Solomon, A., M. Rosenblatt, D. Q. Li, Z. Liu, D. Monroy, Z. Ji, B. L. Lokeshwar, and S. C. Pflugfelder. 2000. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 41:2544–2557. [PubMed] [Google Scholar]
- 29.Sotozono, C., J. He, Y. Matsumoto, M. Kita, J. Imanishi, and S. Kinoshita. 1997. Cytokine expression in the alkali-burned cornea. Curr. Eye Res. 16:670–676. [DOI] [PubMed] [Google Scholar]
- 30.Springer, T. A. 1995. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57:827–872. [DOI] [PubMed] [Google Scholar]
- 31.Stadnyk, A. W. 1994. Cytokine production by epithelial cells. FASEB J. 8:1041–1047. [DOI] [PubMed] [Google Scholar]
- 32.Swenson, P. D., M. S. Lowens, C. L. Celum, and J. C. Hierholzer. 1995. Adenovirus types 2, 8, and 37 associated with genital infections in patients attending a sexually transmitted disease clinic. J. Clin. Microbiol. 33:2728–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran, M. T., D. A. Dean, R. N. Lausch, and J. E. Oakes. 1998. Membranes of herpes simplex virus type-1-infected human corneal epithelial cells are not permeabilized to macromolecules and therefore do not release IL-1α. Virology 244:74–78. [DOI] [PubMed] [Google Scholar]
- 34.Tsubota, K., H. Inoue, K. Ando, M. Ono, K. Yoshino, and I. Saito. 1998. Adenovirus-mediated gene transfer to the ocular surface epithelium. Exp. Eye Res. 67:531–538. [DOI] [PubMed] [Google Scholar]
- 35.Wadell, G. 1979. Classification of human adenoviruses by SDS-polyacrylamide gel electrophoresis of structural polypeptides. Intervirology 11:47–57. [DOI] [PubMed] [Google Scholar]
- 36.Wadell, G., G. Sundell, and J. C. de Jong. 1981. Characterization of candidate adenovirus 37 by SDS-polyacrylamide gel electrophoresis of virion polypeptides and DNA restriction site mapping. J. Med. Virol. 7:119–125. [DOI] [PubMed] [Google Scholar]
- 37.Wigand, R., A. Bartha, R. S. Dreizin, H. Esche, H. S. Ginsberg, M. Green, J. C. Hierholzer, S. S. Kalter, J. B. McFerran, U. Pettersson, W. C. Russell, and G. Wadell. 1982. Adenoviridae: second report. Intervirology 18:169–176. [DOI] [PubMed] [Google Scholar]