Abstract
Initiation of reverse transcription in hepadnaviruses (hepatitis B viruses) depends on the specific binding of an RNA signal (the packaging signal, ɛ) on the pregenomic RNA template by the viral reverse transcriptase (RT) and is primed by the RT itself (protein priming). We have previously shown that the RT-ɛ interaction and protein priming require the cellular heat shock protein, Hsp90. However, additional host factors required for these reactions remained to be identified. We now report that five cellular chaperone proteins, all known cofactors of Hsp90, were sufficient to reconstitute a duck hepatitis B virus RT active in ɛ binding and protein priming in vitro. Four proteins, Hsp90, Hsp70, Hsp40, and Hop, were required for reconstitution of RT activity, and the fifth protein, p23, further enhanced the kinetics of reconstitution. RT activation by the chaperone proteins is a dynamic process dependent on ATP hydrolysis and the Hsp90 ATPase activity. Thus, our results have defined a minimal complement of host factors necessary and sufficient for RT activation. Furthermore, this defined in vitro reconstitution system has now paved the way for future biochemical and structural studies to elucidate the mechanisms of RT activation and chaperone functions.
Reverse transcription in hepadnaviruses (hepatitis B viruses [HBVs]) is carried out by a virally encoded reverse transcriptase (RT), which is unique among all known RTs in several aspects (39, 43). The RT initiates DNA synthesis de novo using a specific tyrosine residue located within its unique N-terminal domain as a protein primer (27, 46, 48, 51; for a recent review, see reference 19). This protein-priming reaction requires interaction between the RT and a specific RNA signal, termed ɛ, located on the viral pregenomic RNA (pgRNA) (the template for reverse transcription) (33, 47). The ɛ RNA forms a conserved stem-loop structure with an internal bulge, which is used as the specific template for protein priming (and thus, the origin of reverse transcription) (29, 45, 47; for a recent review, see reference 21). In addition, ɛ serves as the RNA packaging signal (16, 24) and, through its interaction with the RT, directs the selective encapsidation of both the pgRNA and the RT into viral nucleocapsids (1, 33). Therefore, the specific interaction between the RT and ɛ triggers two essential early steps in hepadnavirus assembly and replication, i.e., the protein-primed initiation of reverse transcription and the assembly of replication-competent nucleocapsids.
Using the duck hepatitis B virus (DHBV) RT as a model system, we have recently found that the RT requires the assistance of host cell factors in order to carry out specific ɛ binding and protein-priming functions (20, 22). One such cellular factor is the 90-kDa heat shock protein (Hsp90). Hsp90 associates with the DHBV RT and is required for RT-ɛ interaction and protein priming in vitro and for pgRNA packaging and DNA synthesis in vivo. Furthermore, the chaperone is specifically incorporated into viral nucleocapsids via association with the RT.
Hsp90 is thought to facilitate the functions of a specific subset of cellular proteins (the target or substrate proteins) by helping to establish and maintain certain poised but labile conformations of these target proteins, through a dynamic, multistage process (2, 4, 32, 44). In so doing, Hsp90 invariably collaborates with other factors (the so-called cochaperones or cofactors) and forms various multicomponent chaperone complexes. The precise composition of the Hsp90 chaperone complexes varies depending upon the nature of the target proteins and the stage of the chaperoning process. In the case of the steroid receptors, which require the Hsp90 chaperone for their hormone binding activity (34), it has been recently shown that five components of the Hsp90 chaperone complex, i.e., Hsp90 itself, Hsp70, Hsp40, Hop/p60, and p23, are sufficient to reconstitute the receptors into a hormone-binding competent conformation in vitro (7, 26, 28). Both Hsp90 and Hsp70 are ATPases and can facilitate protein folding in an ATP-binding and hydrolysis-dependent fashion. Hsp40 can stimulate the ATPase activity of Hsp70 and thus modulate Hsp70’s chaperone function. Hop (heat shock protein organizing protein, also called p60) can bind to both Hsp90 and Hsp70 and is thought to act as a bridge between Hsp90 and Hsp70. The small, acidic phosphoprotein, p23, binds Hsp90 and appears to function, via a still-undefined mechanism, at a late step in steroid receptor folding. On the other hand, a subset of cellular protein kinases also require the Hsp90 chaperone for their functions and involve a different set of Hsp90 cofactors, including CDC37/p50 (12, 41). However, it is not yet clear which Hsp90 cofactors together are sufficient for maintaining kinase functions.
Our previous results showed that two Hsp90 cofactors, Hsp70 and p23, are important for the ɛ binding and protein-priming activities of the RT (20, 22). However, these proteins are not sufficient for RT activation, and additional factors required for RT functions remained to be identified. To facilitate the isolation and identification of these factors, we have recently established an in vitro reconstitution system using purified, bacterially expressed recombinant RT and the rabbit reticulocyte lysate (18), a eukaryotic cytoplasmic extract rich in chaperone components (11, 37) and known to support RT functions (20, 46). These results showed that the RT can be expressed in the absence of a functional Hsp90 complex in bacteria and can be activated posttranslationally in an Hsp90-dependent fashion. Using this in vitro reconstitution system, we have now identified the minimal complement of the Hsp90 cofactors sufficient for reconstituting in vitro a functional DHBV RT active in ɛ binding and protein priming and have developed a defined in vitro system for RT activation using purified RT and chaperone components. We describe here conditions for in vitro reconstitution of a priming-competent RT using purified components and discuss the implications of these results for hepadnavirus replication and Hsp90 functions.
MATERIALS AND METHODS
Bacterial expression and purification of DHBV mini-RT proteins.
Two truncated minimal DHBV RT fusion proteins with the glutathione-S-transferase (GST), GST-miniRT1 and GST-miniRT2, were expressed in Escherichia coli and purified as described previously (18). MiniRT2/CA29 bears two amino acid substitutions that render the mutant RT defective in ɛ RNA binding (38). MiniRT1/YMHA bears two amino acid substitutions in the RT active site (changing the conserved YMDD motif into YMHA) (5) that abolishes the polymerase activity of the RT.
Purification of Hsp90.
Human Hsp90β was expressed in Sf9 cells and purified as described previously (13). Cell lysates were fractionated by DEAE-cellulose column chromatography followed by chromatography on heparin-agarose, mono Q, and Superdex 200 columns. The preparation was more than 99% pure as assessed by densitometry of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. These same procedures were used to purify bacterially expressed tumor necrosis factor receptor-associated protein 1 (TRAP-1), HtpG, and the Hsp90 E46A mutant.
Purification of Hsp70.
Human Hsp70 was expressed in Sf9 cells and purified as described previously for avian Hsp70 (36). Cell lysates were fractionated by DEAE-cellulose and ATP-agarose column chromatography. This was precipitated using ammonium sulfate (75% saturation), and the redissolved Hsp70 was fractionated by 16/60 Superdex 200 fast-performance liquid chromatography. Only the monomer peak of Hsp70 was used. The preparation was approximately 97% pure as assessed by densitometry of SDS-PAGE gels.
Purification of Hop.
Human Hop (p60) expressed in bacteria was prepared essentially as described previously (37). Bacterial lysates were fractionated by DEAE-cellulose and hydroxylapatite column chromatography. Additional purification was achieved by fractionating the pool from hydroxylapatite on a Mono Q column (10/10; Pharmacia). The preparation was approximately 94% pure as assessed by densitometry of SDS-PAGE gels.
Purification of Ydj1.
Ydj1p was expressed in bacteria and purified as described previously (26). Bacterial lysates were fractionated by DEAE-cellulose and hydroxylapatite column chromatography. The preparation was approximately 80% pure as assessed by densitometry of SDS-PAGE gels.
Purification of p23.
The bacterial expression and purification of human p23 has been described (42). The soluble fraction of bacterial lysate was fractionated by DEAE-cellulose column chromatography followed by phenyl-Sepharose (hp 1660) fast-performance liquid chromatography. The preparation was more than 99% pure as assessed by densitometry of SDS-PAGE gels.
Protein priming.
Approximately 10 ng of purified GST-miniRT proteins were used in an in vitro protein-priming reaction in a total volume of 10 μl, as described previously (18). In all reactions, [α-32P]dATP was used as the labeled nucleotide. Various supplements were added to the priming reaction, as indicated in the figures. These included an ATP regenerating system (5 mM ATP, 10 mM creatine phosphate, and 50-μg/ml creatine phosphokinase), an ATP depleting system (2-U/ml hexokinase; 10 mM glucose), and rabbit reticulocyte lysate (nuclease treated; Promega).
RNA binding.
RNA binding assay was performed using glutathione agarose bead-bound GST-miniRT fusion proteins and in vitro-transcribed, 32P-labeled ɛ RNA, as described previously (18).
RESULTS
Reconstitution of protein priming with Hsp90, Hsp70, Hop, and Hsp40.
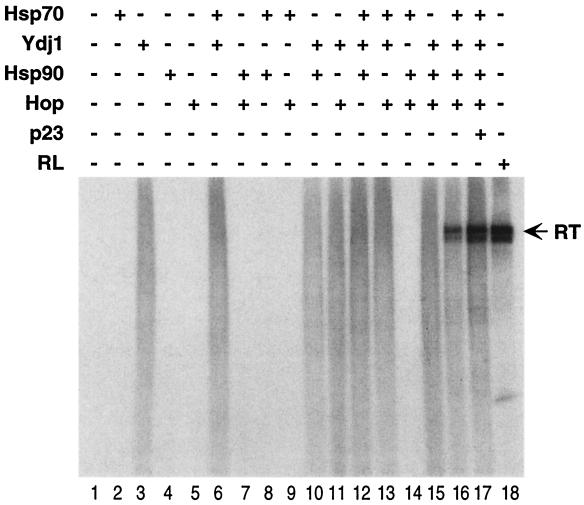
Recently we succeeded in purifying the recombinant DHBV RT expressed in bacteria as truncated minimal proteins fused to GST. One such minimal RT protein, GST-miniRT1, showed strong protein-priming activity in vitro upon reconstitution with the rabbit reticulocyte lysate in an Hsp90-dependent fashion (18) (Fig. 1, lane 18). Using this reconstitution system, we have now attempted to stimulate protein priming using known components of the Hsp90 chaperone complex to substitute for the lysate. For the steroid receptors, it has recently been reported that five proteins of the Hsp90 complex, i.e., Hsp90, Hsp70, p60/Hop, Ydj1 (the yeast Hsp40 homologue), and p23, are sufficient to transform the receptor into a hormone-binding competent conformation (7, 26). We have used these proteins, alone and in various combinations, to reconstitute protein priming. As can be seen in Fig. 1, four proteins, Hsp90, Hsp70, Hop, and Ydj1, together could reconstitute the protein-priming reaction (Fig. 1, lane 16). None of these proteins used alone or in any two- or three-protein combination was effective. Occasionally, the combination of Hsp70 plus Ydj1 could weakly stimulate protein-priming activity (e.g., Fig. 2A, lane 4; data not shown), but they were much less effective than the four proteins added together.
FIG. 1.
Reconstitution of protein priming with different combinations of chaperone proteins. Purified GST-MiniRT1 was used for an in vitro protein-priming assay, with or without reconstitution with the indicated chaperone proteins. All reactions were supplemented with the ɛ RNA and an ATP regenerating system (see Materials and Methods for details). Approximately 10 ng of GST-MiniRT1 was used in each reaction (10-μl reaction volume). The amount of the chaperone proteins used was 350 ng of Hsp70, 1,000 ng of Ydj1, 120 ng of Hsp90, 125 ng of Hop, and 20 ng of p23 per reaction. Reticulocyte lysate (RL) (Promega) was used at 5 μl per reaction. The reaction time in all cases was 1 h at 30°C. The 32P-labeled GST-MiniRT1 protein (the product of protein priming) was detected by resolving the reactions by SDS-PAGE and autoradiography. The labeled GST-MiniRT1 protein is indicated (RT).
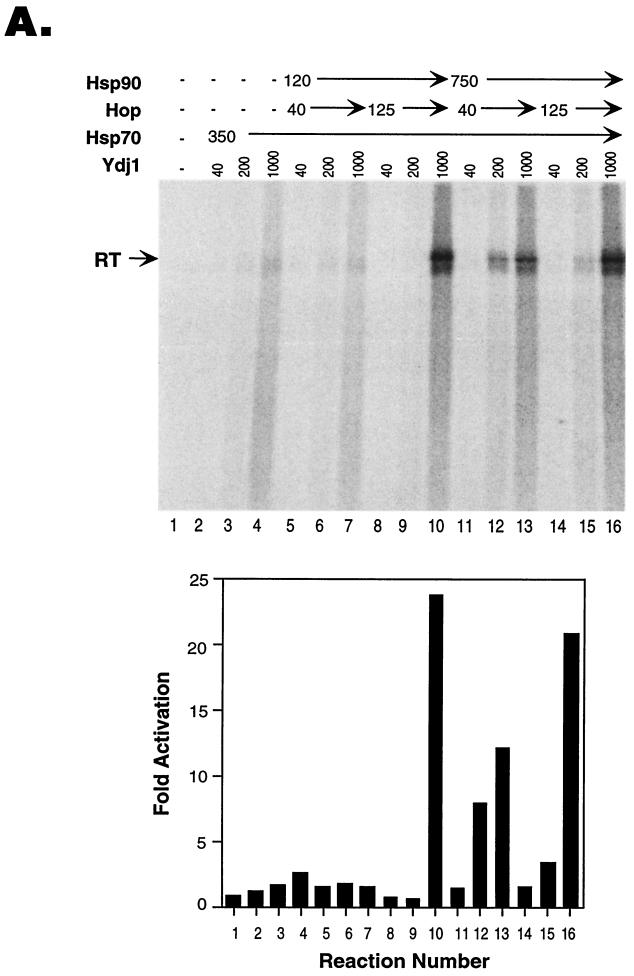
FIG. 2.
Concentration dependence of reconstitution of protein priming. Protein-priming reactions were carried out as for Fig. 1, except that the amounts of the chaperone proteins added to the reactions were varied. (A) The amount of Hsp70 was held constant (350 ng per reaction), and various amounts (in nanograms) of Hsp90, Hop, and Ydj1 were added to the indicated reactions. The graph at the bottom shows the quantitative results (by phospho-imaging) derived from the same reactions shown at the top. The control reaction (reaction 1) signal was set to 1, and the fold activation represents the fold increase in the reaction signal over this background activity. (B) The amounts of Hsp90 and Hop were held constant (at 120 and 125 ng per reaction, respectively), and various amounts (in nanograms) of Ydj1 and Hsp70 were added to the indicated reactions. The graph on the right shows the quantitative results derived from the reactions shown on the left. The control reaction (reaction 7) signal was set to 1.
Concentration dependence on the chaperone proteins for reconstitution of protein priming.
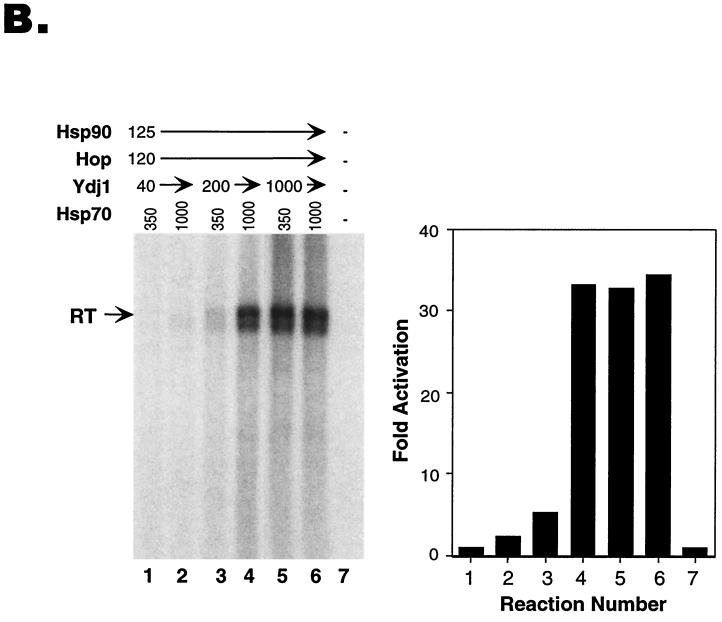
We found that reconstitution of protein priming with the chaperones was highly concentration dependent for each of the four proteins used. We routinely used approximately 10 ng (corresponding to 11 nM in the 10-μl reaction volume, assuming the active RT unit was a monomer) of purified GST-miniRT1 in the reaction. The amounts of the chaperone proteins required for optimal reconstitution were found to be 120 ng (66 nM) for Hsp90, 350 ng (500 nM) for Hsp70, 125 ng (208 nM) for Hop, and 1,000 ng (2.5 μM) for Ydj1 (Fig. 2A, lane 10; additional titration results not shown).
Interestingly, the optimal concentration of one chaperone protein seemed to be dependent on the concentrations of the other proteins. Thus, when the concentration of Hsp90 was increased (from 120 ng [66 nM] to 750 ng [413 nM]), less Hop (40 ng [67 nM], instead of 125 ng [208 nM]) was required to stimulate priming, given the same concentrations of Hsp70 and Ydj1 (compare lanes 12 and 13 to 6 and 7 in Fig. 2A). Furthermore, when the concentration of Hsp70 was increased (1,000 ng [1.4 μM]), less Ydj1 (200 ng [500 nM]) was needed to achieve the same maximal degree of RT reconstitution (Fig. 2B, compare lanes 4 and 6). Conversely, more Ydj1 (1,000 ng [2.5 μM]) was required when less Hsp70 (350 ng [500 nM]) was used (Fig. 2B, compare lanes 3 and 5).
Kinetics of in vitro reconstitution of protein priming.
We noticed that with a 1-h reaction time, the protein-priming activity reconstituted with the four purified chaperone proteins was not as high as that reconstituted with the reticulocyte lysate (Fig. 1, lanes 16 and 18, and Fig. 3A, lanes 5 and 11). One reason for the low efficiency in reconstituting protein priming using the purified proteins, compared to using the reticulocyte lysate, could be kinetic differences. We therefore extended the reaction time to see if that could further increase the extent of reconstitution with the purified chaperone proteins. As shown in Fig. 3, extending the reaction time from 1 h to 2 h did increase the priming activity by approximately twofold when the four chaperone proteins were used for reconstitution. On the other hand, it did not further increase reconstitution with the reticulocyte lysate. Further extension of the reaction time to 3 and 4 hours did not further increase the extent of protein priming reconstituted with either the reticulocyte lysate or purified chaperones (data not shown). On the other hand, when reconstitution was carried out for as little as 30 min, the reticulocyte lysate already significantly stimulated protein priming (approximately 30% of the maximum achieved at 1 h), while the four purified proteins showed little reconstituting activity after this short reaction time. These results thus showed that the kinetics of reconstitution of protein priming with the four chaperone proteins was slower, and the maximal degree lower (two to threefold), than with the reticulocyte lysate.
FIG. 3.
Time dependence of reconstitution of protein priming. (A) Protein-priming reactions, as described in Fig. 1, were carried out for different durations (30 min, 1 h, or 2 h), either without added chaperones or with the addition of four chaperones (Hsp90, Hsp70, Hop, and Ydj1), five chaperones (Hsp90, Hsp70, Hop, Ydj1, and p23) or reticulocyte lysate (RL). The amount of chaperones used was 120 ng for Hsp90, 350 ng for Hsp70, 125 ng for Hop, 1,000 ng for Ydj1, and 50 ng for p23. The amount of RL used was 5 μl per reaction. (B) Quantitative results derived from multiple time course experiments were summarized, and the means and standard errors are shown. The control reaction (without added chaperones or RL) signal was always set to 1, and fold activation represents the fold increase in reaction signal over this background at the respective time points. Filled circles, reconstitution with RL; open circles, reconstitution with Hsp90, Hsp70, Hop, Ydj1, and p23; open diamonds, reconstitution with Hsp90, Hsp70, Hop, and Ydj1.
Enhancement of reconstitution of protein priming by p23.
The above results suggested that additional factors in the reticulocyte lysate, other than the four proteins used, may be able to further increase the speed and extent of reconstitution of protein priming. One such candidate factor we tested was p23. p23 has been shown to be required for the function of some Hsp90 target proteins (23, 26). We have previously observed that p23 could stimulate the protein-priming activity when it was added to the RT expressed in vitro in a wheat germ translation system (22). The data presented in the above sections indicated that p23 was not essential for RT reconstitution under our in vitro conditions. In addition, we found that p23 could not substitute for any of the above four factors in reconstitution (data not shown). However, the results in Fig. 1 and 3 indicate that p23 could enhance the kinetics of RT reconstitution. Thus, in a 30-min or 1-h reaction, the addition of p23 plus the other four chaperone proteins stimulated protein priming to a higher degree than was seen with reactions in its absence (Fig. 1, lanes 16 and 17; Fig. 3A, lanes 4 through 9). Note that the reactions shown in Fig. 1 were performed for 1 h only. After 2 h of reconstitution, the RT priming activity obtained with the four chaperone proteins (without p23) approached that obtained with all five chaperones (Fig. 3). Thus, with all five chaperone proteins, protein-priming reaction reconstituted using the purified proteins showed similar kinetics to and was nearly as efficient (60 to 70%) as that with the reticulocyte lysate.
Dependence of reconstitution of protein priming on RT functions and ɛ RNA.
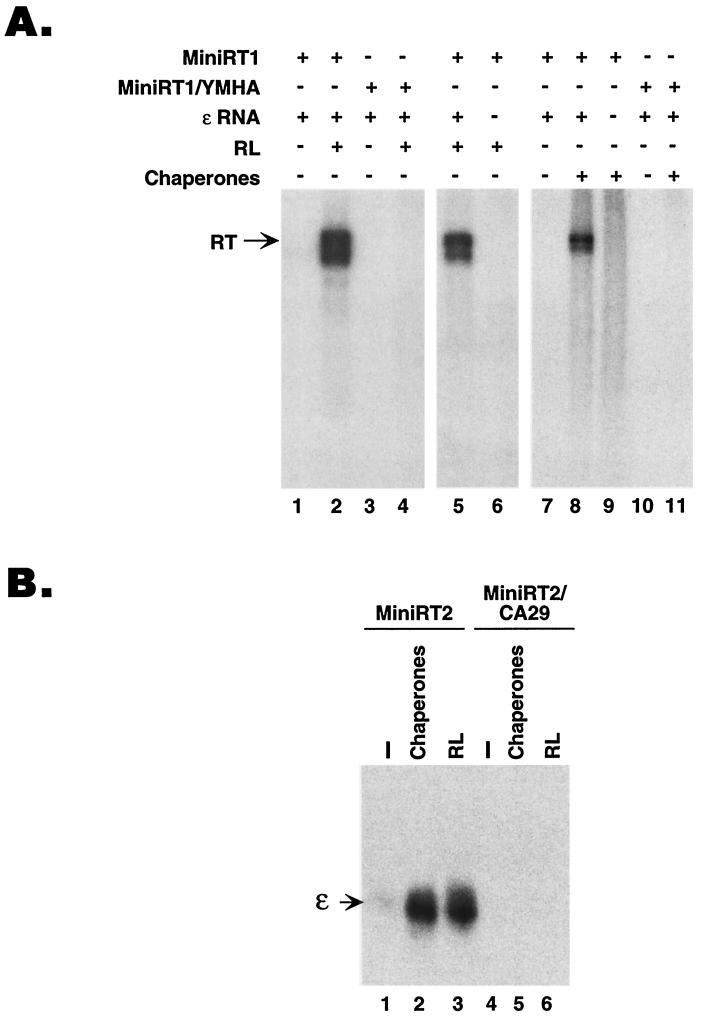
It was important to verify that the protein-priming activity detected after reconstitution using the purified chaperone proteins represented authentic RT function. To do this, we tested well-characterized RT mutants in our reconstitution assay. As expected from previous studies (46), reconstitution of protein priming using the purified RT and cellular proteins was absolutely dependent on the catalytic activity of the RT. A mutant RT (termed YMHA) with two amino acid substitutions in the RT active site (5) completely abolished protein priming (Fig. 4A, lanes 3, 4, 10 and 11). Similarly, reconstitution of protein priming with either the purified chaperone proteins or the reticulocyte lysate required the ɛ RNA (Fig. 4A, lanes 6 and 9) (33, 47).
FIG. 4.
Reconstitution of protein priming and RT-ɛ interaction required RT functions and the ɛ RNA. (A) GST-MiniRT1 or its mutant derivative MiniRT1/YMHA, which harbors two amino acid substitutions in the RT active site, abolishing its catalytic activity, was used in the in vitro protein-priming assay. Reconstitution was done using either reticulocyte lysate (RL) or the five chaperone proteins (Hsp90, Hsp70, Hop, Ydj1, and p23). Where indicated, the ɛ RNA was omitted from some of the reactions shown. (B) Purified GST-MiniRT2 or its mutant derivative MiniRT2/CA29, which harbors two amino acid substitutions abolishing its ɛ RNA binding activity, was assessed for ɛ binding activity by the GST pull-down assay (see Materials and Methods). The minimal RT proteins were prebound to the glutathione beads, and 32P-labeled ɛ RNA was then allowed to bind to the RT proteins with (lanes 1 and 4) or without the addition of the five chaperones proteins (Hsp90, hsp70, Hop, Ydj1, and p23; lanes 2 and 5) or RL (lanes 3 and 6). All reactions were supplemented with the ATP regenerating system. The bound ɛ RNA was then resolved by SDS-PAGE and autoradiography. The ɛ RNA is indicated.
To demonstrate directly that chaperone reconstitution stimulated RT-ɛ interaction, we measured the efficiency of RT-ɛ binding using a coprecipitation assay developed previously (18, 47). For this assay, we used a shorter minimal RT protein, GST-MiniRT2, which is as active in ɛ binding as the full-length RT (18) but is more readily expressed and purified than miniRT1. As shown in Fig. 4B, the five purified chaperones could stimulate RT-ɛ binding as efficiently as the reticulocyte lysate. Similar results were obtained using GST-MiniRT1 (data not shown). As a negative control, we used a ɛ RNA binding-defective RT mutant, MiniRT2/CA29 (18, 38), which showed no ɛ binding activity with or without reconstitution.
Dependence on ATP hydrolysis and the ATPase activity of Hsp90 for reconstitution of protein priming.
Using this defined reconstitution system, we have begun to characterize in detail the requirement for protein priming. Previously we have shown that protein priming reconstituted with the reticulocyte lysate was dependent on ATP (18). Here, we also tested the dependence on ATP for reconstituting protein priming using purified components. Preliminary results showed that the addition of ATP, in particular of an ATP regenerating system (see Materials and Methods), strongly stimulated reconstitution of the protein-priming activity (Fig. 5A, lane 2; data not shown). On the other hand, protein priming could still be reconstituted, albeit much less efficiently, using the purified chaperone proteins even in the absence of any exogenous source of ATP (Fig. 5A, lane 3). However, purified Hsp70 does contain some bound ATP and ADP. In addition, the purified minimal RT proteins still contained two bound bacterial chaperones (DnaK and GroEL) (18; also see Discussion), and these might also have some residual amounts of bound ATP. Thus, we attempted to eliminate these potential sources of ATP that may be introduced into the reaction unintentionally. Inclusion of an ATP-depleting system (hexokinase plus glucose; see Materials and Methods) in the reaction completely inhibited protein priming (data not shown). Furthermore, we used a nonhydrolyzable ATP analog, γ-S-ATP, as a competitive inhibitor for ATP hydrolysis. As shown in Fig. 5A (lane 4), the inclusion of γ-S-ATP completely abolished protein priming, thus demonstrating that ATP hydrolysis, not just ATP binding, was required for reconstituting protein priming.
FIG. 5.
Reconstitution of protein priming required ATP hydrolysis and the ATPase activity of Hsp90. (A) Protein-priming reactions were carried out as for Fig. 1, except that the ATP regenerating system (ATP RS) was omitted from the reactions shown in lanes 3 and 4. Instead, γ-S-ATP (5 mM) was added to reaction 4. All reactions except that shown in lane 1 contained five chaperone proteins (Hsp90, Hsp70, Hop, Ydj1, and p23). (B) Protein-priming reactions were carried out as for Fig. 1. All reactions contained 1 μg of Hsp70, 125 ng of Hop, and 200 ng of Ydj1. The amounts (in nanograms) of Hsp90, HtpG, TRAP, and the Hsp90 E46A ATPase mutant that were added to the reactions are indicated.
Recently Hsp90 has been clearly shown to be an ATPase (31, 35). Indeed, Hsp90 mutants defective in ATP hydrolysis cannot maintain the Hsp90 functions that are essential for cell survival (31), nor can they reconstitute ligand-binding activity of steroid receptors (14), although these mutants retain a “passive” chaperone activity in vitro that is ATP independent. The requirement for ATP hydrolysis in reconstituting protein priming, as shown above, could have reflected the need for the ATPase activity of Hsp90 and/or Hsp70, which is known to require ATP hydrolysis for its function (15). We have previously shown that geldanamycin, a specific Hsp90 inhibitor that binds to the Hsp90 ATP-binding pocket and competes with ATP for Hsp90 binding, can block RT-ɛ interaction and protein priming (18, 20, 22), suggesting that binding of ATP by Hsp90 is important for its chaperoning function towards the RT. To determine directly whether the reconstitution of protein priming specifically required the ATPase-dependent, “active” chaperone activity of Hsp90, we tested a well-characterized Hsp90 mutant, the E46A mutant, which is defective in ATP hydrolysis (14). In addition, we also included two distantly related Hsp90 homologues, the bacterial protein HtpG and the mitochondrial protein TRAP, which is related more to HtpG than to Hsp90 (9, 31). These distant Hsp90 relatives display in vitro chaperone activities and are also active in ATP binding and hydrolysis but are nevertheless deficient in maintaining the functions of authentic Hsp90 target proteins in eukaryotic cells.
As shown in Fig. 5B, neither TRAP nor HtpG could substitute for Hsp90 in the reconstitution of protein priming. The ATPase-defective Hsp90 mutant, carrying the E46A mutation, was also much less (10 to 20%) active than the wild-type Hsp90 in reconstituting protein priming. Furthermore, at higher concentrations, the E46A mutant (but not TRAP or HtpG) significantly inhibited protein priming such that the priming activity in the presence of high concentrations of the E46A mutant was two- to threefold lower than the residual priming activity when Hsp90 was omitted altogether (Fig. 5B, lane 8 versus lane 1). Furthermore, the addition of the E46A protein together with wild-type Hsp90 also resulted in dose-responsive inhibition of Hsp90-mediated reconstitution of protein priming (Fig. 5B, lanes 11 to 14), indicating that the ATPase-defective E46A mutant acted as a dominant-negative inhibitor of Hsp90 function in this system.
DISCUSSION
Protein-primed initiation of reverse transcription represents a unique and critical early step in hepadnavirus replication. Our recent studies have demonstrated that protein priming requires not only the viral reverse transcriptase and the ɛ RNA template but also specific host cell factors, including the Hsp90 chaperone (20, 22). Building on our recent success in expressing and purifying truncated minimal DHBV RT proteins (18), we have now succeeded in efficiently reconstituting the protein-priming reaction with the purified minimal RT proteins and five cellular factors. Four host proteins, Hsp90, Hsp70, Hop, and Hsp40 (all known components of the Hsp90 chaperone complex), were shown to be required and sufficient for significant reconstitution of protein priming, provided that a source of energy (in the form of hydrolyzable ATP) was provided. These four proteins, together with p23 (another Hsp90 chaperone component), could reconstitute protein priming nearly as efficiently as and with similar kinetics to the reticulocyte lysate, a cytoplasmic extract rich in chaperone components and widely used for protein folding and chaperone studies. This defined reconstitution system faithfully recapitulated the known requirements for protein priming in that it required the polymerase activity of the RT protein and the authentic viral ɛ RNA as the template.
Efficient reconstitution of protein priming required each of the chaperone components, used at optimal concentrations. With an RT concentration of 11 nM, the optimal concentrations required for each chaperone protein were 66 nM for Hsp90, 0.5 μM for Hsp70, 208 nM for Hop, 2.5 μM for Ydj1, and 200 nM for p23. However, when the Hsp70 concentration was increased to 1.4 μM, 0.5 μM Ydj1 was sufficient for maximal reconstitution. In addition, nearly maximal reconstitution activity was also obtained with 67 nM Hop when the Hsp90 concentration was increased to 413 nM. The optimal chaperone concentrations found for reconstituting the active progesterone receptor were 0.8 μM for Hsp90, 1.4 μM for Hsp70, 0.08 μM for Hop, 0.2 μM for Ydj1, and 1.3 μM for p23 (26), and similar chaperone concentrations were also required for optimal reconstitution of the glucocorticoid receptor (28). Therefore, the optimal chaperone concentrations found for both the RT and steroid receptor systems are comparable. In both systems, the chaperone proteins are used at a relative molar excess over the target protein. This is commonly observed for chaperone functions due to the cyclic, reiterative nature of chaperone-target interactions (4, 44). Furthermore, the interdependence of the concentration optimums between Hsp90 and Hop and between Hsp70 and Hsp40 suggests that the different chaperone proteins may function in distinct partnerships. Mechanistically, Hsp70, together with Hsp40, is thought to function early in the Hsp90 complex-mediated folding pathway of the steroid receptors, whereas Hop is thought to act as a bridge to bring Hsp90 to the receptor-bound Hsp70 (6, 40). Although the activation/folding pathway of the RT remains to be defined, the observed concentration interdependence is consistent with the notion that Hsp70 and Hsp40 may function cooperatively during one step of the pathway, whereas Hsp90 and Hop may cooperate during a different stage.
The role of p23 as a cofactor in Hsp90-mediated target protein folding is not well defined. Studies with the steroid receptors have shown that p23 is stringently required for the ligand-binding activity of the progesterone receptor (26) but not of the glucocorticoid receptor (7, 28). Genetic analysis in yeast also showed that deletion of the p23 homologue resulted in only minimal effect on Hsp90 target proteins, including steroid receptors and protein kinases (3, 8; but also see reference 25). We have previously shown that when the RT was expressed in the wheat germ extract, which seems to be deficient in p23, protein-priming activity could be stimulated by p23 (22). Here, we have shown that p23 was not absolutely required for reconstitution of protein priming in vitro but could nevertheless enhance the kinetics of reconstitution. It appears that the effect of p23 may depend on the particular target proteins and the assay systems and conditions used. One potential mechanism whereby p23 may enhance the kinetics of RT reconstitution is promotion of recycling of the Hsp90 complex from the RT, since p23 has recently been shown to stimulate substrate release from Hsp90 (50).
We have demonstrated that efficient reconstitution of protein priming required the ATPase-dependent, active chaperone functions of Hsp90 and not just the passive, ATP-independent chaperone activity that Hsp90 displays toward some generic substrates in vitro (10, 49). Furthermore, an Hsp90 mutant (E46A) defective in ATPase activity behaved as a dominant-negative inhibitor in the reconstitution assay when used at higher concentrations. Since the Hsp90 mutant retains the ability to bind to target proteins and to some cochaperones (14, 50), it may inhibit protein priming by sequestering the target (the RT) and/or cofactors (e.g., Hop). In addition, we found that the bacterial and mitochondrial Hsp90 homologues, which have chaperone activities towards some in vitro substrates (9, 30), did not show any effect, either positive or negative, on the reconstitution of RT activity. These results thus provide direct evidence that the interaction between the RT and Hsp90 reflects a specific process and the RT represents a bona fide target of the Hsp90 chaperone complex.
It is remarkable that the same set of proteins is sufficient to reconstitute the protein-priming activity of the RT and the ligand binding activity of the steroid receptors, which have very different structures and functions. It is possible that these factors form a core Hsp90 chaperone complex that may be used to facilitate the folding of a diverse range of substrate proteins, through the participation of additional accessory factors dedicated to individual substrates. The requirement for these additional factors may not be revealed until more in vivo-like assay conditions are developed. On the other hand, it remains possible that other Hsp90 target proteins, such as the protein kinases, may require a very different set of Hsp90 cofactors than the steroid receptors and the RT, as indicated by the importance of CDC37/p50 in kinase functions (12, 41).
It is important to emphasize that the five host proteins identified here represent only the minimal cellular requirement for hepadnavirus protein priming. Additional factors may be required for protein priming in the infected cell in vivo. We attempted to determine the specific activity of the RT in protein priming by measuring the amount of the labeled nucleotides incorporated into the RT, which is a direct result of protein priming. Our estimate showed that under the best in vitro conditions so far, only 10 to 20% of the RT molecules could be activated into a priming-competent state with either the purified chaperone proteins or the reticulocyte lysate. Interestingly, similar specific activities were estimated also for the RT translated directly in the reticulocyte lysate (33; J. Hu, unpublished results). Although it remains possible that we have yet to find the optimal conditions for reconstitution in vitro, these results suggest that additional factors other than the five proteins tested here (and possibly tissue and/or species specific) may be important for protein priming as well. One approach to identifying these additional factors would be to examine purified viral nucleocapsids for the presence of specifically incorporated host factors. We have found that both Hsp90 and p23 are incorporated into the nucleocapsids in an RT-dependent manner (22; J. Hu, unpublished). It is not yet known whether the other chaperone proteins identified here are also incorporated into the nucleocapsids. In addition, as we have reported previously, the purified minimal RT proteins expressed in bacteria are tightly bound to the two bacterial chaperones DnaK and GroEL (18). It has proven difficult so far to completely eliminate these associated bacterial chaperones from the RT while maintaining its potential for activation. It remains formally possible that these bacterial proteins may also play a role in the in vitro reconstitution of RT activity in our system, perhaps by maintaining the RT in a foldable state.
The ability to reconstitute protein priming with defined components should now facilitate mechanistic studies on the mechanism of RT activation by host factors. One of the important questions still unresolved is the mechanism of recognition of the RT (and indeed, of any other target proteins) by the Hsp90 chaperone complex. This defined reconstitution system now provides the opportunity to study in detail the mechanism of RT recognition by the chaperone components. Interestingly, a recent report showed that the telomerase, another specialized reverse transcriptase, may also require the Hsp90 chaperone for its activity (17). The telomerase shares some interesting features with the hepadnavirus RT in that they both can recognize a specific RNA and indeed require their respective endogenous RNAs for activity. It will be interesting to see what common characteristics, if any, are shared by these two reverse transcriptases (as opposed to other RTs) and are recognized by the Hsp90 complex. Furthermore, this defined in vitro reconstitution system has now opened the door for future biochemical and structural studies to elucidate the dynamics of RT-chaperone interactions and the pathway of RT folding and activation.
FIG. 2.
Continued.
Acknowledgments
We thank Nancy McMahon for assistance in protein purification and C. Seeger, R. Corley, and G. Viglianti for critical comments on the manuscript.
J. Hu is a Harcourt General Researcher and the recipient of an American Liver Foundation Liver Scholar Award. This work was supported by Public Health Service grants R01 AI43453 (to J.H.) and DK46249 (to D.T.) from the National Institutes of Health, by a New Investigator Award of The Medical Foundation from the Harcourt General Charitable Foundation (to J.H.), and by the American Liver Foundation (to J.H.).
REFERENCES
- 1.Bartenschlager, R., and H. Schaller. 1992. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 11:3413–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohen, S., A. Kralli, and K. Yamamoto. 1995. Hold ’em and fold ’em: chaperones and signal transduction. Science 268:1303–1304. [DOI] [PubMed] [Google Scholar]
- 3.Bohen, S. P. 1998. Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of Hsp90-dependent signaling proteins. Mol. Cell. Biol. 18:3330–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchner, J. 1999. Hsp90 & Co—a holding for folding. Trends Biochem. Sci. 24:136–141. [DOI] [PubMed] [Google Scholar]
- 5.Chang, L. J., R. C. Hirsch, D. Ganem, and H. E. Varmus. 1990. Effects of insertional and point mutations on the functions of the duck hepatitis B virus polymerase. J. Virol. 64:5553–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, S., and D. F. Smith. 1998. Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J. Biol. Chem. 273:35194–35200. [DOI] [PubMed] [Google Scholar]
- 7.Dittmar, K. D., and W. B. Pratt. 1997. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90 · p60 · hsp70-dependent step is sufficient for creating the steroid binding conformation. J. Biol. Chem. 272:13047–13054. [DOI] [PubMed] [Google Scholar]
- 8.Fang, Y., A. E. Fliss, J. Rao, and A. J. Caplan. 1998. SBA1 encodes a yeast hsp90 cochaperone that is homologous to vertebrate p23 proteins. Mol. Cell. Biol. 18:3727–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felts, S. J., B. A. Owen, P. Nguyen, J. Trepel, D. B. Donner, and D. O. Toft. 2000. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J. Biol. Chem. 275:3305–3312. [DOI] [PubMed] [Google Scholar]
- 10.Freeman, B., and R. Morimoto. 1996. The human cytosolic molecular chaperone hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J. 15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- 11.Frydman, J., E. Nimmesgern, K. Ohtsuka, and F. U. Hartl. 1994. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 370:111–117. [DOI] [PubMed] [Google Scholar]
- 12.Grammatikakis, N., J. H. Lin, A. Grammatikakis, P. N. Tsichlis, and B. H. Cochran. 1999. p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function. Mol. Cell. Biol. 19:1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenert, J. P., W. P. Sullivan, P. Fadden, T. A. Haystead, J. Clark, E. Mimnaugh, H. Krutzsch, H.-J. Ochel, T. W. Schulte, E. Sausville, L. M. Neckers, and D. O. Toft. 1997. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 272:23843–23850. [DOI] [PubMed] [Google Scholar]
- 14.Grenert, J. P., B. D. Johnson, and D. O. Toft. 1999. The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J. Biol. Chem. 274:17525–17533. [DOI] [PubMed] [Google Scholar]
- 15.Hartl, F. 1996. Molecular chaperones in cellular protein folding. Nature 381:571–580. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch, R. C., D. D. Loeb, J. R. Pollack, and D. Ganem. 1991. cis-acting sequences required for encapsidation of duck hepatitis B virus pregenomic RNA. J. Virol. 65:3309–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt, S. E., D. L. Aisner, J. Baur, V. M. Tesmer, M. Dy, M. Ouellette, J. B. Trager, G. B. Morin, D. O. Toft, J. W. Shay, W. E. Wright, and M. A. White. 1999. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 13:817–826. [DOI] [PMC free article] [PubMed]
- 18.Hu, J., and D. Anselmo. 2000. In vitro reconstitution of a functional duck hepatitis B virus reverse transcriptase: posttranslational activation by Hsp90. J. Virol. 74:11447–11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, J., and C. Seeger. 1996. Expression and characterization of hepadnavirus reverse transcriptases. Methods Enzymol. 275:195–208. [DOI] [PubMed]
- 20.Hu, J., and C. Seeger. 1996. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 93:1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, J., and C. Seeger. 1997. RNA signals that control DNA replication in hepadnaviruses. Semin. Virol. 8:205–211.
- 22.Hu, J., D. O. Toft, and C. Seeger. 1997. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 16:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, J. L., T. G. Beito, C. J. Krco, and D. O. Toft. 1994. Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Mol. Cell. Biol. 14:1956–1963. [DOI] [PMC free article] [PubMed]
- 24.Junker-Niepmann, M., R. Bartenschlager, and H. Schaller. 1990. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 9:3389–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knoblauch, R., and M. J. Garabedian. 1999. Role for Hsp90-associated cochaperone p23 in estrogen receptor signal transduction. Mol. Cell. Biol. 19:3748–3759. [DOI] [PMC free article] [PubMed]
- 26.Kosano, H., B. Stensgard, M. C. Charlesworth, N. McMahon, and D. Toft. 1998. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J. Biol. Chem. 273:32973–32979. [DOI] [PubMed] [Google Scholar]
- 27.Lanford, R. E., L. Notvall, and B. Beames. 1995. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J. Virol. 69:4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishima, Y., P. J. Murphy, D. P. Li, E. R. Sanchez, and W. B. Pratt. 2000. Stepwise assembly of a glucocorticoid receptor · hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J. Biol. Chem. 275:18054–18060. [DOI] [PubMed] [Google Scholar]
- 29.Nassal, M., and A. Rieger. 1996. A bulged region of the hepatitis B virus RNA encapsidation signal contains the replication origin for discontinuous first-strand DNA synthesis. J. Virol. 70:2764–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemoto, T. K., T. Ono, and K. Tanaka. 2001. Substrate-binding characteristics of proteins in the 90 kDa heat shock protein family. Biochem. J. 354:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panaretou, B., C. Prodromou, S. M. Roe, R. O’Brien, J. E. Ladbury, P. W. Piper, and L. H. Pearl. 1998. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 17:4829–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearl, L. H., and C. Prodromou. 2000. Structure and in vivo function of Hsp90. Curr. Opin. Struct. Biol. 10:46–51. [DOI] [PubMed] [Google Scholar]
- 33.Pollack, J. R., and D. Ganem. 1994. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J. Virol. 68:5579–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pratt, W., and D. Toft. 1997. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrine Rev. 18:306–360. [DOI] [PubMed] [Google Scholar]
- 35.Prodromou, C., S. Roe, R. O’Brien, J. Ladbury, P. Piper, and L. Pearl. 1997. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90:65–75. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher, R. J., W. J. Hansen, B. C. Freeman, E. Alnemri, G. Litwack, and D. O. Toft. 1996. Cooperative action of Hsp70, Hsp90, and DnaJ proteins in protein renaturation. Biochemistry 35:14889–14898. [DOI] [PubMed] [Google Scholar]
- 37.Schumacher, R. J., R. Hurst, W. P. Sullivan, N. J. McMahon, D. O. Toft, and R. L. Matts. 1994. ATP-dependent chaperoning activity of reticulocyte lysate. J. Biol. Chem. 269:9493–9499. [PubMed] [Google Scholar]
- 38.Seeger, C., E. H. Leber, L. K. Wiens, and J. Hu. 1996. Mutagenesis of a hepatitis B virus reverse transcriptase yields temperature-sensitive virus. Virology 222:430–439. [DOI] [PubMed] [Google Scholar]
- 39.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, D. 1993. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol. Endocrinol. 7:1418–1429. [DOI] [PubMed] [Google Scholar]
- 41.Stepanova, L., X. Leng, S. Parker, and J. Harper. 1996. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 10:1491–1502. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan, W., B. Stensgard, G. Caucutt, B. Bartha, N. McMahon, E. Alnemri, G. Litwack, and D. Toft. 1997. Nucleotides and two functional states of hsp90. J. Biol. Chem. 272:8007–8012. [DOI] [PubMed] [Google Scholar]
- 43.Summers, J., and W. S. Mason. 1982. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell 29:403–415. [DOI] [PubMed] [Google Scholar]
- 44.Toft, D. 1998. Recent advances in the study of hsp90 structure and mechanism of action. Trends Endocrinol. Metab. 9:238–243. [DOI] [PubMed] [Google Scholar]
- 45.Wang, G. H., and C. Seeger. 1993. Novel mechanism for reverse transcription in hepatitis B viruses. J. Virol. 67:6507–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, G. H., and C. Seeger. 1992. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell 71:663–670. [DOI] [PubMed] [Google Scholar]
- 47.Wang, G. H., F. Zoulim, E. H. Leber, J. Kitson, and C. Seeger. 1994. Role of RNA in enzymatic activity of the reverse transcriptase of hepatitis B viruses. J. Virol. 68:8437–8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber, M., V. Bronsema, H. Bartos, A. Bosserhoff, R. Bartenschlager, and H. Schaller. 1994. Hepadnavirus P protein utilizes a tyrosine residue in the TP domain to prime reverse transcription. J. Virol. 68:2994–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiech, H., J. Buchner, R. Zimmermann, and U. Jacob. 1992. Hsp90 chaperones protein folding in vitro. Nature 358:169–170. [DOI] [PubMed] [Google Scholar]
- 50.Young, J. C., and F. U. Hartl. 2000. Polypeptide release by Hsp90 involves ATP hydrolysis and is enhanced by the co-chaperone p23. EMBO J. 19:5930–5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zoulim, F., and C. Seeger. 1994. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J. Virol. 68:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]