Direct cell-to-cell spread of animal viruses in solid tissues is a complex, poorly understood process. A number of viruses have become very adept at moving from an infected cell to an adjoining uninfected cell, specifically at sites of cell-cell contact, moving across epithelial cell or neuronal junctions. Directed cell-to-cell spread is typically rapid and efficient, in part because viruses and their cell surface receptors are in close proximity, and viruses can move across the narrow spaces between cells, protected from the effects of neutralizing antibodies and other immune system components by tight and adherens junctions. Virus cell-to-cell spread and entry of extracellular or cell-free virus particles frequently share mechanistic details, e.g., the use of similar fusion machinery, but cell-to-cell spread can involve intracellular and extracellular events that determine virus delivery to cell junctions and the use of receptors found exclusively at cell junctions. Here, we focus on cell-to-cell spread as defined above and do not discuss virus-induced formation of syncytia, i.e., fusion of infected and uninfected cells.
Three interesting examples of how animal viruses have organized their egress strategies to promote cell-to-cell spread are described here. The alphaherpesviruses provide fascinating examples of viruses that replicate in polarized cells, epithelial cells, and neurons and mimic intracellular sorting pathways to direct nascent virions to cell junctions, promoting infection of adjacent epithelial cells and directed spread within the nervous system. Human immunodeficiency virus (HIV) is a virus that normally replicates in lymphocytes and macrophages, cells that are not usually considered polarized. However, HIV can specifically assemble progeny at sites of transient contact between lymphocytes and macrophages, thereby promoting virus spread. Poxviruses can induce the formation of actin tails that launch virus particles from the cell surface on the tips of microvilli toward neighboring cells.
HIV.
HIV infects CD4+ T lymphocytes and monocytes/macrophages, cells that travel widely throughout the host circulatory and lymphatic systems. T cells and macrophages can interact intimately with one another and with other cell types through transient, but nevertheless robust, bonds. HIV apparently uses this cell adhesion to spread from cell to cell. One interesting example includes anti-HIV CD4+ T cells that form “immunologic synapses,” involving T-cell receptors, LFA-1, intercellular adhesion molecule 1 (ICAM-1), CD43, and other costimulatory and cell adhesion molecules (CAMs) (reviewed in reference 23), with other, HIV-infected CD4+ T cells. In this case, virus spread from infected T cells to uninfected effector T cells may contribute to the loss of anti-HIV CD4+ T cells and collapse of the anti-HIV immune response (reviewed in reference 54). Moreover, cell-to-cell transmission of HIV can occur between other cell types, such as macrophages, endothelial cells, and epithelial cells.
Rapid cell-to-cell spread was observed in early studies of HIV and was compared to processes by which herpesviruses and poxviruses spread (38, 49, 77). HIV is highly cell associated, and the infectivity of cell-associated virus is much higher than that of cell-free virus (15, 19). Furthermore, efficient transmission can occur in the presence of neutralizing antibodies. In many instances, HIV cell-to-cell spread was considered to be largely via cell-cell fusion (syncytium formation). However, while cell fusion may play a role in HIV disease, its extent and its significance in promoting virus spread in vivo are not clear (15, 31, 47). Studies with cultured T cells and macrophages or dendritic cells have provided evidence for cell-to-cell spread, without cell fusion, and apparently across junctions formed between cells (6, 15, 18, 26, 65, 66, 91, 92).
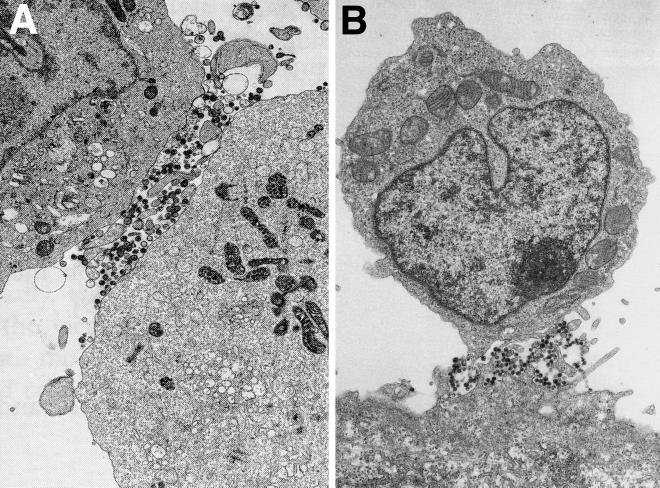
Direct spread of HIV from T cells or macrophages can involve the release of progeny virus at one pole of the cells, at sites of cell-cell contact. T cells and macrophages are not usually considered polarized cells. However, after activation or during motility, T cells and macrophages can establish extensive cytoskeletal networks and form specific polar membrane domains (reviewed in reference 74). HIV budding can be observed at the leading edge or pseudopods of cells (62, 64, 67). Moreover, when T lymphocytes contact antigen-presenting cells, there are further changes in the cell surface, adhesion between cells occurs, and HIV can be localized largely to these sites of cell-cell contact (Fig. 1A). Studies of HIV-infected CD4+ T-cell lines in contact with each other revealed highly polarized expression of viral antigens, virus budding, and particles on the cell surface between microvilli at cell junctions (18, 26, 62). When placed in contact with epithelial cells, HIV-infected monocytes (8, 67) and T lymphocytes (66, 84) displayed numerous virus particles on microvillar structures that interdigitated between epithelial cell microvilli (Fig. 1B). There was extensive polarization of virus budding and release of virus particles at these sites of cell-cell contact, whereas budding of HIV from the same monocytes or T lymphocytes grown in suspension was not observed.
FIG. 1.
Polarized egress of HIV particles on T lymphocytes. (A) HIV-infected C8166 cells, a CD4+ T-cell line, were analyzed by electron microscopy. Virions were found preferentially at sites of cell contact. (Reprinted from AIDS [26] with permission of the publisher.) (B) HIV-infected CD4+ T cells (upper cell) adhering to a BeWo epithelial cell in the lower part of the panel. Virions were observed at sites of cell-cell contact, between microvilli extending from both cells. (Reprinted from AIDS [65] with permission of the publisher.)
Interactions of HIV envelope glycoprotein gp120 with CD4, as well as with CXCR4, CCR5, or other chemokine receptors, are required for virus entry into most host cells. However, CAMs, including LFA-1 and its ligands ICAM-1, -2, and-3, can contribute to both virus entry and cell-to-cell spread. ICAM-1 can be incorporated into the virion envelope, apparently affecting entry of extracellular virus (30, 41, 70), but can also cement cells together, promoting movement of HIV directly across cell junctions (26, 92). It is likely that other T-cell or macrophage CAMs can similarly contribute to cell-to-cell spread of HIV.
The polarized egress of HIV and budding of virions at sites of cell-cell contact appears to be directly related to assembly of viral proteins at specific plasma membrane domains. HIV Gag and Env proteins determine where assembly occurs. It is possible that one or the other, or both, of these viral proteins respond to changes in cytoskeletal architecture or cell-cell contact. Alternatively, Gag and Env proteins associate with membrane lipid rafts, which can accumulate at pseudopods at one pole of HIV-infected T cells after cell activation or cell-cell contact (58). The relative contributions of Gag and Env proteins in HIV polarized assembly are not yet clear. Simultaneous assembly and budding of HIV particles can be driven solely by Gag proteins that associate with the inner surface of the plasma membrane, forming a shell around the viral RNA and promoting budding into the extracellular space (reviewed in reference 32). Expression of Gag in the absence of Env and RNA results in association with the plasma membrane and formation of virus-like particles, implying that Gag contains all the necessary determinants for assembly. There is also preliminary evidence for polarized expression of Gag in the absence of Env (P. Spearman and M. Thali, unpublished results).
Env also appears capable of influencing the spatial distribution of virus assembly and increasing the rate of virus egress through interactions with clathrin adapters (reviewed in reference 103). Env was targeted specifically to the basolateral surfaces of polarized epithelial cells through a tyrosine-based motif (YSPL) in the cytoplasmic (CT) domain of gp41, and there was basolateral assembly of virus particles (51, 61). Moreover, in HIV-infected Jurkat CD4+ lymphocytes, there was polarized localization of p24 and virus budding, and mutation of the tyrosine motif in gp41 reduced this polarization (18). Importantly, mutation of the same tyrosine reduced cell-to-cell transmission of virus without affecting cell-free virus transmission. Mutation of the tyrosine motif caused severe attenuation of HIV in SCID-hu mice (C. Stoddart, J. M. McCune, and M. Thali, personal communication), and mutation of an analogous tyrosine motif in simian immunodeficiency virus markedly affected virulence in vivo without affecting replication in cultured cells (35). Therefore, it appears that this Env tyrosine motif, as well as a dileucine motif (103), may promote interactions with clathrin adapters that sort membranes in the trans-Golgi network (TGN) and endosomes. This appears to be similar to the sorting of alphaherpesvirus glycoproteins that has been associated with directed egress to cell junctions (see below). However, in the case of HIV, it is remarkable that this occurs not in polarized cells but in lymphocytes that form transient contacts.
ALPHAHERPESVIRUSES.
Alphaherpesviruses replicate rapidly in a wide variety of cell types, spread swiftly from cell to cell, and have the capacity to establish latency in sensory or autonomic neurons (71). The best studied of these viruses, herpes simplex virus (HSV), normally infects mucosal oral or genital tissues and less frequently infects the skin and eye, and it spreads to and establishes latency in sensory and autonomic neurons but can also spread into the central nervous system, causing encephalitis. Varicella-zoster virus (VZV), the etiologic agent of chickenpox and shingles, similarly infects mucosal tissues and the upper respiratory tract, and it has the capacity to spread to lymph nodes, other organs, and the skin by passage in leukocytes (3). Pseudorabies virus (PRV) is a highly neurovirulent pig alphaherpesvirus (reviewed in reference 25).
The relative importance of direct cell-to-cell spread versus spread by extracellular virus is illustrated by several salient features of the biology of alphaherpesviruses. First, HSV, VZV, and PRV all remain largely cell associated; large numbers of virus particles accumulate in the cytoplasm or on the surfaces of cultured cells. Second, alphaherpesviruses replicate primarily in cells that are extensively polarized or which form extensive cell-cell contacts. Neutralizing antibodies have little effect on their ability to spread between cells in culture and are ineffective in limiting progression of disease, and antibody titers do not predict the severity of disease or time to recrudescence (reviewed in references 3 and 16). Third, virus mutants with defects in cell-to-cell spread but without defects in entry of extracellular virions are severely attenuated in animal models of HSV and PRV pathogenesis (see below). We and others have proposed that HSV particles can efficiently pass across the junctions formed between epithelial cells and neurons, in spaces that are relatively resistant to the effects of antiviral antibodies (22).
There is good evidence that the egress of herpesviruses can occur such that nascent virions are directed to cell junctions and that this promotes efficient cell-to-cell spread. The pathway or pathways by which alphaherpesviruses exit cells have been controversial, but there is now some agreement based on recent biochemical and genetic studies (reviewed in reference 79). Herpesvirus nucleocapsids assemble in the nucleus and acquire an envelope by budding into the perinuclear space. These particles lose their envelope by fusion with the outer nuclear envelope, releasing capsids into the cytoplasm. The cystosolic capsids then acquire a protein layer known as the tegument and are enveloped by cytoplasmic TGN or endosomal membranes before traveling to the cell surface. At the plasma membrane there is fusion, resulting in delivery of enveloped virions into the extracellular space, although most particles often remain bound to the cell surfaces. As with HIV, there is recent evidence for polarized egress of HSV and PRV; virus particles can be preferentially delivered to lateral surfaces of polarized epithelial cells, to cell junctions rather than to apical surfaces (46).
Cell-to-cell spread of alphaherpesviruses involves several viral membrane glycoproteins. HSV glycoproteins gB, gD, and gH/gL are essential for virus entry into most or all cultured cells, as well as for cell-to-cell spread (12, 29, 50). Apparently, these glycoproteins function in most or all cell types to promote fusion of the virion envelope with the cell as the virus enters the cell, regardless of whether this occurs at apical cell surfaces (entry) or at cell junctions (cell-to-cell spread). HSV and PRV gD acts to bind cell surface receptors required for entry (13, 45, 69), and several classes of these receptors have been described (reviewed in reference 82). gB and gH/gL are involved in subsequent interactions with the plasma membrane of the cell that lead to fusion with the virion envelope (reviewed in reference 93), but this process is poorly understood. However, there is another HSV and PRV glycoprotein, gE/gI, that is involved in cell-to-cell spread but does not obviously function in entry of extracellular virions (reviewed in references 25 and 46). HSV and PRV gE/gI is a heterodimer of two glycoproteins, gE and gI, that functions primarily or exclusively in polarized cells or in cells that form extensive junctions with one another (i.e., keratinocytes, epithelial cells, and neurons), not in highly transformed cells in culture (21, 22, 25, 86, 98). In experimental animal models, HSV and PRV mutants lacking either gE or gI are severely restricted for spread in epithelial tissues and in the nervous system (14, 20, 21, 68, 86, 87). Given that gE/gI specifically functions to promote direct cell-to-cell spread in polarized cells, but not entry of extracellular virions, this glycoprotein provides an excellent molecular tool to study cell-to-cell spread.
Numerous studies have demonstrated that gE/gI is targeted to the TGN or endosomes, sites where virus envelopment occurs, and in some cases this involves endocytosis (1, 2, 55, 60, 85, 86, 105). The accumulation of gE/gI in the TGN or endosomes depends on the CT domains of gE and gI, which have tyrosine-containing motifs (YXXØ, where Ø is a hydrophobic residue), clusters of acidic residues, and dileucine motifs (1, 2, 28, 48, 85, 106) which interact with μ and β subunits of clathrin adapters (reviewed in references 27, 48, and 56). Similar signals in cellular proteins affect intracellular trafficking in endosomes and the TGN, as well as endocytosis, and—importantly—can determine basolateral versus apical sorting (reviewed in references 7 and 28). VZV gE/gI and HSV gD are also modified with mannose-6-phosphate, which can also contribute to intracellular sorting in the TGN, and this has been shown to affect HSV cell-to-cell spread (11, 36). The accumulation of gE/gI in the TGN and endosomes apparently contributes to virus assembly there. A PRV mutant lacking gE, or the gE CT domain, as well as a second glycoprotein, gM, accumulated large numbers of cytosolic nucleocapsids, consistent with a role for gE/gI and gM in reenvelopment (9). Moreover, VZV and HSV mutants lacking gI, or the gI CT tail, accumulated abundant cytosolic nucleocapsids (96; D. C. Johnson and T. Wisner, unpublished results). When HSV gE/gI was expressed by transfection or virus vectors, the glycoprotein accumulated extensively in the TGN or endosomes. The same was true during early stages of HSV infection; however, at intermediate to late times of infection, gE/gI moved to cell junctions (55). Redistribution of gE to cell junctions required gI, and there was also redistribution of a cellular TGN protein, TGN46 (55). Together, these data have led to the hypothesis that gE/gI promotes the initial accumulation of virus structural components in the TGN or endosomes, likely in concert with other virion components, such as gM (37, 55, 95). Envelopment of viruses into specific TGN or endosomal compartments is followed by movement to the cell surface.
Movement of enveloped HSV and PRV particles from TGN and endosomes, the sites of secondary envelopment, to the cell surface appears to be specifically directed to lateral or basolateral surfaces. In monolayers of HSV- or PRV-infected epithelial cells, large numbers of virus particles were observed at cell junctions but not at apical surfaces (46). This was related to expression of gE/gI in that HSV mutants lacking gE, or the CT domain of gE, mislocalized nascent virions, accumulating large numbers of virus particles on the apical surface and in the cytoplasm. A component of the AP-1 clathrin adapter sorting machinery, μ1B, was determined to be involved in this directed egress to lateral surfaces (46). Therefore, it appears that gE/gI mutants are defective for cell-to-cell spread in epithelial cells in part because virions are not sorted properly to cell junctions.
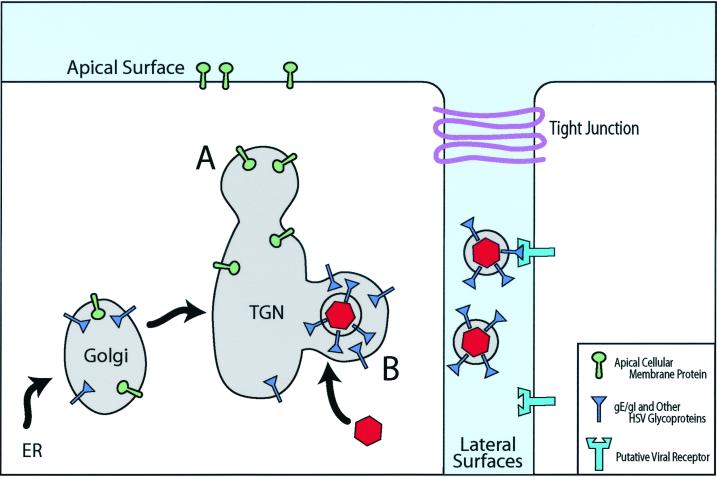
Based on these observations and others (9, 37, 39), we have proposed a model for how gE/gI promotes cell-to-cell spread (55) (Fig. 2). Alphaherpesvirus gE/gI accumulates in the TGN or endosomes; in conjunction with other viral proteins (e.g., gM), this promotes accumulation of other viral glycoproteins and tegument components there. In polarized cells, this accumulation, and ultimately the acquisition of the virion envelope, occurs at specific TGN or endosomal membranes, those that have been or will be sorted to the basolateral or lateral surfaces of epithelial cells, rather than to apical surfaces (Fig. 2). As virus replication proceeds, there is directed delivery of nascent particles, present within TGN-derived transport vesicles, specifically to epithelial cell junctions; virus particles are delivered into the space between adjacent cells and can initiate infection of a neighboring cell. We have also speculated that the large extracellular domain(s) of gE/gI may also play a role in cell-to-cell spread, perhaps by binding to proteins that are components of cell junctions, e.g., CAMs (22, 55). This may explain the preferential localization of gE/gI to cell junctions (55, 98), and there is evidence that gE, gI, and gD may have been derived from a common ancestor (52). By this scenario, gE/gI could act as a receptor-binding protein, much as gD, acting specifically at cell junctions to promote virus entry into recipient cells (Fig. 2).
FIG. 2.
Directed egress of alphaherpesviruses by sorting in the TGN. Alphaherpesvirus glycoproteins accumulate in the TGN, and this process involves the cytoplasmic domains of gE/gI (blue) as well as other viral membrane proteins, such as gM. There is assembly of tegument components around these sites of glycoprotein accumulation and binding of nucleocapsids (red). In epithelial cells, assembly of viral particles occurs selectively in sorting compartments of the TGN that will ultimately be delivered to cell basolateral or lateral surfaces (B), e.g., through gE/gI coupling to μ1B-substituted AP1 clathrin adapters (46). Other viral or cellular membrane proteins are sorted in different domains of the TGN to apical surfaces (A). Virions reach the lateral surfaces of cells in transport vesicles, which fuse with the plasma membrane and deliver virus particles into the space between cells. There are specific interactions of HSV glycoproteins with components of cell junctions—gD with nectins and apparently gE/gI with other molecules—which facilitate entry into adjacent cells. When both cells are infected, there can be accumulation of HSV particles between cells as receptors are blocked. ER, endoplasmic reticulum.
Evidence for directed egress of HSV has also been obtained with an ocular model of virus infection, although the architecture of the epithelial cells appears to be different from that of the cultured cells described above. When HSV particles move from underlying neurons into the deeper layers of the corneal epithelium, virions are transported predominantly onto the apical surfaces of the epithelial cells, but subsequently virions are delivered laterally between epithelial cells that form the surface of the cornea (59). In cultured neurons, HSV and PRV capsids and envelope glycoproteins are transported separately by microtubules to axon termini, where envelopment occurs (63, 80). PRV gE/gI and a second membrane protein, US9, which also contains tyrosine, dileucine, and acidic motifs (reviewed in reference 10), are involved in sorting decisions made in neurons (reviewed in reference 89). Elegant studies in the rodent nervous system have shown that gE/gI and US9 are involved in determining movement of nascent virions within the complex neuronal circuitry (10, 14, 25, 44, 85–88). There are many similarities between sorting decisions made in neurons and those made in epithelial cells, but there are also significant differences (14, 21, 68, 85–88). In summary, alphaherpesvirus gE/gI and US9 proteins apparently function to escort structural components to sites of virus envelopment, in neurons at axon termini, and in epithelial cells at TGN compartments that are ultimately delivered to cell junctions.
POXVIRUSES.
Poxviruses are a family of large DNA viruses, including variola (smallpox), cowpox, monkeypox, ectromelia, and vaccinia viruses, that cause epithelial lesions and, in some cases, disseminated systemic diseases in animals or humans (27). Rapid cell-to-cell spread of poxviruses in the epidermis and endothelium is an important characteristic of these viruses that replicate and spread extremely rapidly, outstripping host immune responses.
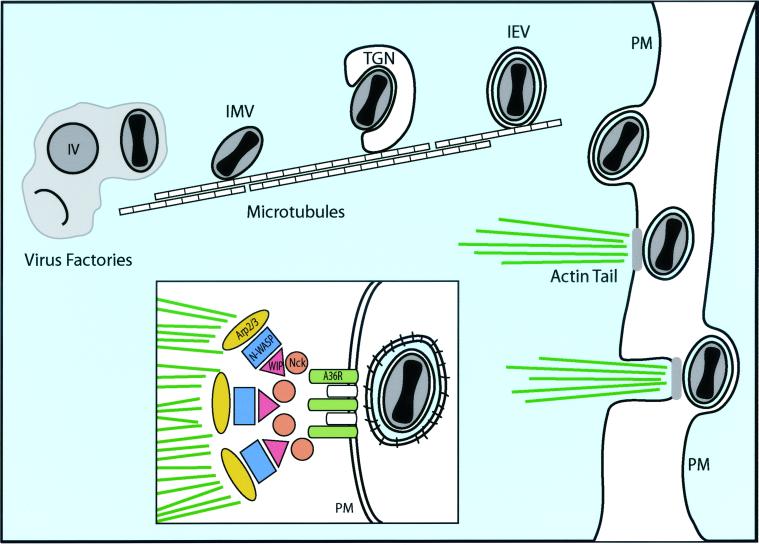
Most of what is known about poxvirus egress and cell-to-cell spread comes from studies of vaccinia virus (VV). Assembly of VV is complex. VV proteins and DNA accumulate in cytoplasmic virus “factories” in which there appear to be crescent-shaped membrane structures studded with spicules that apparently assemble away from other, cellular membranes (57). These structures extend into ovals, acquire nucleocapsids in becoming immature virions, and then mature into electron-dense intracellular mature virus (IMV) (Fig. 3). It was reported that crescents were derived as a tightly apposed pair of membranes from the intermediate endoplasmic reticulum or Golgi compartment (81). However, it has also been reported that IMV contain only a single membrane, possibly constructed in virus factories without acquisition of cellular membranes (17a, 42). The majority of IMV remain in the cytoplasm and can be infectious upon release by cell lysis; however, a fraction of them are transported via microtubules (76) to the TGN (40, 78) or tubular endosomes (90) and are wrapped by these membranes. This produces intracellular enveloped viruses (IEV), which contain two more envelopes than IMV and are modified with various virus-encoded membrane proteins (Fig. 3). IEV move to the cell surface, where the outer envelope fuses with the plasma membrane, releasing cell surface enveloped viruses (CEV) and extracellular enveloped viruses (EEV), both with one more envelope than IMV. CEV are frequently coupled to cytoplasmic actin tails that produce microvilli extending from the cell surface and projecting CEV toward apposing cells. CEV are crucial for VV cell-to-cell spread, which is measured by the size of plaques in cultured cells. EEV are important for long-range virus spread, which is evidenced by formation of comets extending from plaques.
FIG. 3.
Egress of VV particles from cells on actin tails. Poxvirus nucleocapsids are formed and acquire envelopes in virus factories, producing IMV, which subsequently acquire two additional membranes by budding into the TGN, forming IEV. Microtubules are involved in the transport of IMV and IEV to the plasma membrane (PM). There is fusion of the outer envelope with the plasma membrane, and CEV (bound to the cell surface) rest on platforms, composed in part of A33R, A34R, and A36R, that sit at one pole of the virus particle. A36R promotes the nucleation of actin filaments through interactions with Nck and recruitment of WIP, N-WASP, and Arp2/3 (inset), leading to actin polymerization. Actin tails produce microvilli that project CEV toward adjacent cells, promoting virus entry.
Studies of VV mutants lacking components of the IEV or EEV envelope have provided molecular details of the final stages of VV egress. There are at least seven VV proteins found in the IEV and EEV but not in the IMV, and these proteins are all targeted, directly or indirectly, to the TGN. These proteins loosely fall into two classes: (i) F13L and B5R are required for wrapping of the IEV envelope around the IMV (4, 24, 99), while (ii) F12L, A33R, A34R, A36R, and A56R are not required for this envelopment. F12L, A33R, A34R, and A36R are required for the assembly of actin tails; IEV, CEV, and EEV are formed in the absence of these viral proteins, but CEV do not extend toward other cells on microvilli, and plaques are small (53, 72, 75, 100, 101, 104). Therefore, CEV and actin tails are crucial for VV cell-to-cell spread.
It has been proposed that A33R, A34, and A36R form a platform for assembly of actin tails (101). Actin tail formation occurs by VV-induced local activation of cellular signaling pathways, which normally induces cell surface actin polymerization. Specifically, A36R becomes phosphorylated at tyrosine 112 in the cytoplasmic domain by Src family kinases, resulting in direct interactions with adapter protein Nck and recruitment of N-WASP, which in turn activates and recruits Arp2/3, a primary factor in nucleation of actin assembly (34) (Fig. 3, inset). The roles of A33R and A34R in actin tail formation are somewhat less clear. Both A33R and A34R interact with A36R and may be responsible for accumulation of A36R in the IEV envelope (73, 102), and A33R and A34R mutants may play additional roles in morphogenesis, wrapping of the IEV envelope, and release of CEV (5, 53, 72, 100).
Earlier microscopic analyses of VV particles suggested that actin tails form on cytoplasmic IEV and transport the particles to the plasma membrane and into microvilli that project into neighboring cells (17, 83), as described previously for Listeria and Shigella (reviewed in reference 33). However, more recent studies have produced refinements to this model, supporting the view that microtubules, instead of actin filaments, propel VV to the cell surface. First, A36R is targeted to the outer envelope of the IEV, but it remains in the plasma membrane at one pole of the CEV, orchestrating actin tails (73, 94, 102). Second, confocal z-axis analyses and electron microscopy have provided evidence that actin tails form exclusively at the cell surface and not in the cytoplasm (43, 97; E. J. Wolffe and B. Moss, unpublished data). Third, inhibitors of microtubules, but not inhibitors of actin polymerization, reduced movement of VV to the cell surface (43, 97). Therefore, it appears that VV moves via microtubules from virus factories to the TGN, where envelopment occurs, and then microtubules ferry IEV to the cell surface (Fig. 3). Actin polymerization apparently occurs only after fusion of the IEV outer envelope with the plasma membrane and is nucleated around A36R, which remains in the plasma membrane (73, 94) (Fig. 3, inset). This explains the polar nature of actin tails, the absence of A36R in EEV, and the use of plasma membrane tyrosine kinase signaling to initiate actin polymerization. Actin tails project CEV at the tips of microvilli into neighboring cells, and this process is apparently required for efficient cell-to-cell spread. This was confirmed in a recent microscopic analysis showing VV atop actin tails projecting across cell junctions (43). However, it is not yet clear whether there is preferential transport of VV particles to sites of epithelial cell contact or specific production of actin tails there. It certainly makes sense that poxviruses would direct progeny specifically to other cells, as do the alphaherpesviruses, but this remains to be tested.
Acknowledgments
We are most grateful to Markus Thali, Geoff Smith, and Bernard Moss for suggestions and critical review of the HIV and VV sections. We thank our colleagues Todd Wisner and Tommy (B. B.) McMillan for providing valuable insights.
Our research was supported by a grants CA73996 from the National Cancer Institute and EY11245 from the National Eye Institute to D.C.J. and by grant EY07029 from the National Eye Institute to M.T.H.
REFERENCES
- 1.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvin, A. M. 1996. Varicella-zoster virus, p. 2547–2585. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 4.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J. Virol. 65:5910–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasco, R., J. R. Sisler, and B. Moss. 1993. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein: effect of a point mutation in the lectin homology domain of the A34R gene. J. Virol. 67:3319–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom, J., C. Nielsen, and J. M. Rhodes. 1993. An ultrastructural study of HIV-infected human dendritic cells and monocytes/macrophages. APMIS 101:672–680. [DOI] [PubMed] [Google Scholar]
- 7.Bonifacino, J. S., and E. C. Dell’Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145:923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourinbaiar, A. S., and D. M. Phillips. 1991. Transmission of human immunodeficiency virus from monocytes to epithelia. J. Acquir. Immune Defic. Syndr. 4:56–63. [PubMed] [Google Scholar]
- 9.Brack, A. R., B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter. 2000. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J. Virol. 74:4004–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brideau, A. D., M. G. Eldridge, and L. W. Enquist. 2000. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J. Virol. 74:4549–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunetti, C. R., R. L. Burke, B. Hoflack, T. Ludwig, K. S. Dingwell, and D. C. Johnson. 1995. Role of mannose-6-phosphate receptors in herpes simplex virus entry into cells and cell-to-cell transmission. J. Virol. 69:3517–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai, W., S. Person, S. C. Warner, J. Zhou, and N. A. DeLuca. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campadelli-Fiume, G., M. Arsenakis, F. Farabegoli, and B. Roizman. 1988. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 62:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr, J. M., H. Hocking, P. Li, and C. J. Burrell. 1999. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265:319–329. [DOI] [PubMed] [Google Scholar]
- 16.Corey, L., and P. G. Spear. 1986. Infections with herpes simplex viruses. N. Engl. J. Med. 314:686–691. [DOI] [PubMed] [Google Scholar]
- 17.Cudmore, S., P. Cossart, G. Griffiths, and M. Way. 1995. Actin-based motility of vaccinia virus. Nature 378:636–638. [DOI] [PubMed] [Google Scholar]
- 17a.Dales, S., and B. G. T. Pogo. 1981. Biology of poxviruses, vol. 18. Springer-Verlag, Berlin, Germany.
- 18.Deschambeault, J., J. P. Lalonde, G. Cervantes-Acosta, R. Lodge, E. A. Cohen, and G. Lemay. 1999. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J. Virol. 73:5010–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 67:2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dingwell, K. S., C. R. Brunetti, R. L. Hendricks, Q. Tang, M. Tang, A. J. Rainbow, and D. C. Johnson. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087–7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dingwell, K. S., and D. C. Johnson. 1998. Herpes simplex virus gE/gI facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 72:8933–8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dustin, M. L., and J. A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23–29. [DOI] [PubMed] [Google Scholar]
- 24.Engelstad, M., and G. L. Smith. 1993. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology 194:627–637. [DOI] [PubMed] [Google Scholar]
- 25.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of α-herpesviruses in the nervous system. Adv. Virus Res. 51:237–347. [DOI] [PubMed] [Google Scholar]
- 26.Fais, S., M. R. Capobianchi, I. Abbate, C. Castilletti, M. Gentile, P. Cordiali Fei, F. Ameglio, and F. Dianzani. 1995. Unidirectional budding of HIV-1 at the site of cell-to-cell contact is associated with co-polarization of intercellular adhesion molecules and HIV-1 viral matrix protein. AIDS 9:329–335. [PubMed] [Google Scholar]
- 27.Fenner, F. 1996. Poxviruses, p. 2673–2702. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 2nd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 28.Folsch, H., H. Ohno, J. S. Bonifacino, and I. Mellman. 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99:189–198. [DOI] [PubMed] [Google Scholar]
- 29.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortin, J. F., R. Cantin, and M. J. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM. J. Virol. 72:2105–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannan, L. D. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272:115–117. [DOI] [PubMed] [Google Scholar]
- 32.Freed, E. O. 1998. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251:1–15. [DOI] [PubMed] [Google Scholar]
- 33.Frischknecht, F., S. Cudmore, V. Moreau, I. Reckmann, S. Rottger, and M. Way. 1999. Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella. Curr. Biol. 9:89–92. [DOI] [PubMed] [Google Scholar]
- 34.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401:926–929. [DOI] [PubMed] [Google Scholar]
- 35.Fultz, P. N., P. J. Vance, M. J. Endres, B. Tao, J. D. Dvorin, I. C. Davis, J. D. Lifson, D. C. Montefiori, M. Marsh, M. H. Malim, and J. A. Hoxie. 2001. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol. 75:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabel, C. A., L. Dubey, S. P. Steinberg, D. Sherman, M. D. Gershon, and A. A. Gershon. 1989. Varicella-zoster virus glycoprotein oligosaccharides are phosphorylated during posttranslational maturation. J. Virol. 63:4264–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta, P., R. Balachandran, M. Ho, A. Enrico, and C. Rinaldo. 1989. Cell-to-cell transmission of human immunodeficiency virus type 1 in the presence of azidothymidine and neutralizing antibody. J. Virol. 63:2361–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiller, G., and K. Weber. 1985. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J. Virol. 55:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hioe, C. E., P. C. Chien, C. Lu, T. A. Springer, X. H. Wang, J. Bandres, and M. Tuen. 2001. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J. Virol. 75:1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollinshead, M., A. Vanderplasschen, G. L. Smith, and D. J. Vaux. 1999. Vaccinia virus intracellular mature virions contain only one lipid membrane. J. Virol. 73:1503–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollinshead, M., G. Rodger, H. Van Eijl, M. Law, R. Hollinshead, D. J. T. Vaux, and G. L. Smith. 2001. Vaccinia virus utilizes microtubules for movement to the cell surface. J. Cell Biol. 154:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husak, P. J., T. Kuo, and L. W. Enquist. 2000. Pseudorabies virus membrane proteins gI and gE facilitate anterograde spread of infection in projection-specific neurons in the rat. J. Virol. 74:10975–10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson, D. C., and M. W. Ligas. 1988. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J. Virol. 62:4605–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koenig, S., H. E. Gendelman, J. M. Orenstein, M. C. Dal Canto, G. H. Pezeshkpour, M. Yungbluth, F. Janotta, A. Aksamit, M. A. Martin, and A. S. Fauci. 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233:1089–1093. [DOI] [PubMed] [Google Scholar]
- 48.Le Borgne, R., and B. Hoflack. 1998. Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway. Curr. Opin. Cell Biol. 10:499–503. [DOI] [PubMed] [Google Scholar]
- 49.Li, P., and C. J. Burrell. 1992. Synthesis of human immunodeficiency virus DNA in a cell-to-cell transmission model. AIDS Res. Hum. Retrovir. 8:253–259. [DOI] [PubMed] [Google Scholar]
- 50.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lodge, R., J. P. Lalonde, G. Lemay, and E. A. Cohen. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGeoch, D. J., A. Dolan, S. Donald, and F. J. Rixon. 1985. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 181:1–13. [DOI] [PubMed] [Google Scholar]
- 53.McIntosh, A. A., and G. L. Smith. 1996. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J. Virol. 70:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980–987. [DOI] [PubMed] [Google Scholar]
- 55.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molloy, S. S., E. D. Anderson, F. Jean, and G. Thomas. 1999. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 9:28–35. [DOI] [PubMed] [Google Scholar]
- 57.Moss, B. (ed.). 1996. Poxviridae: the viruses and their replication, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 58.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Hara, P. T., M. S. Chin, and J. H. LaVail. 2000. The spread of herpes simplex virus type 1 from trigeminal neurons to the murine cornea: an immunoelectron microscopy study. J. Virol. 74:4776–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owens, R. J., J. W. Dubay, E. Hunter, and R. W. Compans. 1991. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 88:3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearce-Pratt, R., D. Malamud, and D. M. Phillips. 1994. Role of the cytoskeleton in cell-to-cell transmission of human immunodeficiency virus. J. Virol. 68:2898–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perotti, M. E., X. Tan, and D. M. Phillips. 1996. Directional budding of human immunodeficiency virus from monocytes. J. Virol. 70:5916–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phillips, D. M. 1994. The role of cell-to-cell transmission in HIV infection. AIDS 8:719–731. [DOI] [PubMed] [Google Scholar]
- 66.Phillips, D. M., and A. S. Bourinbaiar. 1992. Mechanism of HIV spread from lymphocytes to epithelia. Virology 186:261–273. [DOI] [PubMed] [Google Scholar]
- 67.Phillips, D. M., X. Tan, M. E. Perotti, and. V. R. Zacharopoulos. 1998. Mechanism of monocyte-macrophage-mediated transmission of HIV. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S67–S70. [PubMed] [Google Scholar]
- 68.Rajcani, J., U. Herget, and H. C. Kaerner. 1990. Spread of herpes simplex virus (HSV) strains SC16, ANG, ANGpath and its glyC minus and GlyE minus mutants in DBA-2 mice. Acta Virol. 34:305–320. [PubMed] [Google Scholar]
- 69.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J. Virol. 65:5348–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71:4847–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roizman, B., and A. E. Sears. 1996. Herpes simplex viruses and their replication, p. 2231–2296. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 72.Roper, R. L., E. J. Wolffe, A. Weisberg, and B. Moss. 1998. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J. Virol. 72:4192–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rottger, S., F. Frischknecht, I. Reckmann, G. L. Smith, and M. Way. 1999. Interactions between vaccinia virus IEV membrane proteins and their roles in IEV assembly and actin tail formation. J. Virol. 73:2863–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez-Madrid, F., and M. A. del Pozo. 1999. Leukocyte polarization in cell migration and immune interactions. EMBO J. 18:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanderson, C. M., F. Frischknecht, M. Way, M. Hollinshead, and G. L. Smith. 1998. Roles of vaccinia virus EEV-specific proteins in intracellular actin tail formation and low pH-induced cell-cell fusion. J. Gen. Virol. 79:1415–1425. [DOI] [PubMed] [Google Scholar]
- 76.Sanderson, C. M., M. Hollinshead, and G. L. Smith. 2000. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particles. J. Gen. Virol. 81:47–58. [DOI] [PubMed] [Google Scholar]
- 77.Sato, H., J. M. Orenstein, D. Dimitrov, and D. Martin. 1992. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology 186:712–724. [DOI] [PubMed] [Google Scholar]
- 78.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J. Virol. 68:130–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 75:5697–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 98:3466–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sodeik, B., R. W. Doms, M. Ericsson, G. Hiller, C. E. Machamer, W. van’t Hof, G. van Meer, B. Moss, and G. Griffiths. 1993. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J. Cell Biol. 121:521–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for α-herpesvirus entry. Virology 275:1–8. [DOI] [PubMed] [Google Scholar]
- 83.Stokes, G. V. 1976. High-voltage electron microscope study of the release of vaccinia virus from whole cells. J. Virol. 18:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan, X., R. Pearce-Pratt, and D. M. Phillips. 1993. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J. Virol. 67:6447–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tirabassi, R. S., and L. W. Enquist 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tirabassi, R. S., and L. W. Enquist. 1998. Role of the envelope protein gE in the pseudorabies virus life cycle. J. Virol. 72:4571–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tirabassi, R. S., and L. W. Enquist. 2000. Role of the pseudorabies virus gI cytoplasmic tail in neuroinvasion, virulence, and posttranslational N-linked glycosylation. J. Virol. 74:3505–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tirabassi, R. S., R. A. Townley, M. G. Eldridge, and L. W. Enquist. 1997. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J. Virol. 71:6455–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of α-herpesviruses in axons. Traffic 2:429–436. [DOI] [PubMed] [Google Scholar]
- 90.Tooze, J., M. Hollinshead, B. Reis, K. Radsak, and H. Kern. 1993. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 60:163–178. [PubMed] [Google Scholar]
- 91.Tsai, W. P., S. R. Conley, H. F. Kung, R. R. Garrity, and P. L. Nara. 1996. Preliminary in vitro growth cycle and transmission studies of HIV-1 in an autologous primary cell assay of blood-derived macrophages and peripheral blood mononuclear cells. Virology 226:205–216. [DOI] [PubMed] [Google Scholar]
- 92.Tsunetsugu-Yokota, Y., S. Yasuda, A. Sugimoto, T. Yagi, M. Azuma, H. Yagita, K. Akagawa, and T. Takemori. 1997. Efficient virus transmission from dendritic cells to CD4+ T cells in response to antigen depends on close contact through adhesion molecules. Virology 239:259–268. [DOI] [PubMed] [Google Scholar]
- 93.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Eijl, H., M. Hollinshead, and G. L. Smith. 2000. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology 271:26–36. [DOI] [PubMed] [Google Scholar]
- 95.Wang, Z., M. D. Gershon, O. Lungu, C. A. Panagiotidis, Z. Zhu, Y. Hao, and A. A. Gershon. 1998. Intracellular transport of varicella-zoster glycoproteins. J. Infect. Dis. 178(Suppl. 1):S7–S12. [DOI] [PubMed] [Google Scholar]
- 96.Wang, Z.-H., M. D. Gershon, O. Lungu, Z. Zhu, S. Mallory, A. M. Arvin, and A. A. Gershon. 2001. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J. Virol. 75:323–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ward, B. M., and B. Moss. 2001. Visualization of intracellular movement of vaccinia virus virions containing a green fluorescent protein-B5R membrane protein chimera. J. Virol. 75:4802–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 74:2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolffe, E. J., S. N. Isaacs, and B. Moss. 1993. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J. Virol. 67:4732–4741. (Erratum, 67:5709–5711.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolffe, E. J., E. Katz, A. Weisberg, and B. Moss. 1997. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J. Virol. 71:3904–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolffe, E. J., A. S. Weisberg, and B. Moss. 1998. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology 244:20–26. [DOI] [PubMed] [Google Scholar]
- 102.Wolffe, E. J., A. S. Weisberg, and B. Moss. 2001. The vaccinia virus A33R protein provides a chaperone function for viral membrane localization and tyrosine phosphorylation of the A36R protein. J. Virol. 75:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wyss, S., C. Berlioz-Torrent, M. Boge, G. Blot, S. Honing, R. Benarous, and M. Thali. 2001. The highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adapter. J. Virol. 75:2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang, W. H., D. Wilcock, and G. L. Smith. 2000. Vaccinia virus F12L protein is required for actin tail formation, normal plaque size, and virulence. J. Virol. 74:11654–11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu, Z., M. D. Gershon, Y. Hao, R. T. Ambron, C. A. Gabel, and A. A. Gershon. 1995. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J. Virol. 69:7951–7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu, Z., Y. Hao, M. D. Gershon, R. T. Ambron, and A. A. Gershon. 1996. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J. Virol. 70:6563–6575. [DOI] [PMC free article] [PubMed] [Google Scholar]