Abstract
Colicin E9 is a microbial toxin that kills bacteria through random degradation of chromosomal DNA. Within the active site of the cytotoxic endonuclease domain of colicin E9 (the E9 DNase) is a 32 amino acid motif found in the H-N-H group of homing endonucleases. Crystal structures of the E9 DNase have implicated several conserved residues of the H-N-H motif in the mechanism of DNA hydrolysis. We have used mutagenesis to test the involvement of these key residues in colicin toxicity, metal ion binding and catalysis. Our data show, for the first time, that the H-N-H motif is the site of DNA binding and that Mg2+-dependent cleavage of double-stranded DNA is responsible for bacterial cell death. We demonstrate that more active site residues are required for catalysis in the presence of Mg2+ ions than transition metals, consistent with the recent hypothesis that the E9 DNase hydrolyses DNA by two distinct, cation-dependent catalytic mechanisms. The roles of individual amino acids within the H-N-H motif are discussed in the context of the available structural information on this and related DNases and we address the possible mechanistic similarities between caspase-activated DNases, responsible for the degradation of chromatin in eukaryotic apoptosis, and H-N-H DNases.
INTRODUCTION
Homing endonucleases promote the homing of genetic elements coding for them into intronless/inteinless allelic sites and are classified into four families based on active site sequences: LAGLIDAG, GIY-YIG, His-Cys box and H-N-H (1). Structural and mechanistic studies have revealed striking similarities between homing endonucleases and nucleases found in widely different biological settings. The non-specific DNA/RNA nuclease from Serratia marcescens, for example, is a homologue of the His-Cys box enzyme I-PpoI from Physarum polycephalum (2,3). Although both enzymes are dimers, there is no structural similarity between them except at their active sites, at which they cleave DNA by the same basic mechanism (4). Another example of active site similarity is that between the H-N-H family of homing endonucleases (DNases) and colicin DNases (5). The mechanism by which H-N-H enzymes cleave DNA is poorly understood and so we addressed this through a mutagenic scan of the endonuclease domain of colicin E9.
Colicins are SOS-induced protein toxins produced by strains of Escherichia coli and closely related bacteria that provide a selective advantage in the intense competition between bacteria in the environment. They are classified into groups corresponding to the cell surface receptor to which they bind on sensitive E.coli cells; colicins that bind the BtuB protein, the high affinity receptor for vitamin B12, are known as E colicins (6). E colicins are 60 kDa proteins that have three functional domains, each implicated in one of the three stages of cell killing (7). A small, centrally located domain is responsible for receptor binding (8), whilst an N-terminal domain mediates translocation of the cytotoxic C-terminal domain into the cell (9,10). E colicins exhibit one of three cytotoxic activities: (i) a pore-forming ion channel that depolarises the inner membrane (colicin E1) (10); (ii) an H-N-H endonuclease activity that degrades chromosomal DNA (colicins E2, E7, E8 and E9) (11); (iii) a RNase activity that specifically cleaves 16S rRNA (colicins E3, E4 and E6) (12,13) or specific tRNAs (colicin E5) (14).
E colicin-producing bacteria are protected against suicide by the co-synthesis of an immunity protein which binds to the C-terminal domain and neutralises its activity (15,16). In the case of colicin E1, this process occurs in the cytoplasmic membrane, whilst in the case of the enzymatic E colicins (DNases and RNases) it occurs on synthesis in the cytoplasm, the resulting colicin–immunity protein complex being released from the producing cells. Work on the colicin E9-specific immunity protein Im9 shows that this family of nuclease inhibitors is very efficient at preventing bacterial cell death. Im9 is a 9.5 kDa, distorted four helical protein that folds in milliseconds and associates with its target DNase at the rate of diffusion to form an inactivated complex with an equilibrium dissociation constant (Kd) of 9.3 × 10–17 M at pH 7 and 25°C (17).
After binding to E.coli cell surface receptors, colicins are translocated to their site of toxicity by one of two translocation routes (6,18). Group A colicins, the group to which the E colicins belong, require the tol-dependent translocation system (consisting of the proteins TolA, TolB, TolQ, TolR and PAL), whilst Group B colicins, such as colicin B and Ia, use the ton-dependent translocation system. It is not known how the cytotoxic C-terminal domains of E colicins are translocated to the cytoplasm of E.coli cells, a process that requires the tightly bound Im protein to be jettisoned prior to entry.
Recent crystal structures of the 15 kDa DNase domains of colicins E9 and E7 in complex with their cognate immunity proteins, Im9 (16) and Im7 (19), have revealed that immunity proteins are exosite inhibitors (20). In both cases, the Im proteins bind adjacent to the active site cleft, inhibiting colicin activity indirectly by steric and electrostatic hindrance. Kleanthous and Walker (20) have speculated that exosite binding by Im proteins is a selective advantage to enzymatic colicin-producing bacteria since it permits extensive sequence variability to evolve at colicin–immunity protein interfaces. The two DNase structures also revealed that these enzymes are metalloproteins, containing a single transition metal ion within their active sites. Zinc was found in the E7 DNase structure (19), whilst nickel was observed in the E9 DNase (16), the latter due to the purification strategy used in the preparation of the complex.
The core of a colicin DNase active site is the 32 amino acid H-N-H motif (16), located at the C-terminus of the enzyme which envelops the bound metal ion and includes two or three histidine ligands (see Figs 1 and 2). His102 and His127 are both ligands in the Ni and Zn structures but His131 is only a clearly defined ligand in the Zn-bound E7 DNase crystal structure, though solution NMR studies show that His131 does bind to the Ni2+ ion in E9 (21). The final coordination site for the nickel ion in the E9 DNase structure was a phosphate molecule, replaced by a water molecule in the Zn-bound E7 DNase structure. Isothermal titration calorimetry has shown that the H-N-H motif can bind various first row transition metals, the order of affinity being Zn2+ > Ni2+ ≈ Co2+ (22). The role of transition metals in catalysis is not resolved, however, given that zinc does not support E9 DNase catalysis, and indeed inhibits the enzyme at high concentration, while both nickel and cobalt are catalytic metal ions (22–24). This is further complicated by the fact that the E9 DNase preferentially uses Mg2+ or Ca2+ ions for the degradation of double-stranded substrates, while transition metals such as Ni2+ are optimal for cleavage of single-stranded (ss)DNA. Spectroscopic, proteolytic and calorimetric data have demonstrated that the thermodynamic stability of the domain increases substantially on binding transition metal ions (the melting temperature of the enzyme at pH 7.5 increases by 22°C on binding zinc) and a model has been proposed in which removal of the transition metal destabilises the DNase domain thereby facilitating its translocation across bacterial membranes (22).
Figure 1.
H-N-H motif consensus sequence. Alignment of sequences containing the H-N-H motif from a PSI_Blast search using residues 96–131 of the E9 DNase as the search sequence. Spaces are included in the sequences to optimise the alignment. The consensus sequence of the majority of the sequences is shown at the bottom of the figure. The H-N-H motif residues are shown in bold. The numbers refer to the residues of the colicin E9 DNase.
Figure 2.
Structure of the H-N-H motif of the E9 DNase. The apoenzyme structure, showing the catalytic tetrad and bound phosphate molecule (A) in the absence of metal ion (31) and (B) in the presence of nickel (16). The position of H-N-H residues mutated in the present study are indicated. Figures were generated using MOLSCRIPT and Raster3D.
The basic enzymological characteristics of the H-N-H enzyme from colicin E9 have been described, including details of its metal ion requirements, pH optimum, substrate preferences and DNA cleavage specificity (22–24). Along with the available structural information on the H-N-H motif of colicin DNases, this has led to putative mechanistic roles being proposed for individual amino acids within the motif (23). The aim of the present work was to use mutagenesis to test the importance of these conserved H-N-H amino acids in catalysis and metal ion binding, provide definitive proof as to the metal-dependent activity responsible for bacterial cell death by E colicin DNases and, through the use of acidic mutations, demonstrate that the H-N-H motif is the site of DNA binding. These data are discussed in the context of sequence, structural and mechanistic similarities between colicin DNases and homing endonucleases. We also draw attention, for the first time, to the likely mechanistic similarities between H-N-H enzymes and caspase-activated DNase, CAD, an enzyme that functions in metazoan apoptosis (25,26).
MATERIALS AND METHODS
Site-directed mutagenesis of the E9 DNase domain
Site-directed mutants were constructed using the ‘megaprimer’ PCR method (27). A mutagenic primer was used in conjunction with a suitable forward or reverse primer to obtain a PCR product, which was then used as a ‘megaprimer’ in a second stage PCR. The final PCR product containing the desired mutation was isolated using a QIAEX-II gel extraction kit (Qiagen) and cloned into the required plasmid vector.
Protein purification and protein determinations of the E9 DNase domain
pET vectors (Novagen) encoding either the full-size mutant colicins or the mutant DNase domains along with a poly(His)-tagged Im9 (constructed from plasmids pCS4 and pRJ353, respectively) were transformed into E.coli ER2566 or BL21 DE3 cells (Novagen). The mutant DNase domains were purified as previously described (28). Full-size mutant complexes were purified in a similar fashion, however, the 6 M guanidine hydrochloride binding buffer used in the elution step was replaced with elution buffer (1 M imidazole, 0.5 M NaCl, 20 mM Tris–HCl, pH 7.9). To produce metal-free E9 DNase apoproteins, the purified DNase domains were treated with EDTA (10 mM final concentration) before extensive dialysis against 200 mM NaCl in AnalaR water and finally against AnalaR water alone and the lyophilised protein stored at –20°C. Protein concentrations were determined by absorbance at 280 nm (17).
Colicin titres
Colicin titres were determined by spotting 2 µl samples of protein complex (serially diluted in 20 mM Tris–HCl, pH 7.0, 0.1 mg/ml BSA) onto a soft agar lawn of a sensitive indicator strain (E.coli JM83 carrying pUC18). The colicin titre is the reciprocal of the highest dilution of a 1 µg/ml solution of colicin complex that produces a clear zone of inhibition in the indicator lawn.
Nuclease activity
Plasmid-nicking activity of the E9 DNase mutants was determined essentially as previously described (22,24). Duplicate Kunitz assays (29) were performed as described previously (23,24). Briefly, assays were carried out in volumes of 1 ml containing 50 mM Tris–HCl, pH 7.5, 1 mM NiCl2 and 50 µg calf thymus DNA at a temperature of 25°C. The reaction was initiated by the addition of 2.5 µg (167 nM) E9 DNase and DNA hydrolysis monitored by the change in hyperchromicity at 260 nm using a Philips PU 8730 spectrophotometer over a 10 min time course. DNA hydrolysis under these conditions yields linear kinetic traces which were converted into Kunitz units (ΔA260/min/µg) and the data presented relative to wild-type enzyme.
Fluorescence spectroscopy
Zinc binding to the E9 DNase was monitored using the change in fluorescence of the extrinsic fluorophore 8-anilinonaphthalene-1-sulfonic acid (ANS) as described previously (22). Measurements were performed in duplicate using an excitation wavelength of 365 nm and emission spectra recorded from 390 to 600 nm using a Spex Fluoromax 3 spectrofluorimeter. Quartz cuvettes were used containing 2 ml TEA–HCl buffer, pH 7.5, 15 µM ANS and 10 nmol (5 µM) E9 DNase. The degree of fluorescence quench was measured following the addition of increasing increments of ZnCl2 to a maximum of 2- to 3-fold molar excess of zinc over protein.
Trypsin digestion of E9 DNase
Proteolytic digestions of the E9 DNase mutants were carried out as described by Pommer et al. (22).
Gel shift assays
DNase mutants were assayed for their ability to bind to ssDNA. Reactions, containing 80 pmol 5′-fluorescein-labelled ssDNA (GACGTAAGAG) and 320 pmol E9 DNase in 10 mM Tris–HCl, pH 8, 50 mM KCl, 10 mM MgCl2, 0.1 mg/ml BSA and 20% (v/v) glycerol, were incubated for 50 min at room temperature, separated on a 15% acrylamide gel and analysed on a phosphoimager (Storm 840; Molecular Dynamics).
RESULTS
Rationale for mutagenesis of E9 DNase H-N-H motif residues
A sequence alignment delineating the H-N-H motif of colicin E9 and other endonucleases is shown in Figure 1, with the structure of the E9 DNase motif in the presence and absence of bound metal ion presented in Figure 2. Eight residues were chosen for substitution to alanine, Arg5, Arg96, Glu100, His102, His103, Asn118, His127 and His131, six of which are part of the consensus sequence itself, with a further two (Arg5 and Arg96) external to the consensus but shown by crystallography to be part of the structural motif (16). His103, Asn118 and His127 constitute the amino acids of the H-N-H motif itself and are highlighted in bold in Figure 1. In the absence of metal ions, His127 is part of a hydrogen bonded tetrad in the active site that includes Arg96, Glu100 and His102 (30); three residues from this tetrad (Arg96, Glu100 and His127) have previously been identified as important catalytic residues by random mutagenesis (28). Side chain adjustments occur when transition metals such as nickel or zinc bind to the H-N-H motif resulting in the tetrad reorganising into a triad where Arg96, Glu100 and His127 are now hydrogen bonded to each other and His102 is co-opted as a ligand for the metal ion along with His127 (Fig. 2B; 16,19). The H-N-H motif of colicin DNases is structurally homologous to the active sites of other endonucleases, including those of the homing endonuclease I-PpoI, a His-Cys box enzyme, and the non-specific nuclease from Serratia (30). However, only two amino acids appear to be conserved between these enzymes, identified by structural superposition as His103 and Arg5, the latter residue coordinating the bound phosphate molecule in the E9 DNase (23,30). His131 was chosen for substitution since it is conserved amongst H-N-H enzymes, acting as one of the zinc coordination sites in the crystal structure of the E7 DNase (19).
Alanine mutants were first analysed in terms of colicin toxicity and then the individual domains were expressed and purified and the effects on metal-dependent DNase activity and metal binding assessed. Although alanine mutants of Arg96, Glu100 or His127 have previously been found to abolish biological and catalytic activity (28), only limited in vitro characterisation of these mutants was reported since it was not known at the time that the activity of the enzyme differs depending on the cation. We now report more detailed analyses of these three mutants along with the construction and characterisation of a further five mutants of putative catalytic residues within the H-N-H motif.
Biological activity of alanine mutants
The biological activity of the DNase domain alanine mutants was determined using an agar plate assay (see Materials and Methods) in which their activity against a sensitive strain of E.coli was measured relative to wild-type toxin (Table 1). Both H102A and H103A were completely devoid of biological activity, as are R96A, E100A and H127A, as previously reported (28), while R5A and H131A had greatly reduced activity (<2%) and N118A had 10% of wild-type activity.
Table 1. Summary of biological activity data, metal binding and endonuclease activities of E9 DNase domain H-N-H mutants.
| Mutation |
Colicin activitya |
Kunitz activityb |
Plasmid nickingc |
Zn2+ bindingd |
|
|---|---|---|---|---|---|
| Mg2+ | Ni2+ | ||||
| None | 100 | 100 | + | + | ++ |
| R5A | 2 | 26 | – | + | ++ |
| R96A | 0 | 14 | – | + | ++ |
| E100A | 0 | 1 | – | – | + |
| H102A | 0 | 6 | – | – | – |
| H103A | 0 | 0 | – | – | ++ |
| N118A | 10 | 86 | + | + | ++ |
| H127A | 0 | 0 | – | – | – |
| H131A | <1 | 27 | – | + | + |
aBiological activity is shown as a percentage of wild-type activity (titre) recorded from a serial dilution of toxin (1 µg/ml stock) that produces a clear zone of inhibition on an indicator culture.
bDNase activity as determined by the Kunitz assay expressed as a percentage relative to wild-type E9 DNase assayed in the presence of Ni2+.
cPlasmid nicking activity data from Figure 4 identifying whether activity is observed (+) or absent (–) with either Mg2+ or Ni2+.
dZinc binding as determined by the ANS fluorescence quench assay and protease protection (Fig. 3). ++, stoichiometric binding of zinc as for wild-type; +, binding affected by ∼3 orders of magnitude; –, no binding detected by ANS fluorescence or zinc-mediated protease protection.
Metal binding capability of H-N-H mutants
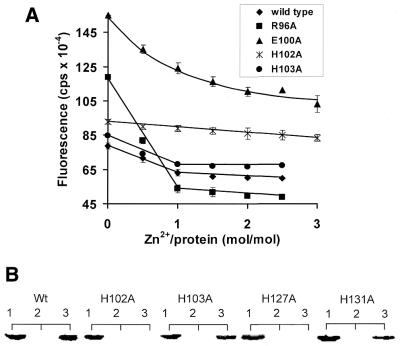
Concomitant with the binding of transition metals to the H-N-H motif of the E9 DNase is the sequestration of non-polar side chains from solvent that are exposed in the apoprotein, an effect that is readily monitored using the hydrophobic dye ANS (22). Binding of transition metals to the E9 DNase causes a significant quenching of the ANS fluorescence signal, providing a more sensitive measure of metal binding than the change in intrinsic tryptophan fluorescence that also accompanies metal ion binding to the DNase (22). Metal-induced ANS fluorescence quench data were determined for each mutant DNase domain at a protein concentration of 5 µM, titrating in a 2- to 3-fold excess of zinc, a concentration well above the estimated Kd for Zn2+, ∼3 nM (22). Data for selected mutants are presented in Figure 3A, with data for all mutants relative to wild-type summarised in Table 1. The alanine mutants fall into three distinct metal-binding classes. The first group comprises four sites (Arg5, Arg96, His103 and Asn118) where alanine mutations yield stoichiometric metal binding similar to that seen for the wild-type protein, although in some instances, such as for R96A, the ANS fluorescence of the apoprotein and the total fluorescence change differed from the wild-type (suggesting that the apoprotein of the mutant DNase may be more unstable than the wild-type protein). The second group includes mutants E100A and H131A, which were markedly affected in their ability to bind metal since excess zinc was required to quench the ANS fluorescence (E100A is shown in Fig. 3A), indicating that these mutations likely affect binding by ≥1000-fold (Fig. 3). This was confirmed by subsequent zinc titration experiments where we determined the approximate Kd for Zn binding to H131A as ∼10–20 µM (data not shown). The third group included H102A and H127A, where metal binding could not be detected by the ANS fluorescence quench assay. The ability of these latter mutants to bind metal ions was further evaluated by a protease protection assay under conditions where zinc binding protects the DNase against tryptic digestion (15). Even in the presence of millimolar zinc, no protease protection could be seen for H102A or H127A, whereas H131A was protected commensurate with its estimated Kd (Fig. 3B). These data indicate that alanine mutations at only two amino acids, His102 and His127, reduce zinc binding by ≥106-fold.
Figure 3.
Zinc binding by H-N-H motif mutants. (A) ANS fluorescence quench induced by Zn2+ binding to wild-type and a selection of alanine mutants. The protein concentration used in these experiments was 5 µM, which is 3 orders of magnitude greater than the wild-type Kd for zinc (22). Stoichiometric binding is seen for wild-type (filled diamonds), R96A (filled squares) and H103A (filled circles) E9 DNases under these conditions, whereas binding is highly compromised in the E100A mutant (filled triangles) and abolished in the H102A mutant (denoted by ×). Each fluorescence measurement was recorded in duplicate and the error bars from these are shown in the figure. Data for all H-N-H alanine mutants are presented in Table 1. (B) Comparing the trypsin susceptibility of the wild-type (Wt) and histidine-to-alanine mutant E9 DNases (5 µg) in the presence of zinc, analysed by SDS–PAGE (20%). Lane 1, apo-DNase control; lane 2, apo-DNase plus trypsin (1%); lane 3, apo-DNase incubated with 2 mM ZnCl2 plus trypsin. In each case incubation with trypsin was for 10 min and as described by Pommer et al. (22).
DNase activity of H-N-H motif mutants
The DNase activity displayed by colicin E9 is very dependent on the metal ion used in the assay. Mg2+ ions are optimal for cleavage of supercoiled plasmids while transition metals such as Ni2+ show poor activity against such substrates (24). Conversely, Ni2+ is the optimal metal ion for the cleavage of highly polymerised calf thymus DNA and ssDNA, whereas Mg2+ is a poor cofactor with these substrates (23,24). Pommer et al. (23) postulated that colicin DNase domains, and so by analogy all H-N-H enzymes, catalyse the hydrolysis of DNA by different mechanisms depending on the metal ion regime, with some residues adopting different catalytic roles depending on whether Mg2+ or Ni2+ is the metal cofactor, and this is addressed in the Discussion.
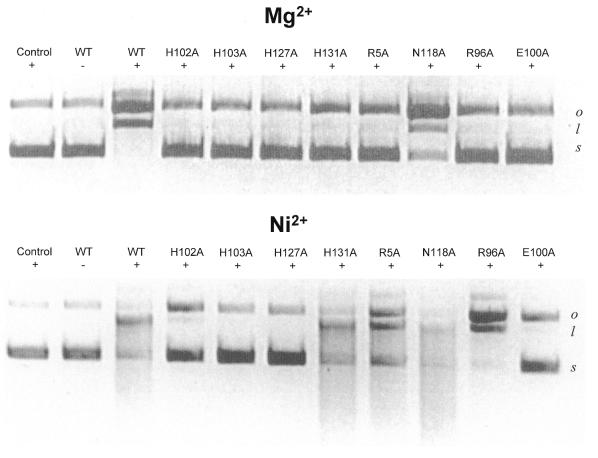
Each of the E9 DNase alanine mutants was assayed initially for catalytic activity in the presence of Mg2+ or Ni2+ using a plasmid nicking assay (Fig. 4). All but one of the eight alanine mutants (N118A) were inactive in the presence Mg2+, whereas only four mutants (E100A, H102A, H103A and H127A) were inactive in the presence of Ni2+. In order to verify the residual activity shown by some of the alanine mutants, the Ni2+-dependent activity of the mutants was also compared (at a higher protein concentration) using calf thymus DNA in the spectrophotometric Kunitz assay, an assay commonly used to study the enzymology of non-specific endonucleases (29). Kunitz data for R96A, E100A and H127A in the presence of Mg2+ ions have previously been reported (28), where they were inactive. These mutant proteins were assayed again in the present study, along with the other mutants, but in the presence of Ni2+ ions (Table 1). H103A and H127A were completely devoid of activity, while E100A and H102A had low activity (<6%). The remaining mutants possessed appreciable activity; N118A was almost as active as the wild-type DNase, while R96A and H131A had 14 and 27% of wild-type activity, respectively.
Figure 4.
DNase activity assays. Plasmid nicking assay of DNase activity. Agarose gel (1.2%) showing the nicking of 1 µg pUC18 DNA by 2 nM apo-E9 DNase (WT) and the indicated alanine mutants in the presence of 10 mM Mg2+ (upper) or 2 mM Ni2+ (lower). Metal ion concentrations have previously been optimised (24). Supercoiled plasmid pUC18 is present in all lanes and DNase omitted from the left hand control lane of each panel. s, supercoiled DNA; o, open circle DNA; l, linear DNA.
The H-N-H motif is the site of DNA binding
At the present time there is no direct structural evidence that DNA binds to the H-N-H motif of the E9 DNase. This is exacerbated by the fact that although Im9 abolishes DNA binding it does not contact any of the residues mutated in this study (16) and that alanine mutations that abolish DNase activity (e.g. H127A) do not abolish DNA binding as deduced by gel shift experiments (28). This latter observation could be explained by the very high positive electrostatic potential of the H-N-H motif itself (see Fig. 6A). Importantly, we have demonstrated previously that the E9 DNase readily binds ssDNA (Kd 0.5 µM), regardless of the DNA sequence, but that binding is dependent on the length of DNA, with oligonucleotides >10 bases being optimal (23). These observations are consistent with the physicochemical properties of the E9 DNase (pI 9.5) and imply that the enzyme recognises DNA primarily through interactions with the phosphate backbone.
Figure 6.
Molecular surface representations of the E9 DNase. (A) Electrostatic potentials for apo-E9 DNase, with the bound phosphate removed for the calculation, where blue and red represent positive and negative potential, respectively (31). (B) Residues that abolish Mg2+-dependent activity (coloured purple) including the bound phosphate molecule (yellow) in the apo-E9 DNase structure (31). (C) Residues that abolish Ni2+-dependent activity (coloured purple) mapped onto the Ni-bound structure of the E9 DNase (16). All structures of the DNase originate from inactive complexes bound with the immunity protein Im9 that does not contact the H-N-H region of the enzyme. Figures were generated using SPOCK.
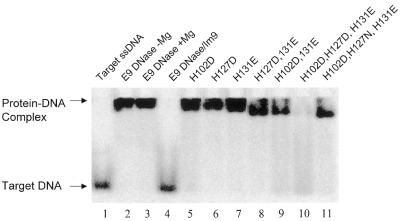
In order to address the question of whether the H-N-H motif is indeed the site of DNA binding we replaced several amino acids with acidic residues, making single, double and triple mutants, and assayed their DNA binding activity using fluoroscein-labelled 10mer ssDNA in a gel shift experiment (Fig. 5). The positions chosen were the three residues His102, His127 and His131 that span the sequence of the H-N-H motif and lie close to each other in the folded structure of the enzyme. His102 and His127 were substituted by aspartic acid while His131 was replaced with glutamic acid. The single acidic mutations at each histidine largely abolished Mg2+-dependent catalytic activity (data not shown) but not DNA binding (Fig. 5). Moreover, double acidic mutations also failed to prevent DNA binding to the H-N-H motif. It was only with a triple acidic mutant that DNA binding was finally abolished, and this was clearly dependent on the additional negative charge introduced by the third acidic mutation, since a single asparagine substitution within the triple mutant largely recovered DNA binding (Fig. 5). These data suggest that the H-N-H motif is indeed the site of DNA binding and that binding is likely to involve extensive electrostatic interactions with the DNA backbone.
Figure 5.
Multiple acidic mutations identify the H-N-H motif as the site of DNA binding. A series of histidine-to-acidic residue mutations of the E9 DNase, in combinations of one, two or three, were assayed for ssDNA binding in a gel shift experiment using fluorescently labeled ssDNA (see Materials and Methods for further details). E9 DNase does not cleave the ssDNA target under the conditions of this assay (compare lanes 2 and 3) and the gel shift is only inhibited completely by Im9 binding (lane 4). The replacement of three histidine residues in the H-N-H motif (His102, His127 and His131) with acidic groups (lane 10) severely compromises binding, as deduced from the loss of the gel shift signal and the smearing of DNA fluorescence throughout the lane. Even two acidic mutations (lanes 9 and 11) within the H-N-H motif does not abolish DNA binding by the DNase. The gel shift data with fluorescently labelled oligonucleotide were highly reproducible (n = 4), with identical data also obtained using radiolabelled oligos (data not shown).
DISCUSSION
Mg2+-mediated catalysis is responsible for colicin DNase-induced bacterial cell death
The introduction of acidic mutations into the H-N-H motif of the E9 DNase identifies it as the site of DNA binding, and this is consistent with crystallographic data showing phosphate bound within the site and with the loss of DNase activity that results from substituting H-N-H residues with alanine. Seven of the eight residues investigated in this study have previously been postulated to be part of the mechanism of DNA hydrolysis or metal ion binding but these roles had yet to be tested prior to this work (16,19,23,28,31). The remaining residue, Asn118, has a structural role, stabilising the two β-strands of the motif through the formation of bridging hydrogen bonds to backbone atoms (16). Although alanine substitution of Asn118 reduces biological activity, this mutant retains essentially wild-type DNase and metal binding activity (Table 1). This is in contrast to the seven other mutants, all of which have little or no biological activity and are defective either in DNase activity, metal coordination or both (Table 1). Alanine substitution at two residues, His102 and His127, produce the most severely affected E9 DNase domains, lacking both metal binding and DNase activity, while E100A is largely inactive and highly compromised in its ability to bind metal ions. H103A is able to bind transition metal ions but lacks DNase activity. Alanine mutations at Arg5, Arg96 and His131 lack plasmid nicking activity in the presence of Mg2+ but display activity in the presence of Ni2+ ions (and indeed all bind transition metals, albeit weakly in the case of H131A). As all three of these latter residues are biologically inactive, this provides definitive proof that Mg2+-dependent nuclease activity is responsible for the killing action of DNase colicins against E.coli cells. These data are also in line with the observation that the E9 DNase shows the highest enzymatic activity in plasmid nicking assays in the presence of Mg2+, detectable at protein concentrations of 1–2 nM (24). Placed in the context of a bacterial cell, this is equivalent to the concentration of one protein molecule in a cell, which is consistent with the pseudo-first order cell death kinetics that classically describe colicin action.
Different combinations of H-N-H amino acids are needed for Mg2+- and Ni2+-mediated DNA hydrolysis
We have proposed previously that the mechanism by which the E9 DNase cleaves DNA in the presence of Mg2+ or Ni2+ ions is different (23). The H-N-H motif mutant data reported in the present study support the idea of distinct mechanisms for the two metal ions since they show that while four amino acids (Glu100, His102, His103 and His127) are essential for Mg2+- and Ni2+-dependent activities, a further three (Arg5, Arg96 and His131) are required for Mg2+-dependent activity. Hence, a greater number of H-N-H residues are needed for DNase activity in the presence of Mg2+ ions than for DNase activity in the presence of transition metal ions. This is readily appreciated when the sites that affect each type of activity are mapped onto the molecular surface of the E9 DNase (Fig. 6). This comparison highlights how both sides of the positively charged active site cleft are required for Mg2+-dependent DNase activity (Fig. 6A and B) where double-stranded (ds)DNA is the preferred substrate (24), but only one side of the cleft is used for Ni2+-dependent activity (Fig. 6A and C) where ssDNA is the preferred substrate (23).
Mechanism of colicin DNases and H-N-H enzymes in relation to other endonucleases
In the following sections we examine the structural and mechanistic similarities between colicin DNases and other endonucleases and relate this to the effects of the alanine mutations. The H-N-H motif of the E9 DNase is structurally homologous to the His-Cys box homing endonuclease I-PpoI and the non-specific endonuclease from Serratia. This led Kühlmann et al. (30) to rename the motif the ‘ββα-Me’ motif to reflect its three structural elements and the identically placed metal ion, Mg2+ in I-PpoI and Serratia, Ni2+ in the E9 DNase. The mechanism of DNA hydrolysis for both I-PpoI and Serratia involves general base activation of a hydrolytic water molecule by a histidine residue and protonation of the 3′ oxygen by a magnesium-bound water molecule, where the magnesium is coordinated by a single enzyme asparagine residue (4,32–34). The structural alignment identified His103 as the general base in the E9 DNase mechanism, with His127 equivalent to the metal-coordinating asparagine (30). Pommer et al. (23) proposed that in the presence of transition metals such as nickel or cobalt, the H-N-H motif of colicin E9 cleaves DNA by a mechanism similar to that of I-PpoI, where the metal ion is involved in activating both the scissile phosphodiester and a water molecule that protonates the 3′ oxygen. In the crystal structure of the E9 DNase, the Ni2+ ion is clearly liganded to His127, His102 and a bound phosphate molecule, with His127 part of a hydrogen bonded triad that includes Glu100 and Arg96, the latter residues interacting through a salt bridge (Fig. 2B). Removal of the transition metal from the active site of the E9 DNase leaves the Glu100–Arg96 salt link intact but results in the movement of His102 to become hydrogen bonded to Glu100, while His127 becomes hydrogen bonded to Arg96 (Fig. 2A). This hydrogen bonded tetrad within the apoenzyme is postulated to be that which undergoes Mg2+-dependent hydrolysis in the E9 DNase (23), with His102 now acting as a general acid in the protonation of the 3′ oxygen and His127 the sole ligand for the magnesium ion (see below). The role of the Glu100–Arg96 salt link appears to be that of modulating the ionisation states of the two key histidine residues, His102 and His127. Arg5, seen in both the Ni-bound and apoprotein forms of the enzyme to hydrogen bond to the bound phosphate, is predicted to be involved in stabilising the pentacoordinate transition state in both Mg2+- and Ni2+-dependent catalysis (23).
The effect of alanine mutations of the four central amino acids of the E9 DNase H-N-H motif, Glu100, His102, His103 and His127, are in broad agreement with their predicted roles in the mechanism. All are required for catalysis in the presence of Mg2+ and Ni2+, with only H103A, the putative general base, retaining its ability to bind transition metals. Both His102 and His127 are transition metal ligands and so it is not surprising that alanine substitutions here essentially abolish metal ion binding (Table 1). The reasons for the loss of DNase activity for His102 under different metal conditions is likely to be different, however, since it is a metal ligand in Ni2+-dependent catalysis but presumed to be a general acid in Mg2+-dependent catalysis. The role of His127 as a metal ligand for Ni2+ and Mg2+ is controversial, since direct ligation of a magnesium ion by a histidine residue has yet to be demonstrated for any enzyme, with this dual capability brought about by the modulatory effects of the Glu100–Arg96 salt bridge (23). Although Glu100 is not thought to have a central mechanistic role it is clearly an important amino acid since substitution to alanine substantially reduces both catalysis and transition metal ion binding, emphasising that its mutually exclusive hydrogen bonds to His127 or His102 play a critical role in the function of the H-N-H motif of colicin DNases.
As well as forming a salt bridge with Glu100, Arg96 forms a hydrogen bond to His127 in the apo-E9 DNase, an interaction that likely serves to depress the pKa of this histidine residue. The R96A mutation does not affect the E9 DNase as dramatically as E100A, since it is capable of binding transition metal ions as well as supporting Ni2+-mediated catalysis. It does not, however, possess Mg2+-dependent activity (Fig. 4), highlighting the likely importance of the interaction with His127 seen in the apoenzyme (Fig. 2A). Similarly, H131A, which binds zinc, albeit weakly, shows substantial Ni2+-mediated catalysis and is inactive with Mg2+ ions. The role of His131 in Mg2+-dependent DNA cleavage remains uncertain, but may be involved in binding the phosphate backbone of dsDNA. Finally, although Arg5 contacts the bound phosphate in both the Ni2+-liganded and apo-E9 DNase structures, the R5A mutation only affects Mg2+ activity, suggesting that this residue is not involved in stabilising the pentacoordinate transition state when Ni2+ is the metal cofactor, emphasising the likely different mechanisms by which the transition state for phosphodiester cleavage is stabilised for the different metal ion regimes.
Structural and mechanistic similarities between H-N-H enzymes and CAD
We have found through manual sequence alignments that H-N-H enzymes share limited active site sequence similarity with CAD enzymes, DNases involved in the degradation of chromatin during the terminal stages of apoptosis (25,26; Fig. 7). These alignments are not extensive (and are not, for example, found using Phi_BLAST searches) but include the three H-N-H motif residues themselves and several other conservatively substituted amino acids. The alignment suggests that an additional 20–21 residues are present in CAD enzymes, with the position of this sequence corresponding to the loop connecting the β-strands in the H-N-H motif (Fig. 2). Smaller insertions at this position of the H-N-H motif had been reported previously in the sequence alignment of a number of H-N-H endonucleases (35) and even larger substitutions of up to 32 residues have been described recently in H-N-H motifs from a wide variety of mostly eukaryotic organisms (36). The validity of the alignment is reinforced by recent mutagenesis reports that have identified important CAD active site residues. As with H-N-H enzymes, CADs have a preponderance of histidines in their active sites, and these have been mutated either to alanine (37) or asparagine (38) with similar results but specific differences that likely reflect the different chemical properties of the mutating amino acid. Since all the mutations in the present study involved alanine substitutions, we largely restrict our comparison to the mutagenesis experiments on mouse CAD where alanine mutants were also constructed (37).
Figure 7.
Alignment of colicin E9 H-N-H motif with CAD proteins. H-N-H motif residues are shown in bold with spaces included to optimise the alignment. The secondary structure elements and numbers at the top refer to those of the colicin E9 DNase, whilst the numbering at the bottom refers to residues in mouse CAD.
Of the five conserved C-terminal histidine residues that were mutated, four were found to inactivate mouse CAD (37); the only mutant to retain catalytic activity was His304, which is not conserved between CAD and H-N-H enzymes (Fig. 7). The equivalent residue in the E9 DNase is a threonine that has previously been shown not to be essential for catalysis (28). One of the inactivating mutants (His242), which has no obvious counterpart in H-N-H enzymes, is thought to have either a structural role (38) or to be involved in DNA binding (37). The remaining three conserved histidines in mouse CAD, His263, His308 and His313, all have counterparts in H-N-H enzymes and are shown by the present study to be critical for activity. His263 is equivalent to the E9 DNase general base His103, its role predicted to be that of activating the hydrolytic water molecule. His308 is equivalent to His127 in E9 and as such may be the Mg2+-binding residue as predicted for the E9 DNase. His313 is not strictly conserved between H-N-H and CAD enzymes, although it is only one amino acid removed from His131 in the alignment with the E9 DNase (Fig. 7) and so may take on a similar role, such as DNA binding. The conserved Asn118, involved in stabilising the motif, is equivalent to Asn299 in mouse CAD. His102, the putative general acid in the E9 DNase, is not conserved in CADs, but there is a conserved aspartic acid residue in an equivalent position that could accommodate such a catalytic role. Glu100, the hydrogen bonding networks of which serve to modulate the roles of active site residues in H-N-H enzymes, is replaced by an asparagine residue and so could fulfil such a role in CAD enzymes; indeed, a glutamine is found at this position in some H-N-H enzymes (Fig. 1). Finally, we note that the DNA cleavage products of CAD have 3′-OH and 5′-phosphate termini and that the enzyme is most active at alkaline pH, requires Mg2+ ions for activity and is inactivated by Zn2+ (38,39), enzymological properties that are analogous to those of the E9 DNase (22–24). Hence, we speculate that colicin DNases (and indeed all H-N-H enzymes, including CADs) cleave DNA by the same basic mechanism. This is an intriguing possibility given that the role of both families of enzymes is the destruction of chromosomal DNA leading to cell death, the former in the context of bacterial intoxication by a protein antibiotic, the latter in the context of mammalian apoptosis.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Christine Moore and Ann Reilly for technical assistance with protein purification, Charles Strange for preliminary metal binding data and Anthony Keeble for helpful comments on the manuscript. This work was supported by a studentship awarded by the Biotechnology and Biological Sciences Research Council of the UK (to D.C.W.) and a grant from The Wellcome Trust, UK and from the EU (contract no. BIO4-98-0156).
REFERENCES
- 1.Chevalier B.S., and Stoddard,B.L. (2001) Homing endonucleases: structural and functional insight into the catalysts of intron/intein mobility. Nucleic Acids Res., 29, 3757–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedhoff P., Franke,I., Meiss,G., Wende,W., Krause,K.L. and Pingoud,A. (1999) A similar active site for non-specific and specific endonucleases. Nature Struct. Biol., 6, 112–113. [DOI] [PubMed] [Google Scholar]
- 3.Friedhoff P., Franke,I., Krause,K.L. and Pingoud,A. (1999) Cleavage experiments with deoxythymidine 3′,5′-bis-(p-nitrophenyl phosphate) suggest that the homing endonuclease I-PpoI follows the same mechanism of phosphodiester bond hydrolysis as the non-specific Serratia nuclease. FEBS Lett., 443, 209–214. [DOI] [PubMed] [Google Scholar]
- 4.Galburt E.A., Chevalier,B., Tang,W., Jurica,M.S., Flick,K.E., Monnat,R.J.,Jr and Stoddard,B.L. (1999) A novel endonuclease mechanism directly visualized for I-PpoI. Nature Struct. Biol., 6, 1096–1099. [DOI] [PubMed] [Google Scholar]
- 5.Shub D.A., Goodrich-Blair,H. and Eddy,S.R. (1994) Amino acid sequence motif of group I intron endonucleases is conserved in open reading frames of group II introns. Trends Biochem. Sci., 19, 402–404. [DOI] [PubMed] [Google Scholar]
- 6.James R., Kleanthous,C. and Moore,G.R. (1996) The biology of E colicins: paradigms and paradoxes. Microbiology, 142, 1569–1580. [DOI] [PubMed] [Google Scholar]
- 7.Soelaiman S., Jakes,K., Wu,N., Li,C. and Shoham,M. (2001) Crystal structure of Colicin E3: implications for cell entry and ribosome inactivation. Mol. Cell, 8, 1053–1062. [DOI] [PubMed] [Google Scholar]
- 8.Penfold C.N., Garinot-Schneider,C., Hemmings,A.M., Moore,G.R., Kleanthous,C. and James,R. (2000) A 76-residue polypeptide of colicin E9 confers receptor specificity and inhibits the growth of vitamin B12-dependent Escherichia coli 113/3 cells. Mol. Microbiol., 38, 639–649. [DOI] [PubMed] [Google Scholar]
- 9.Garinot-Schneider C., Penfold,C.N., Moore,G.R., Kleanthous,C. and James,R. (1997) Identification of residues in the putative TolA box which are essential for the toxicity of the endonuclease toxin colicin E9. Microbiology, 143, 2931–2938. [DOI] [PubMed] [Google Scholar]
- 10.Elkins P., Bunker,A., Cramer,W.A. and Stauffacher,C.V. (1997) A mechanism for toxin insertion into membranes is suggested by the crystal structure of the channel-forming domain of colicin E1. Structure, 5, 443–458. [DOI] [PubMed] [Google Scholar]
- 11.Schaller K., and Nomura,M. (1976) Colicin E2 is DNA endonuclease. Proc. Natl Acad. Sci. USA, 73, 3989–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boon T., (1971) Inactivation of ribosomes in vitro by colicin E3. Proc. Natl Acad. Sci. USA, 68, 2421–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senior B.W., and Holland,I.B. (1971) Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc. Natl Acad. Sci. USA, 68, 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa T., Tomita,K., Ueda,T., Watanabe,K., Uozumi,T. and Masaki,H. (1999) A cytotoxic ribonuclease targeting specific transfer RNA anticodons. Science, 283, 2097–2100. [DOI] [PubMed] [Google Scholar]
- 15.Kleanthous C., Hemmings,A.M., Moore,G.R. and James,R. (1998) Immunity proteins and their specificity for endonuclease colicins: telling right from wrong in protein-protein recognition. Mol. Microbiol., 28, 227–233. [DOI] [PubMed] [Google Scholar]
- 16.Kleanthous C., Kûhlmann,U.C., Pommer,A.J., Ferguson,N., Radford,S.E., Moore,G.R., James,R. and Hemmings,A.M. (1999) Structural and mechanistic basis of immunity towards endonuclease colicins. Nature Struct. Biol., 6, 243–252. [DOI] [PubMed] [Google Scholar]
- 17.Wallis R., Moore,G.R., James,R. and Kleanthous,C. (1995) Protein-protein interactions in colicin E9 DNase-immunity protein complexes. 1. Diffusion-controlled association and femtomolar binding for the cognate complex. Biochemistry, 34, 13743–13750. [DOI] [PubMed] [Google Scholar]
- 18.Lazdunski C.J., Bouveret,E., Rigal,A., Journet,L., Lloubès,R. and Bénédetti,H. (1998) Colicin import into Escherichia coli cells. J. Bacteriol., 180, 4993–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko T.P., Liao,C.C., Ku,W.Y., Chak,K.F. and Yuan,H.S. (1999) The crystal structure of the DNase domain of colicin E7 in complex with its inhibitor Im7 protein. Structure, 7, 91–102. [DOI] [PubMed] [Google Scholar]
- 20.Kleanthous C., and Walker,D. (2001) Immunity proteins: enzyme inhibitors that avoid the active site. Trends Biochem. Sci., 26, 624–631. [DOI] [PubMed] [Google Scholar]
- 21.Hannan J.P., Whittaker,S.B., Hemmings,A.M., James,R., Kleanthous,C. and Moore,G.R. (2000) NMR studies of metal ion binding to the Zn-finger-like HNH motif of colicin E9. J. Inorg. Biochem., 79, 365–370. [DOI] [PubMed] [Google Scholar]
- 22.Pommer A.J., Kûhlmann,U.C., Cooper,A., Hemmings,A.M., Moore,G.R., James,R. and Kleanthous,C. (1999) Homing in on the role of transition metals in the HNH motif of colicin endonucleases. J. Biol. Chem., 274, 27153–27160. [DOI] [PubMed] [Google Scholar]
- 23.Pommer A.J., Cal,S., Keeble,A.H., Walker,D., Evans,S.J., Kühlmann,U.C., Cooper,A., Connolly,B.A., Hemmings,A.M., Moore,G.R. et al. (2001) Mechanism and cleavage specificity of the H-N-H endonuclease colicin E9. J. Mol. Biol., 314, 735–749. [DOI] [PubMed] [Google Scholar]
- 24.Pommer A.J., Wallis,R., Moore,G.R., James,R. and Kleanthous,C. (1998) Enzymological characterization of the nuclease domain from the bacterial toxin colicin E9 from Escherichia coli. Biochem. J., 334, 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enari M., Sakahira,H., Yokoyama,H., Okawa,K., Iwamatsu,A. and Nagata,S. (1998) A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature, 391, 43–50. [DOI] [PubMed] [Google Scholar]
- 26.Sakahira H., Enari,M. and Nagata,S. (1998) Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature, 391, 96–99. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar G., and Sommer,S.S. (1990) The megaprimer method of site-directed mutagenesis. Biotechniques, 8, 404–407. [PubMed] [Google Scholar]
- 28.Garinot-Schneider C., Pommer,A.J., Moore,G.R., Kleanthous,C. and James,R. (1996) Identification of putative active-site residues in the DNase domain of colicin E9 by random mutagenesis. J. Mol. Biol., 260, 731–742. [DOI] [PubMed] [Google Scholar]
- 29.Kunitz M., (1950) Crystalline deoxyribonuclease I. J. Gen. Physiol., 33, 249–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhlmann U.C., Moore,G.R., James,R., Kleanthous,C. and Hemmings,A.M. (1999) Structural parsimony in endonuclease active sites: should the number of homing endonuclease families be redefined? FEBS Lett., 463, 1–2. [DOI] [PubMed] [Google Scholar]
- 31.Kuhlmann U.C., Pommer,A.J., Moore,G.R., James,R. and Kleanthous,C. (2000) Specificity in protein-protein interactions: the structural basis for dual recognition in endonuclease colicin-immunity protein complexes. J. Mol. Biol., 301, 1163–1178. [DOI] [PubMed] [Google Scholar]
- 32.Flick K.E., Jurica,M.S., Monnat,R.J.,Jr and Stoddard,B.L. (1998) DNA binding and cleavage by the nuclear intron-encoded homing endonuclease I-PpoI. Nature, 394, 96–101. [DOI] [PubMed] [Google Scholar]
- 33.Mannino S.J., Jenkins,C.L. and Raines,R.T. (1999) Chemical mechanism of DNA cleavage by the homing endonuclease I-PpoI. Biochemistry, 38, 16178–16186. [DOI] [PubMed] [Google Scholar]
- 34.Miller M.D., Cai,J. and Krause,K.L. (1999) The active site of Serratia endonuclease contains a conserved magnesium-water cluster. J. Mol. Biol., 288, 975–987. [DOI] [PubMed] [Google Scholar]
- 35.Dalgaard J.Z., Klar,A.J., Moser,M.J., Holley,W.R., Chatterjee,A. and Mian,I.S. (1997) Statistical modeling and analysis of the LAGLIDADG family of site-specific endonucleases and identification of an intein that encodes a site-specific endonuclease of the HNH family. Nucleic Acids Res., 25, 4626–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik H.S., and Henikoff,S. (2000) Dual recognition-incision enzymes might be involved in mismatch repair and meiosis. Trends Biochem. Sci., 25, 414–418. [DOI] [PubMed] [Google Scholar]
- 37.Sakahira H., Takemura,Y. and Nagata,S. (2001) Enzymatic active site of caspase-activated DNase (CAD) and its inhibition by inhibitor of CAD. Arch. Biochem. Biophys., 388, 91–99. [DOI] [PubMed] [Google Scholar]
- 38.Meiss G., Scholz,S.R., Korn,C., Gimadutdinow,O. and Pingoud,A. (2001) Identification of functionally relevant histidine residues in the apoptotic nuclease CAD. Nucleic Acids Res., 29, 3901–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widlak P., and Garrard,W.T. (2001) Ionic and cofactor requirements for the activity of the apoptotic endonuclease DFF40/CAD. Mol. Cell. Biochem., 218, 125–130. [DOI] [PubMed] [Google Scholar]