Abstract
1. Potassium-depleted muscles have been analysed for cations, phosphocreatine, adenosine triphosphate and lactate before or after an exposure to a medium with 10 mM potassium salt.
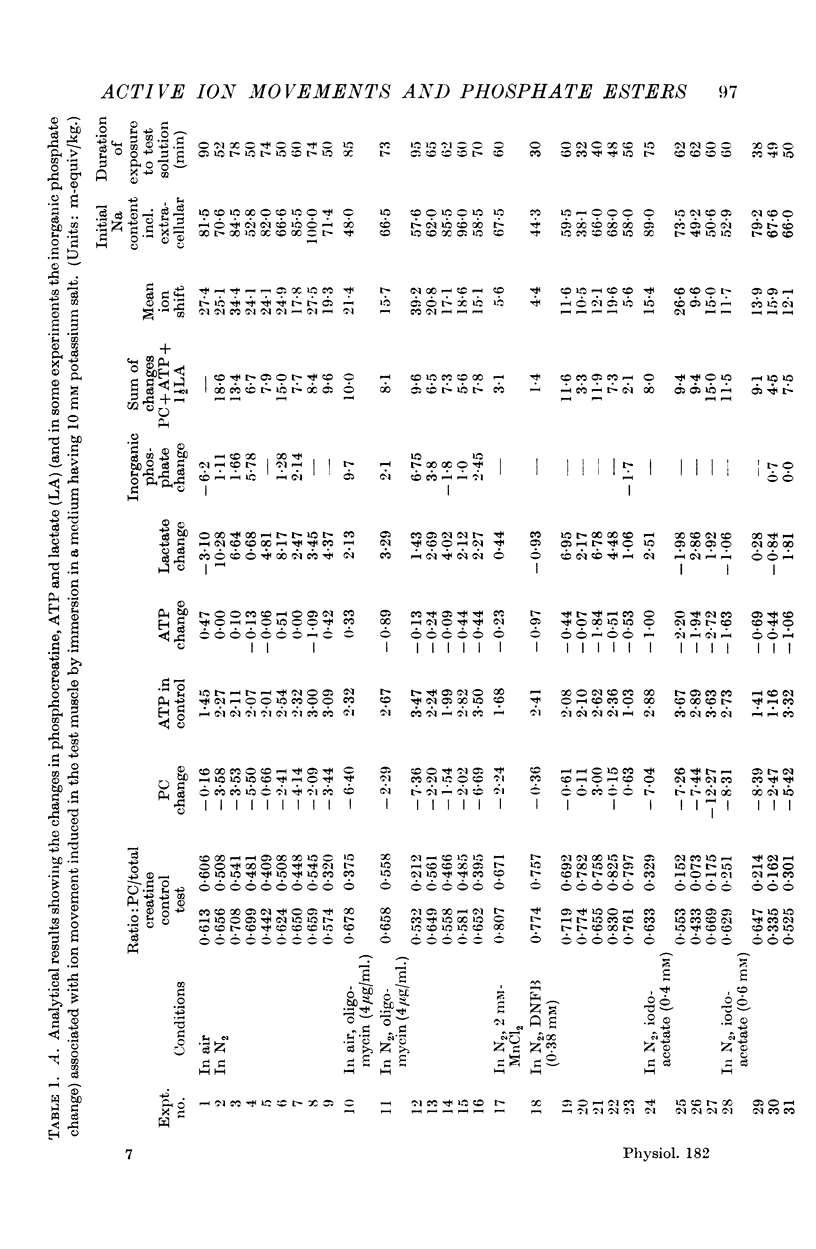
2. The net movements of sodium out and potassium in when the system is anaerobic but not otherwise poisoned are accompanied by break-down of phosphocreatine and formation of lactate.
3. In bicarbonate media oligomycin has little perceptible effect upon these observed changes, which is taken to indicate that mitochondrial phosphorylation is not essential. An inhibition by oligomycin was noted in media buffered with Tris.
4. Dinitrofluorobenzene, which poisons creatine phosphotransferase, leads to the cation changes being accompanied by break-down of ATP and formation of lactate. This indicates that ATP is more directly concerned with energizing the ion movements than is phosphocreatine.
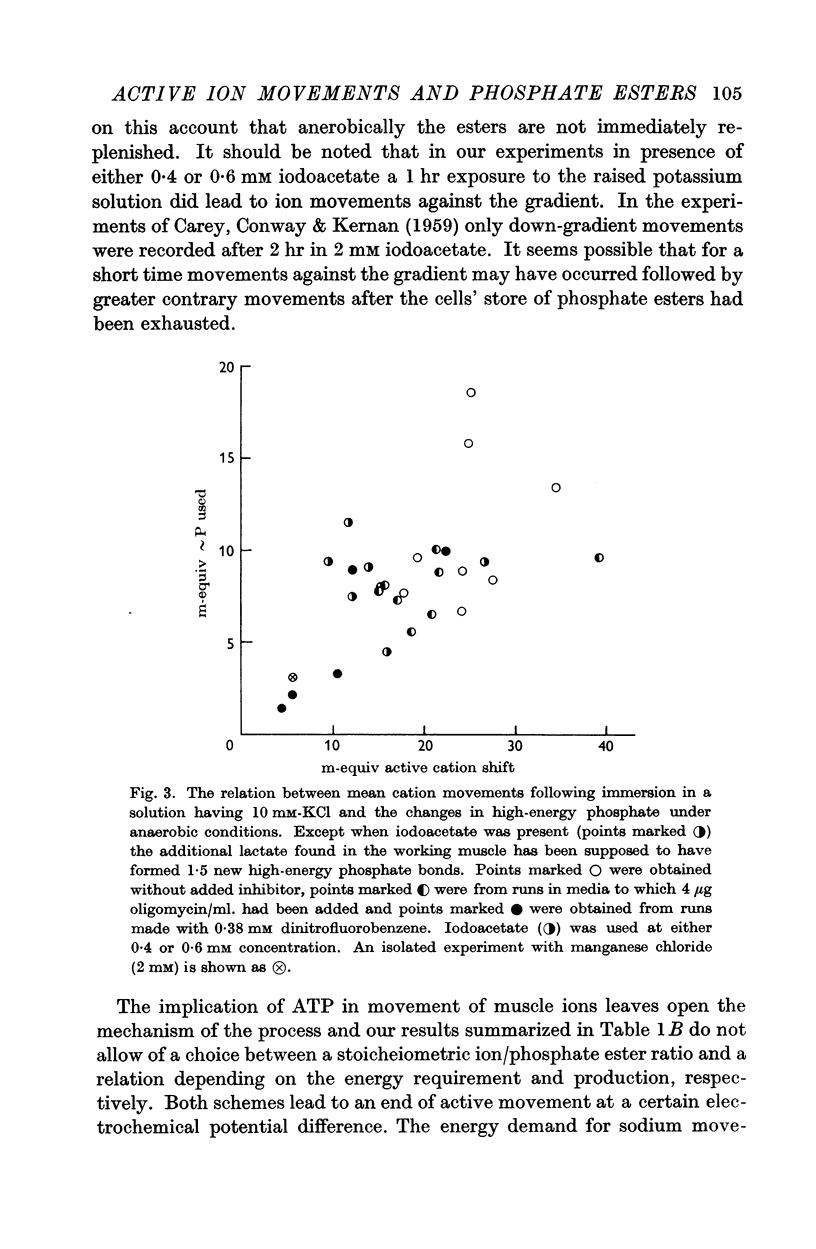
5. Iodoacetate inhibits the glycolytic process and the ion movement is then accompanied by more phosphocreatine break-down than in the other conditions; the level of ATP also falls.
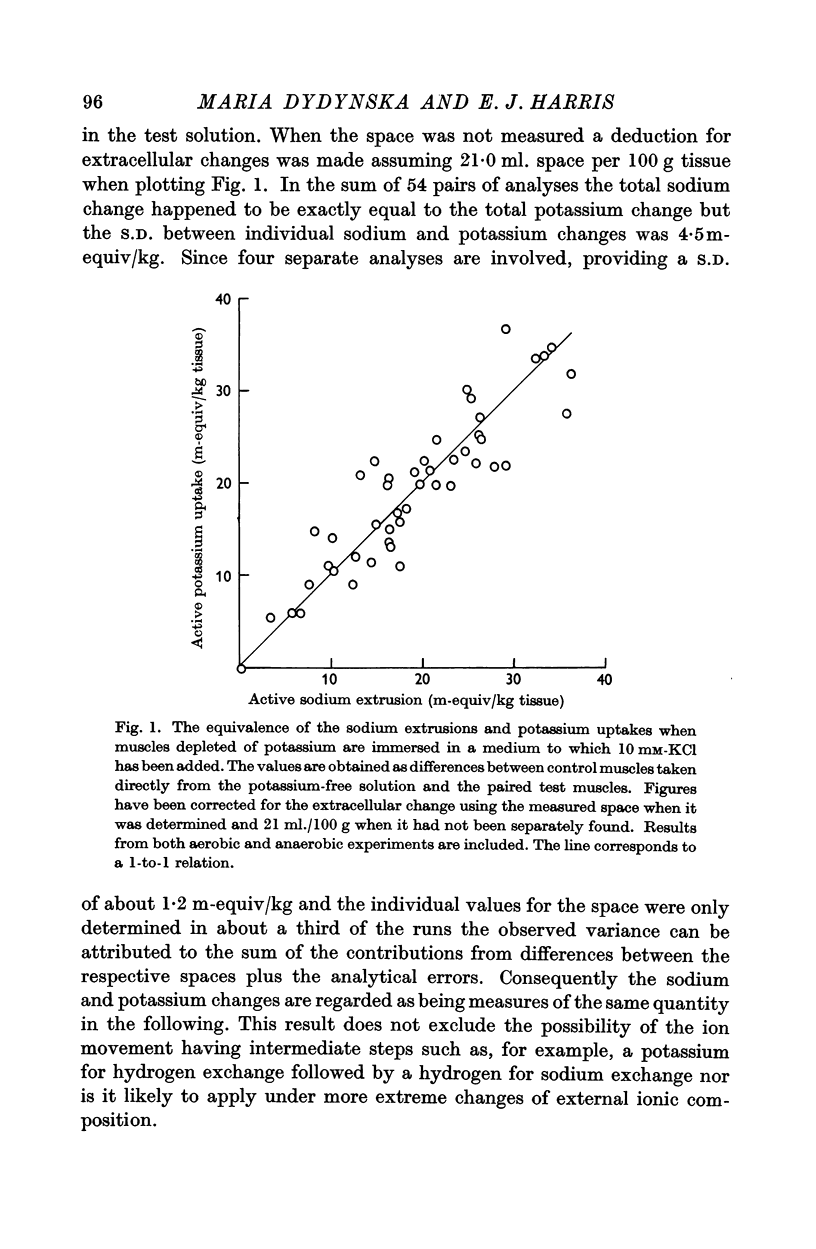
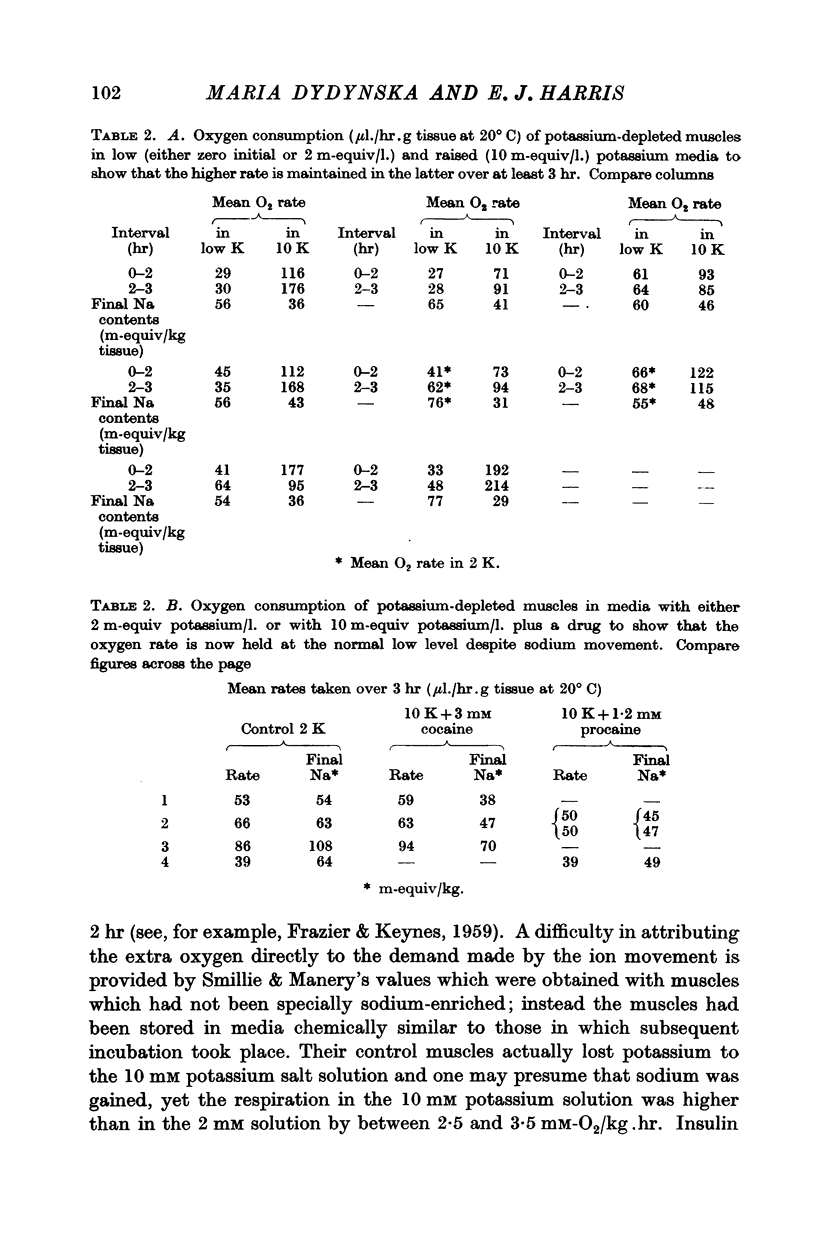
6. The mean number of sodium ions moved out is closely equal to the number of potassium ions moved in. Conditions mentioned in (2) and (3) above lead to about 2·5 sodium ions being moved out per high-energy phosphate bond hydrolysed provided allowance is made for the glycolytic resynthesis of ATP.
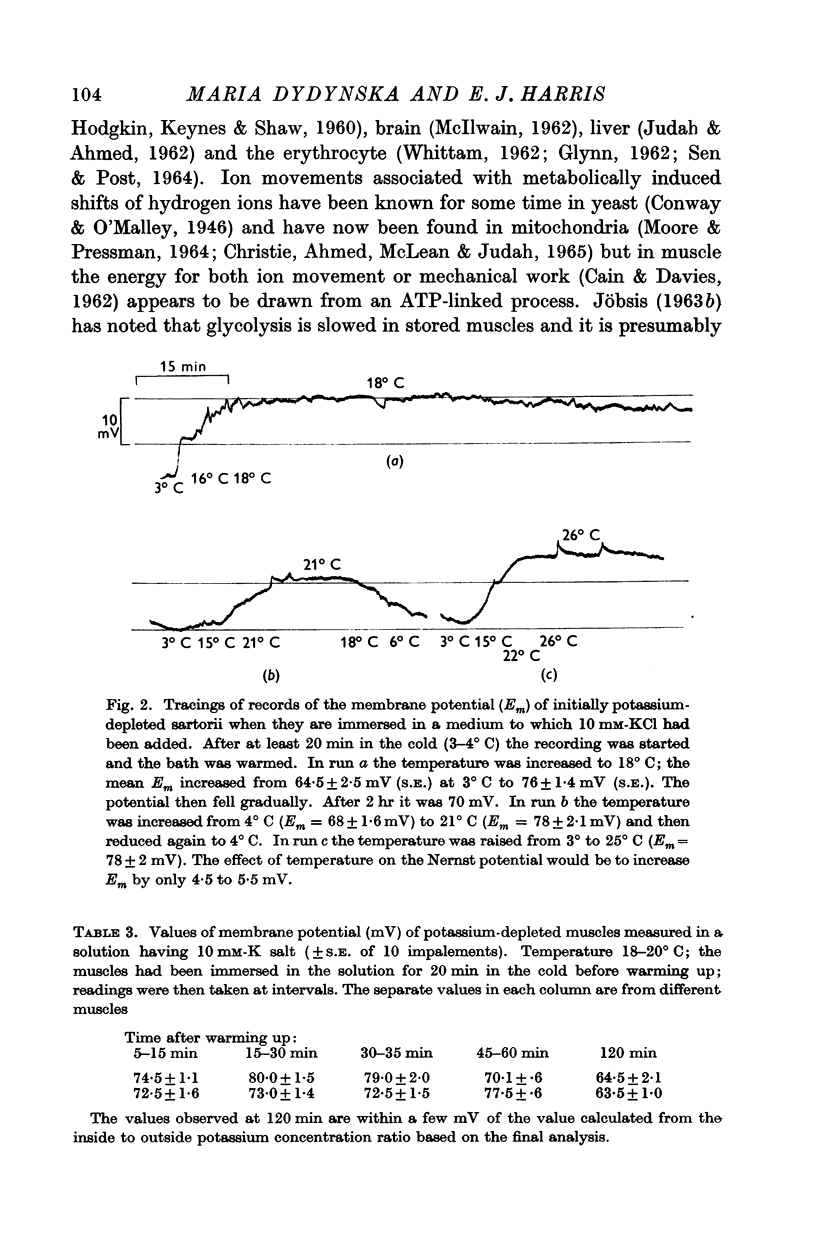
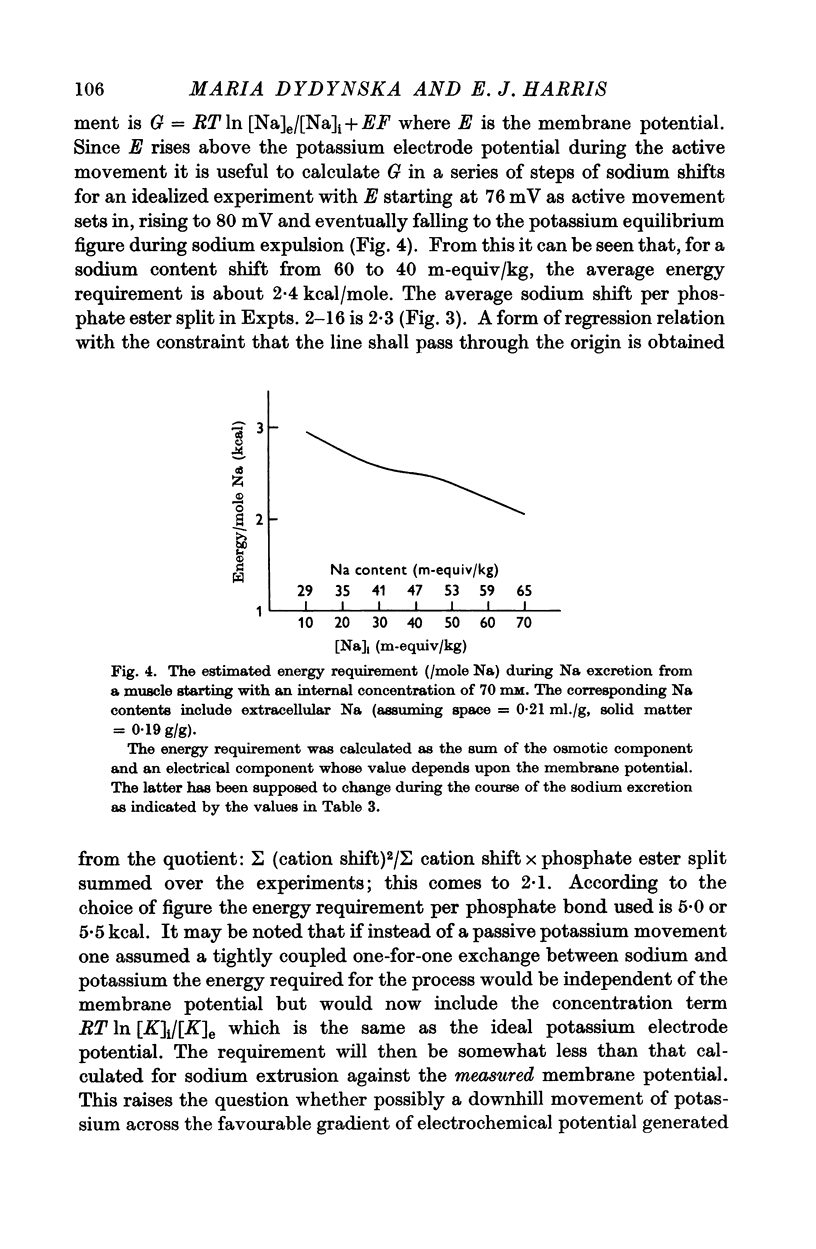
7. Some measurements of membrane potential under comparable conditions of ion movement are reported and these are used to calculate the energy requirement of the process of sodium—potassium interchange.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED K., JUDAH J. D. Role of phosphoproteins in ion transport in liver slices. Biochim Biophys Acta. 1962 Feb 26;57:245–252. doi: 10.1016/0006-3002(62)91117-4. [DOI] [PubMed] [Google Scholar]
- CAIN D. F., DAVIES R. E. Breakdown of adenosine triphosphate during a single contraction of working muscle. Biochem Biophys Res Commun. 1962 Aug 7;8:361–366. doi: 10.1016/0006-291x(62)90008-6. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY M. J., CONWAY E. J., KERNAN R. P. Secretion of sodium ions by the frog's sartorius. J Physiol. 1959 Oct;148:51–82. doi: 10.1113/jphysiol.1959.sp006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON F. D., HARDY D. J., WILKIE D. R. Total energy production and phosphocreatine hydrolysis in the isotonic twitch. J Gen Physiol. 1963 May;46:851–882. doi: 10.1085/jgp.46.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSTEN M. E., MOMMAERTS W. F. THE ACCUMULATION OF CALCIUM IONS BY SARCOTUBULAR VESICLES. J Gen Physiol. 1964 Nov;48:183–197. doi: 10.1085/jgp.48.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., HOLLUNGER G. The interaction of energy and electron transfer reactions in mitochondria. VI. The efficiency of the reaction. J Biol Chem. 1961 May;236:1577–1584. [PubMed] [Google Scholar]
- CHANCE B., WEBER A. THE STEADY STATE OF CYTOCHROME B DURING REST AND AFTER CONTRACTION IN FROG SARTORIUS. J Physiol. 1963 Nov;169:263–277. doi: 10.1113/jphysiol.1963.sp007255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIE G. S., AHMED K., MCLEAN A. E., JUDAH D. ACTIVE TRANSPORT OF POTASSIUM BY MITOCHONDRIA. I. EXCHANGE OF K+ AND H+. Biochim Biophys Acta. 1965 Mar 29;94:432–440. doi: 10.1016/0926-6585(65)90051-8. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J. NEW LIGHT ON THE ACTIVE TRANSPORT OF SODIUM IONS FROM SKELETAL MUSCLE. Fed Proc. 1964 May-Jun;23:680–688. [PubMed] [Google Scholar]
- Conway E. J., O'malley E. The nature of the cation exchanges during yeast fermentation, with formation of 0.02n-H ion. Biochem J. 1946;40(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E. REGULATION OF GLYCOLYSIS IN MUSCLE. I. THE CONVERSION OF PHOSPHORYLASE BETA TO PHOSPHORYLASE ALPHA IN FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3133–3138. [PubMed] [Google Scholar]
- FRAZIER H. S., KEYNES R. D. The effect of metabolic inhibitors on the sodium fluxes in sodium-loaded frog sartorius muscle. J Physiol. 1959 Oct;148:362–378. doi: 10.1113/jphysiol.1959.sp006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUMENTO A. S. SODIUM PUMP: ITS ELECTRICAL EFFECTS IN SKELETAL MUSCLE. Science. 1965 Mar 19;147(3664):1442–1443. doi: 10.1126/science.147.3664.1442. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. TRANSPORT ADENOSINETRIPHOSPHATASE' IN ELECTRIC ORGAN. THE RELATION BETWEEN ION TRANSPORT AND OXIDATIVE PHOSPHORYLATION. J Physiol. 1963 Nov;169:452–465. doi: 10.1113/jphysiol.1963.sp007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V., HOWARTH J. V. The effect of potassium on the resting metabolism of the frog's sartorius. Proc R Soc Lond B Biol Sci. 1957 Aug 24;147(926):21–43. doi: 10.1098/rspb.1957.0034. [DOI] [PubMed] [Google Scholar]
- JOBSIS F. F. Spectrophotometric studies on intact muscle. I. Components of the respiratory chain. J Gen Physiol. 1963 May;46:905–928. doi: 10.1085/jgp.46.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUDAH J. D., AHMED K. THE BIOCHEMISTRY OF SODIUM TRANSPORT. Biol Rev Camb Philos Soc. 1964 May;39:160–193. doi: 10.1111/j.1469-185x.1964.tb00953.x. [DOI] [PubMed] [Google Scholar]
- KARPATKIN S., HELMREICH E., CORI C. F. REGULATION OF GLYCOLYSIS IN MUSCLE. II. EFFECT OF STIMULATION AND EPINEPHRINE IN ISOLATED FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3139–3145. [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- MULLER M. H. Metabolic aspects of ionic shifts in toad muscle. Biochim Biophys Acta. 1962 Mar 12;57:475–494. doi: 10.1016/0006-3002(62)91156-3. [DOI] [PubMed] [Google Scholar]
- NOVOTNY I., VYSKOCIL F., VYKLICKY L., BERANEK R. Potassium and caffeine induced increase of oxygen consumption in frog muscle and its inhibition by drugs. Physiol Bohemoslov. 1962;11:277–284. [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- SLATER E. C. Mechanism of phosphorylation in the respiratory chain. Nature. 1953 Nov 28;172(4387):975–978. doi: 10.1038/172975a0. [DOI] [PubMed] [Google Scholar]
- SMILLIE L. B., MANERY J. F. Effect of external potassium concentrations, insulin and lactate on frog muscle potassium and respiratory rate. Am J Physiol. 1960 Jan;198:67–77. doi: 10.1152/ajplegacy.1960.198.1.67. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUMG THE EFFECT OF OLIGOMYCIN ON NET MOVEMENTS OF SODIUM AND POTASSIUM IN MAMMALIAN CELLS IN VITRO. Biochim Biophys Acta. 1964 Mar 16;82:556–571. doi: 10.1016/0304-4165(64)90447-7. [DOI] [PubMed] [Google Scholar]
- WATTS D. C. STUDIES ON THE MECHANISM OF ACTION OF ADENOSINE 5'-TRIPHOSPHATE-CREATINE PHOSPHOTRANSFERASE. INHIBITION BY MANGANESE IONS AND BY P-NITROPHENYL ACETATE. Biochem J. 1963 Nov;89:220–229. doi: 10.1042/bj0890220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]


