Abstract
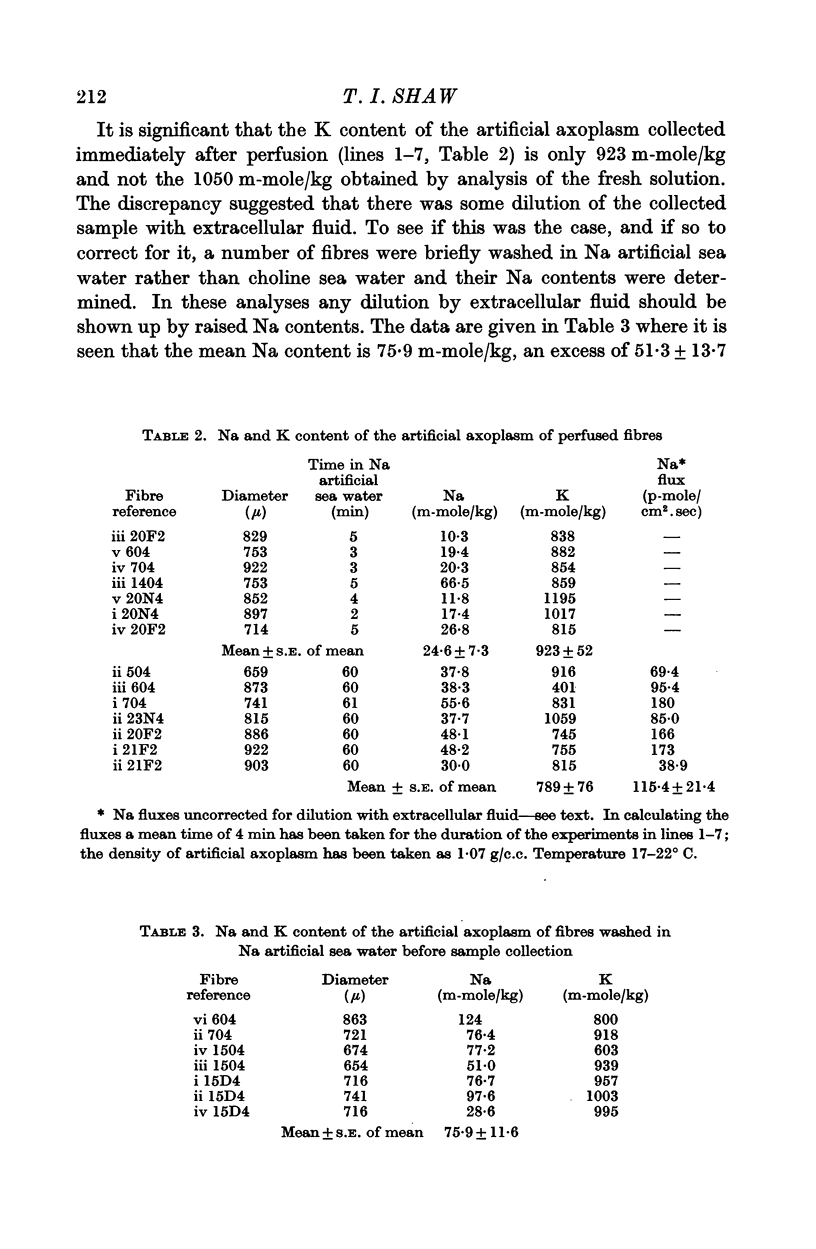
1. The net movements of Na and K into resting squid giant axons perfused with a K2SO4 artificial axoplasm have been determined. Na enters at a rate of 131 ± 25 p-mole/cm2.sec. The K leakage from the fibre is 942 ± 566 p-mole/cm2.sec; clearly this latter figure shows how large is the uncertainty in the exact value for the potassium leakage.
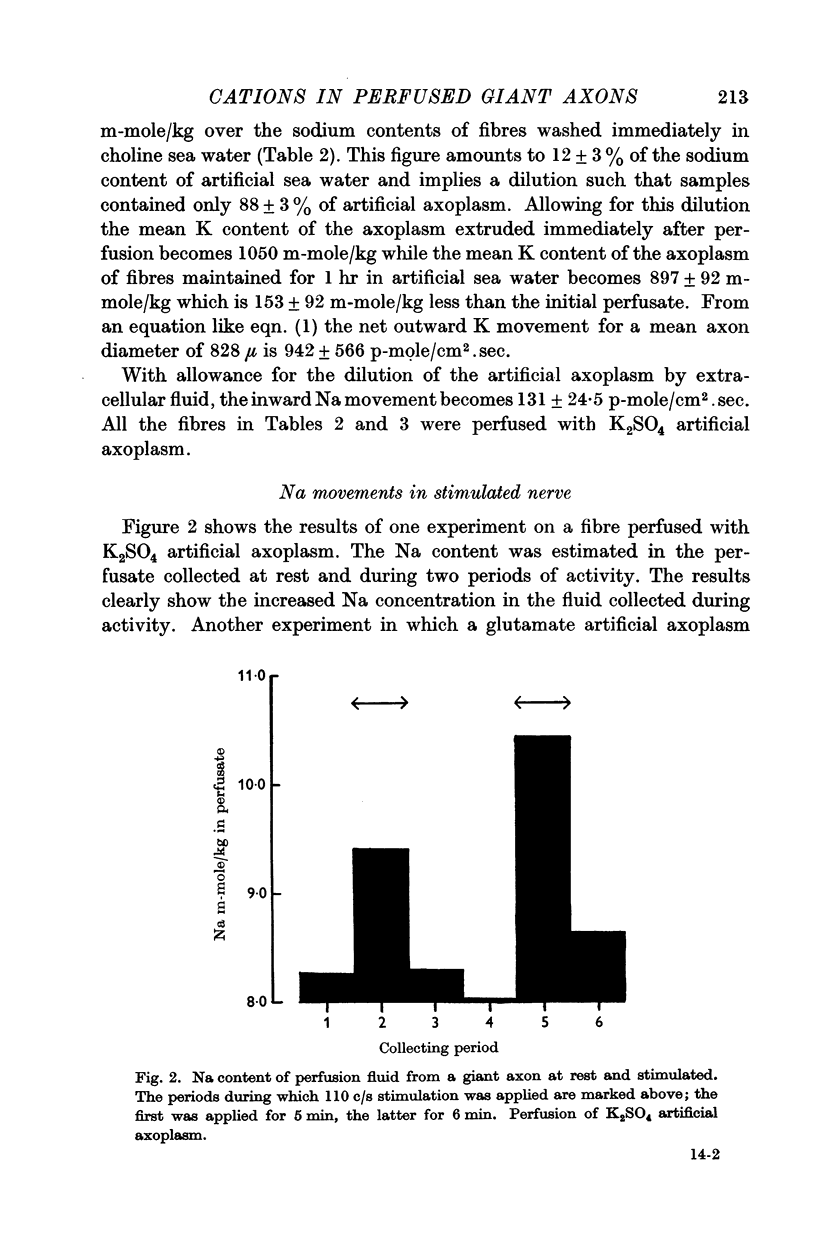
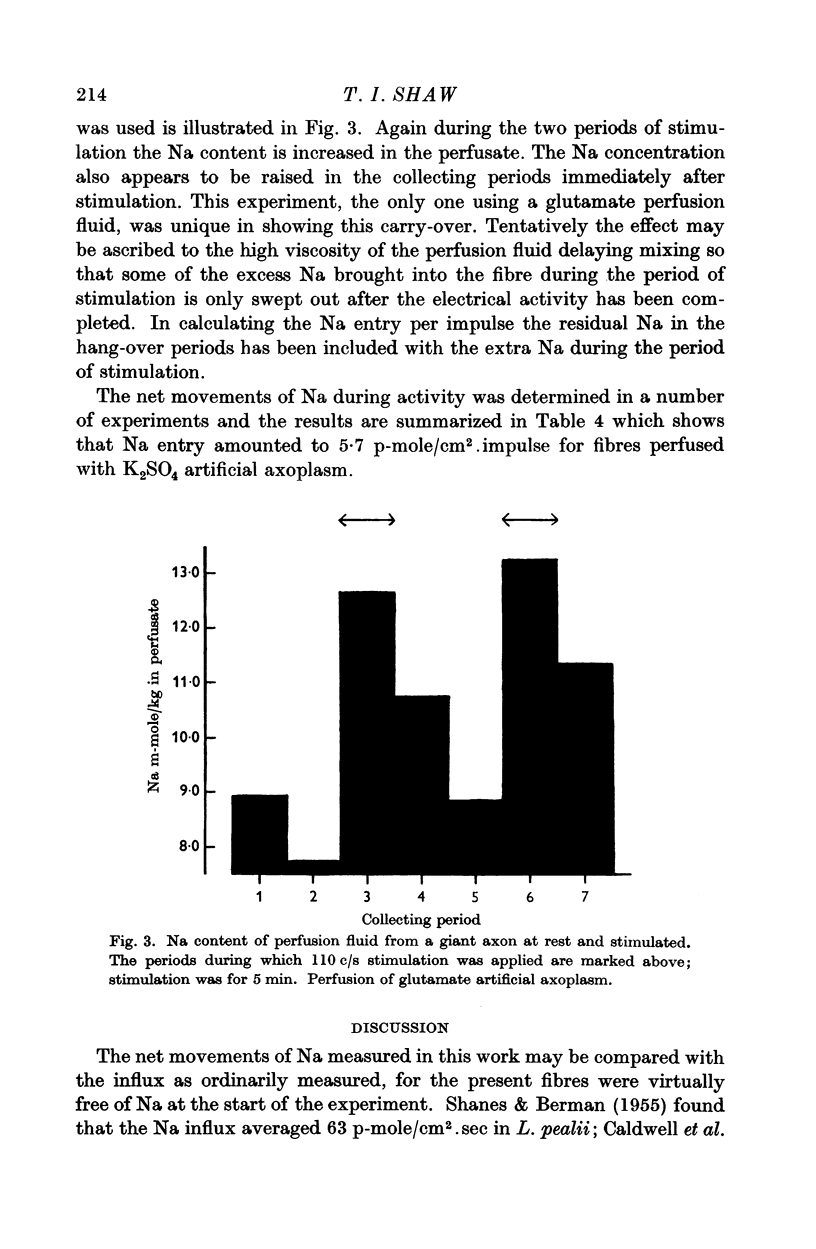
2. The net entry of sodium associated with activity in such fibres has been measured and is 5·7 ± 0·7 p-mole/cm2.impulse.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. The effects of changes in internal ionic concentrations on the electrical properties of perfused giant axons. J Physiol. 1962 Nov;164:355–374. doi: 10.1113/jphysiol.1962.sp007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE J. W., ADELMAN W. J., Jr Electronic measurement of the intracellular concentration and net flux of sodium in the squid axon. J Gen Physiol. 1961 Sep;45:77–92. doi: 10.1085/jgp.45.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHENBERG M. A. Studies on permeability in relation to nerve function, ionic movements across exonal membranes. Biochim Biophys Acta. 1950 Jan;4(1-3):96–114. doi: 10.1016/0006-3002(50)90012-6. [DOI] [PubMed] [Google Scholar]
- SHANES A. M., BERMAN M. D. Kinetics of ion movement in the squid giant axon. J Gen Physiol. 1955 Nov 20;39(2):279–300. doi: 10.1085/jgp.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKI I., TAKENAKA T. EFFECTS OF VARIOUS POTASSIUM SALTS AND PROTEASES UPON EXCITABILITY OF INTRACELLULARLY PERFUSED SQUID GIANT AXONS. Proc Natl Acad Sci U S A. 1964 Sep;52:804–810. doi: 10.1073/pnas.52.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]


