Abstract
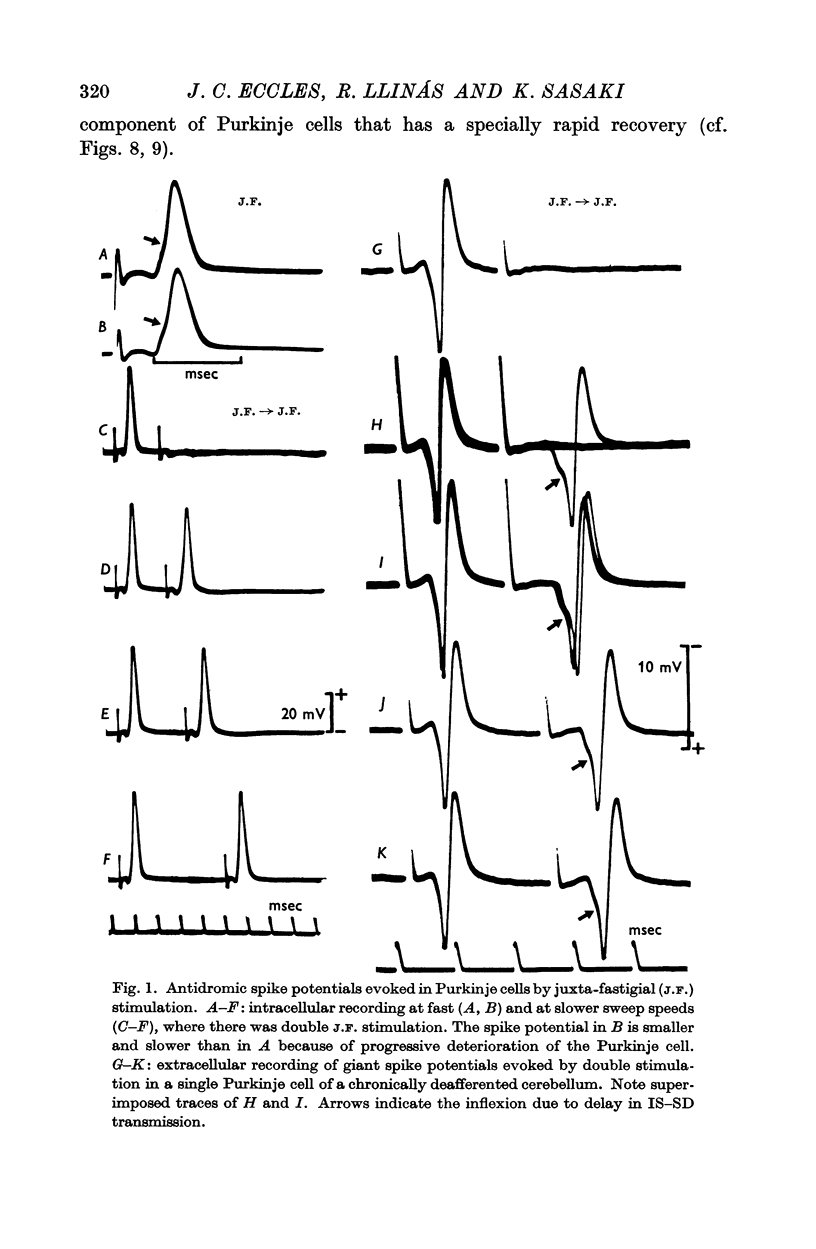
1. Antidromic impulses have been set up in the axons of Purkinje cells of the cerebellar vermis by stimulation in the juxta-fastigial (J.F.) region. Most experiments were performed on the normal cat cerebellum, but in nine the cerebellum was chronically deafferented by bilateral pedunculotomy 9-23 days previously.
2. Intra- and extracellular recording from Purkinje cells both showed a characteristic inflexion on the rising phase of the spike potential (the characteristic IS—SD inflexion) that presumably signals a delay in invasion between the axon and the large soma-dendritic expansion.
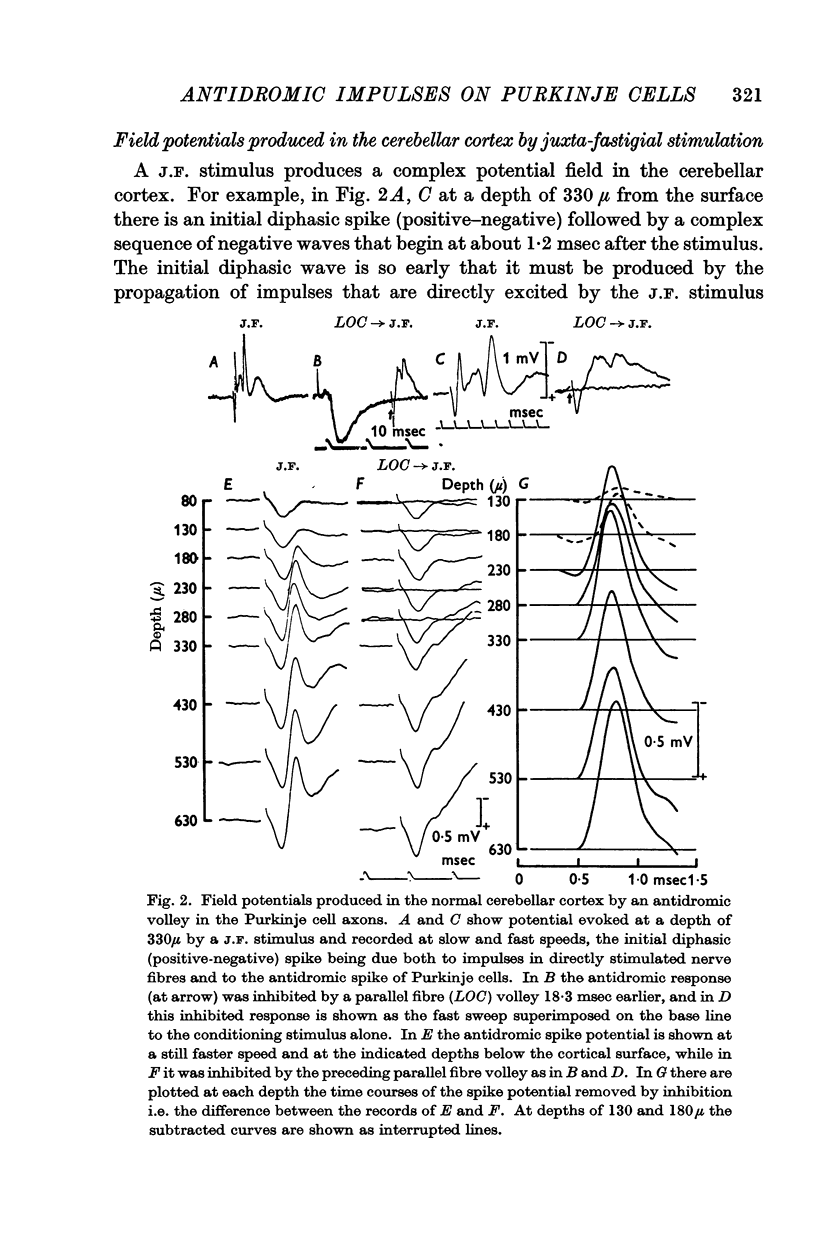
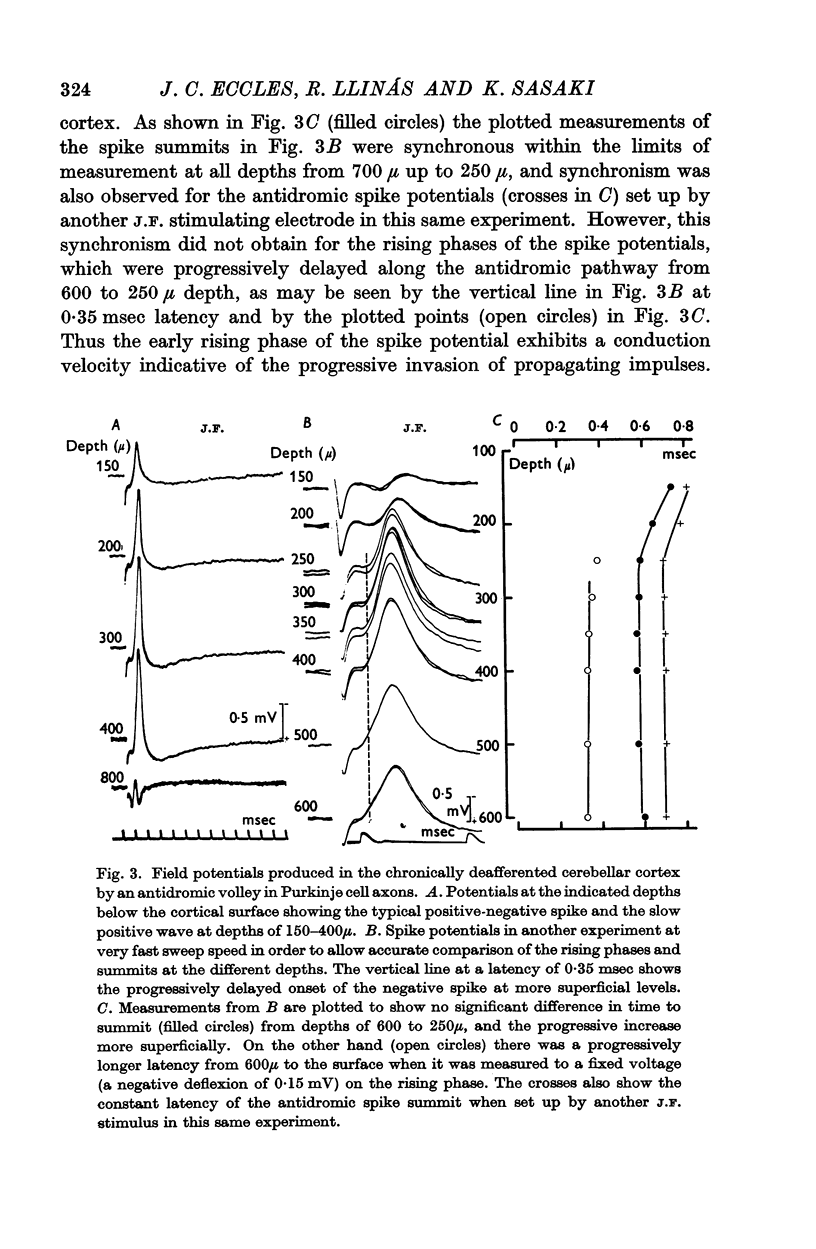
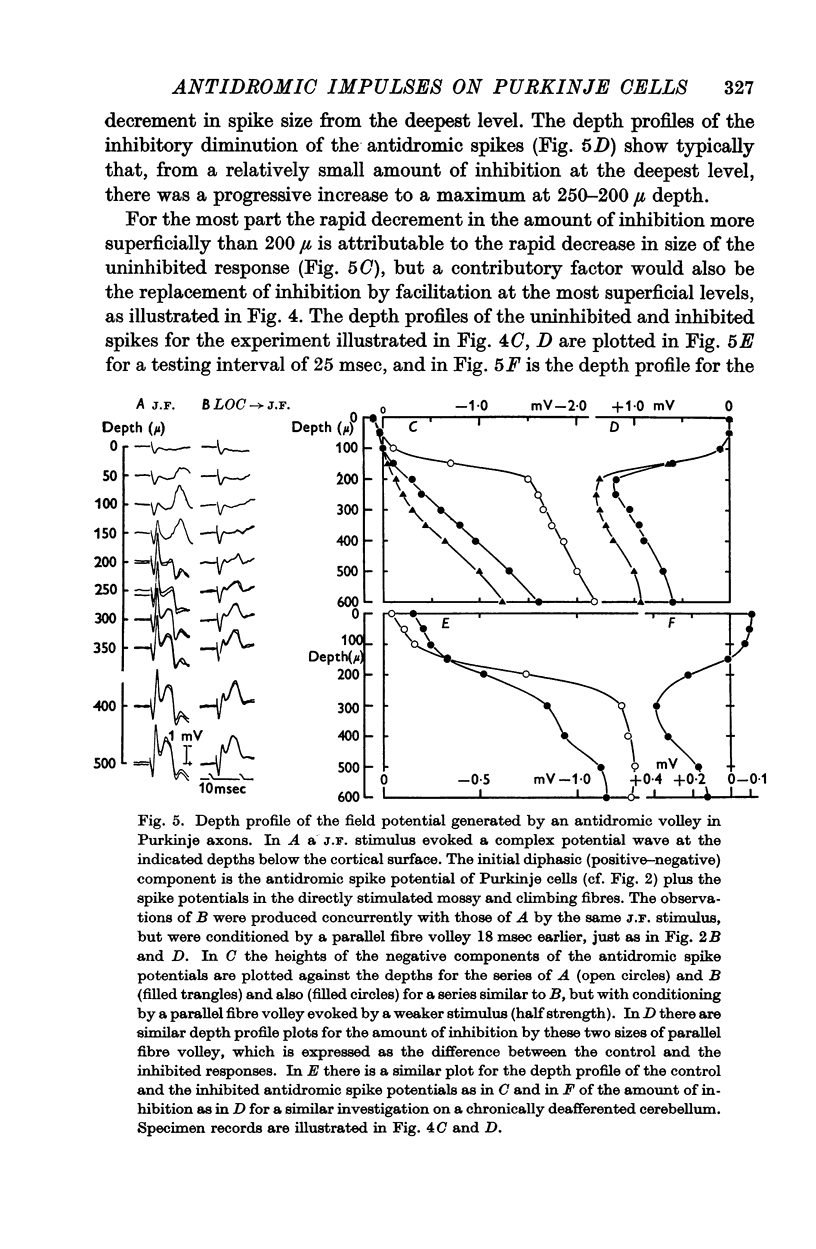
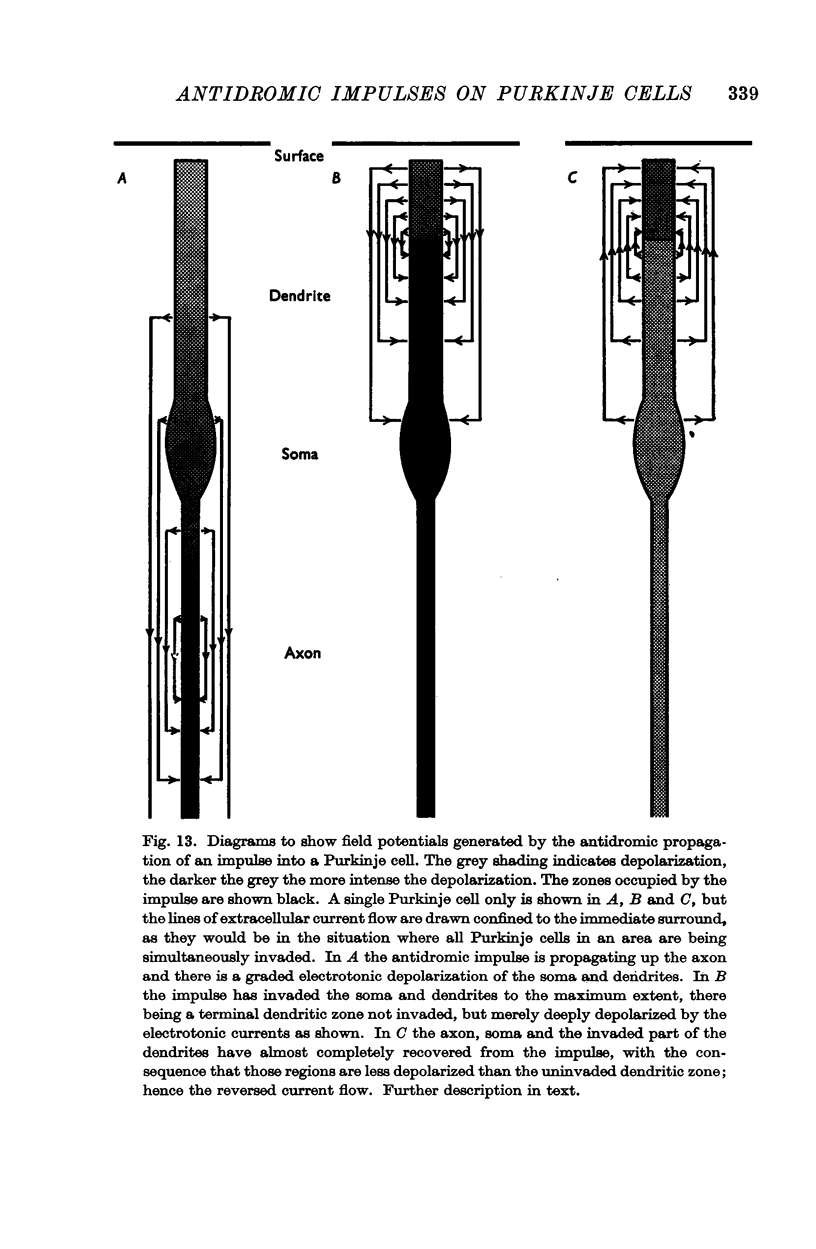
3. Laminar field analysis of the antidromic spike potentials showed that the antidromic impulses invaded at least 200 μ of the main dendrites as well as the soma, there being then a steep decrement to the surface. At superficial levels there was even an inverse antidromic spike potential. There appeared to be a synchronous invasion of the soma-dendritic complex, perhaps due to trigger zones of low threshold on the dendrites.
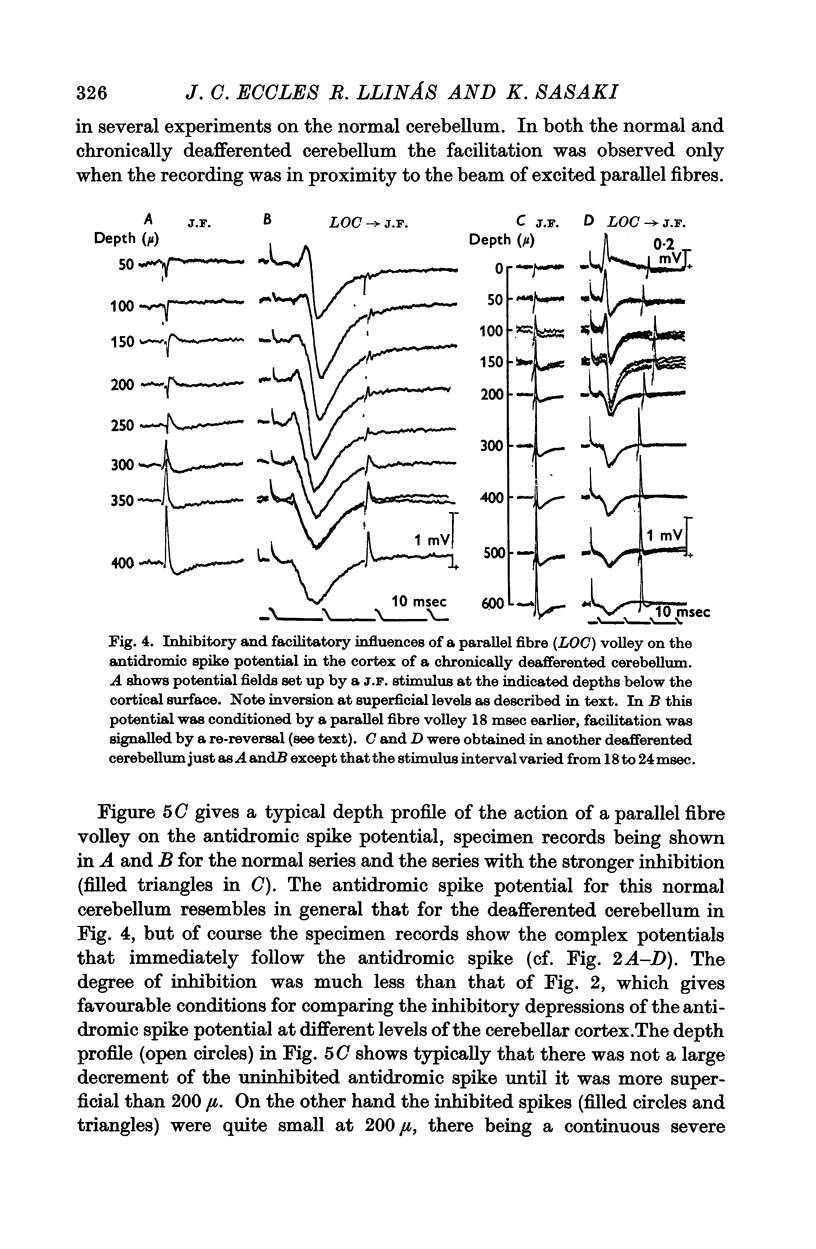
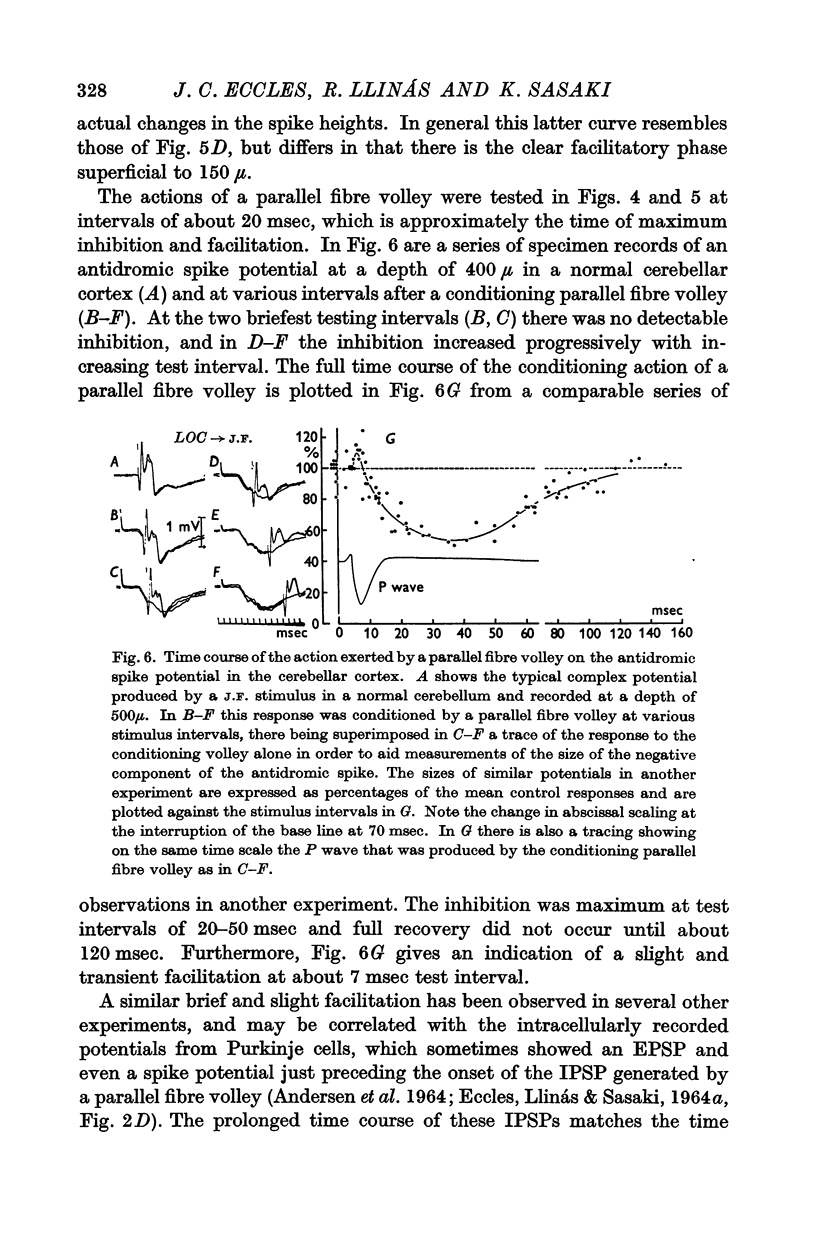
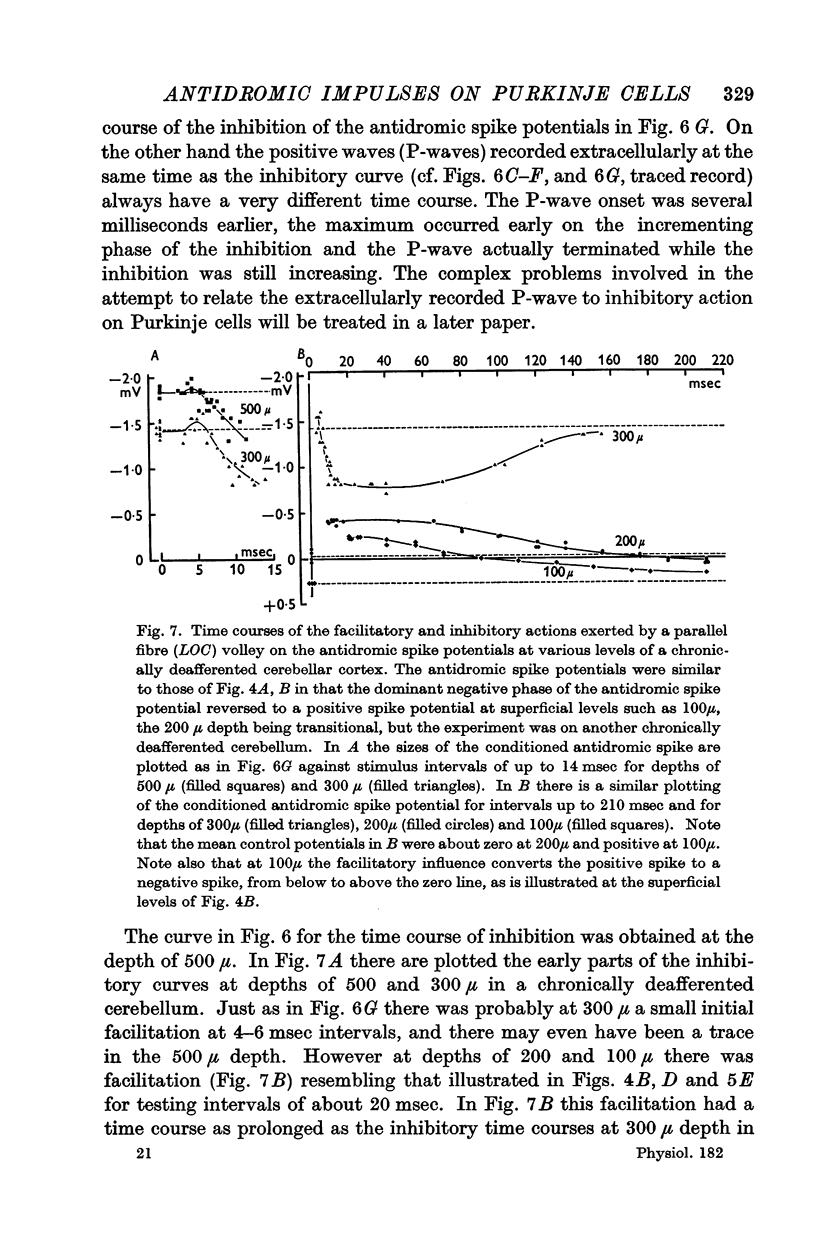
4. Antidromic soma-dendritic invasion was modified in the expected manner by a volley in the parallel fibres; there was inhibition of transmission into the soma and up the main dendrites (maximum effect at 200-300 μ depth) due to the inhibitory action of the basket and superficial stellate cells that are excited by the parallel fibres; there was facilitation of transmission in the dendrites at levels superficial to 200 μ due to the direct excitatory action of parallel fibres. Both the inhibitory and excitatory actions had a duration in excess of 100 msec.
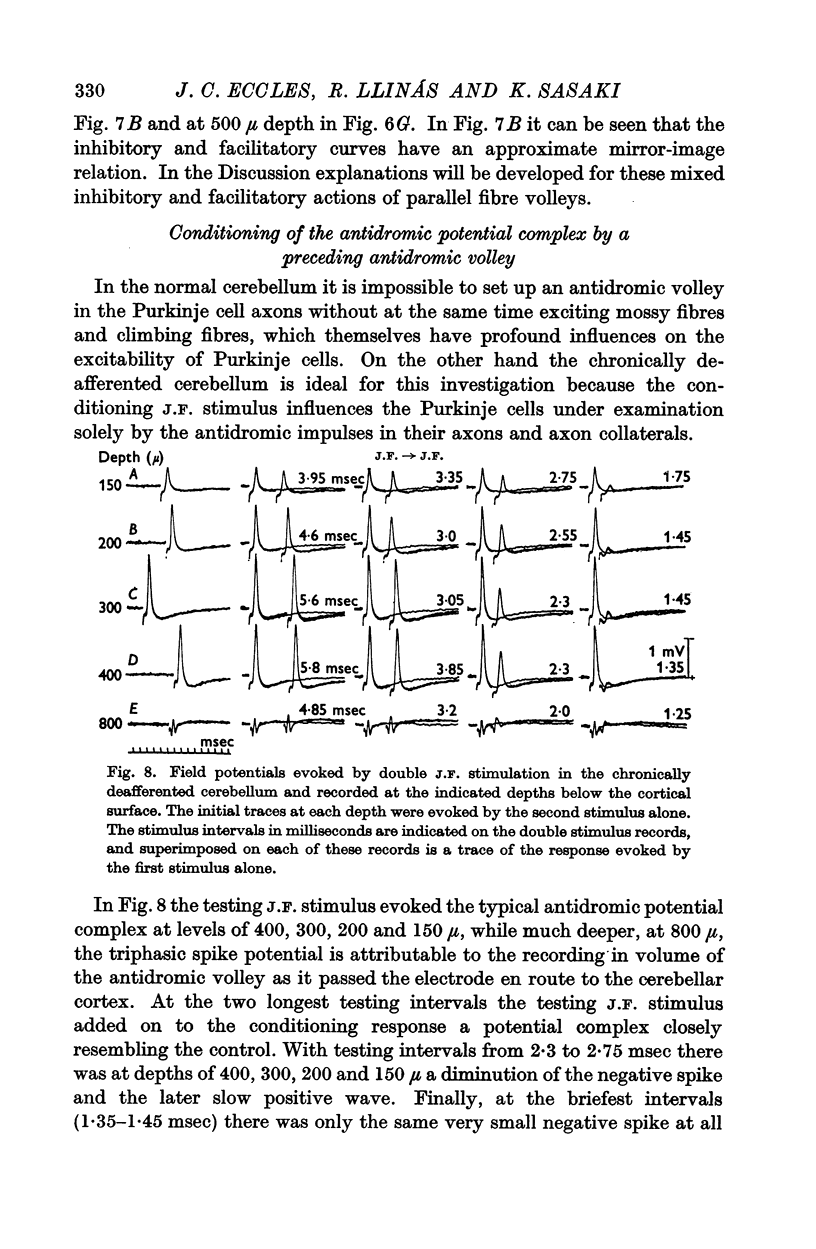
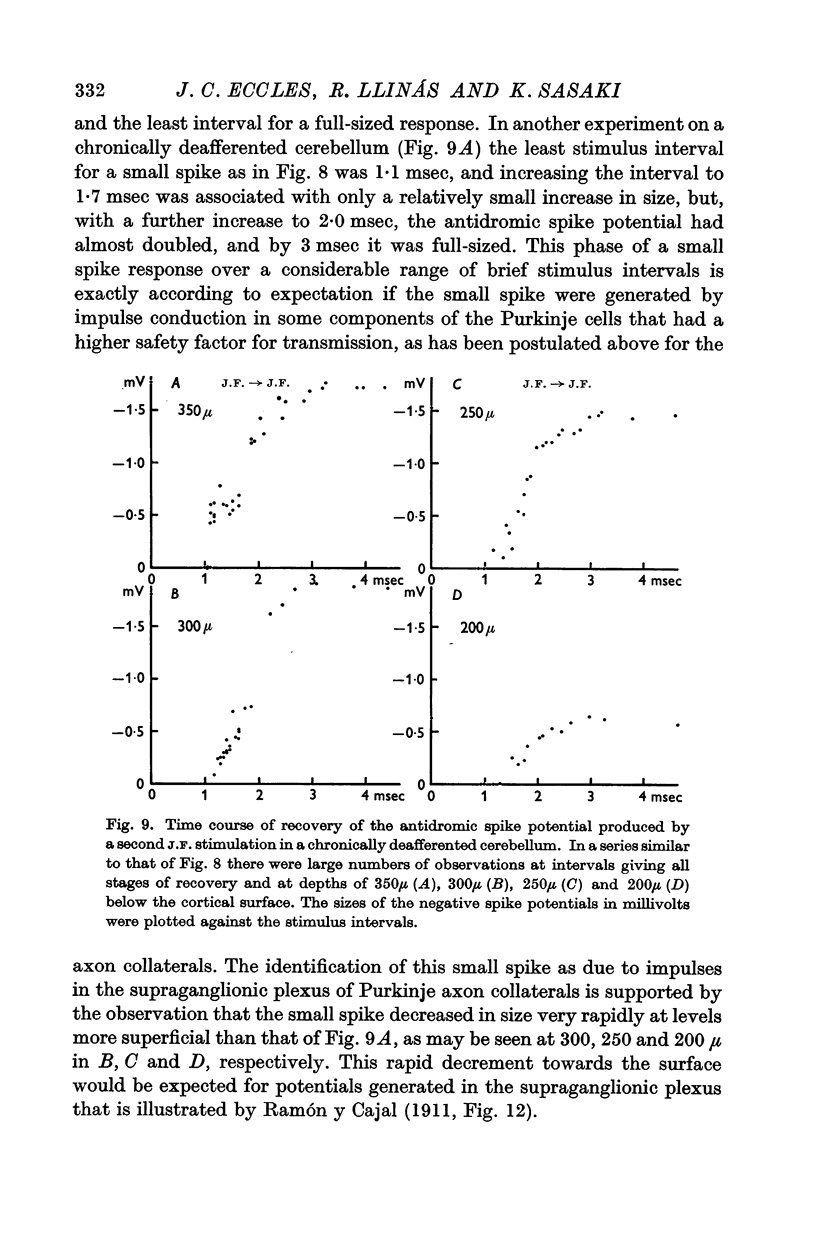
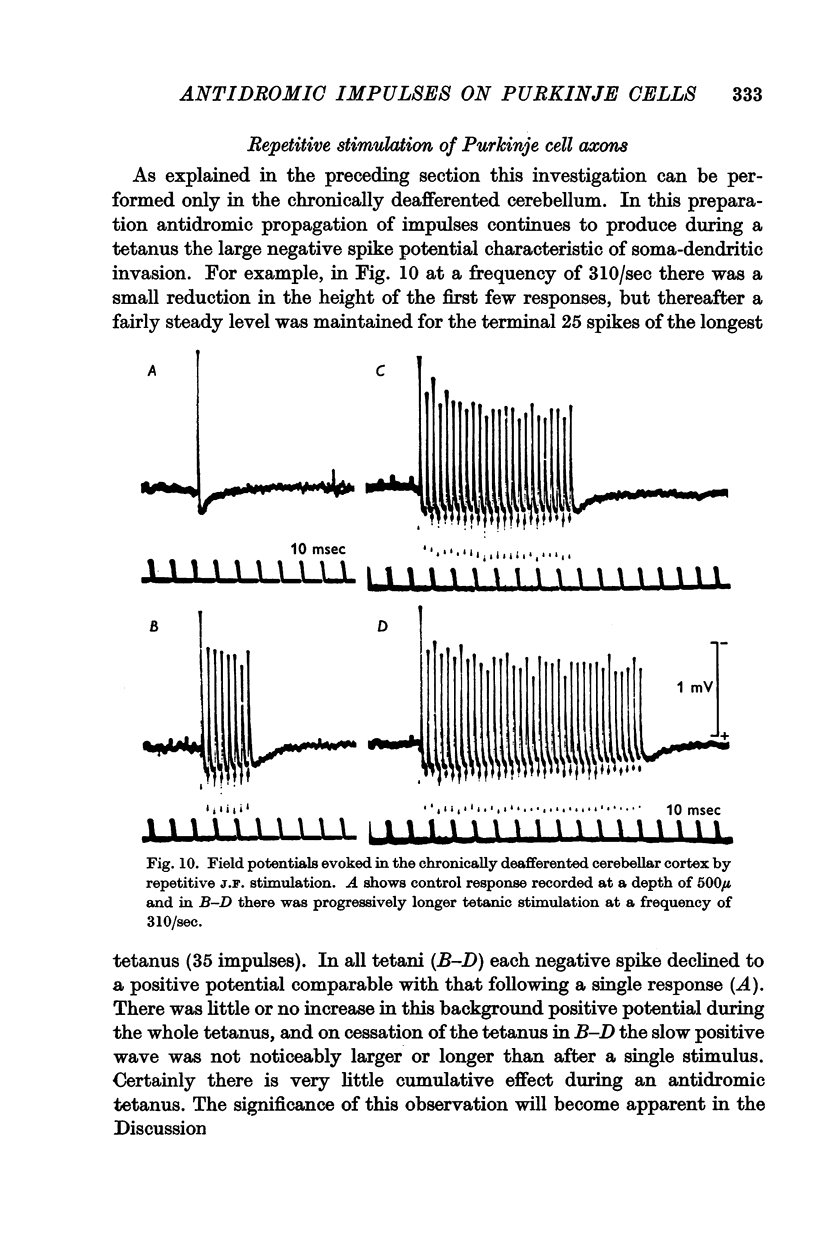
5. In the chronically deafferented cerebellum a second J.F. stimulation evoked a full size antidromic spike potential at an interval of 3 msec. There was a gradual decline in size down to intervals of about 2 msec, and at briefer intervals, to 1 msec, there was a small residual spike potential that possibly is due to transmission into the Purkinje cell axon collaterals at intervals too brief for soma-dendritic invasion. With repetitive stimulation there was a well maintained soma-dendritic invasion at a frequency as high as 300/sec.
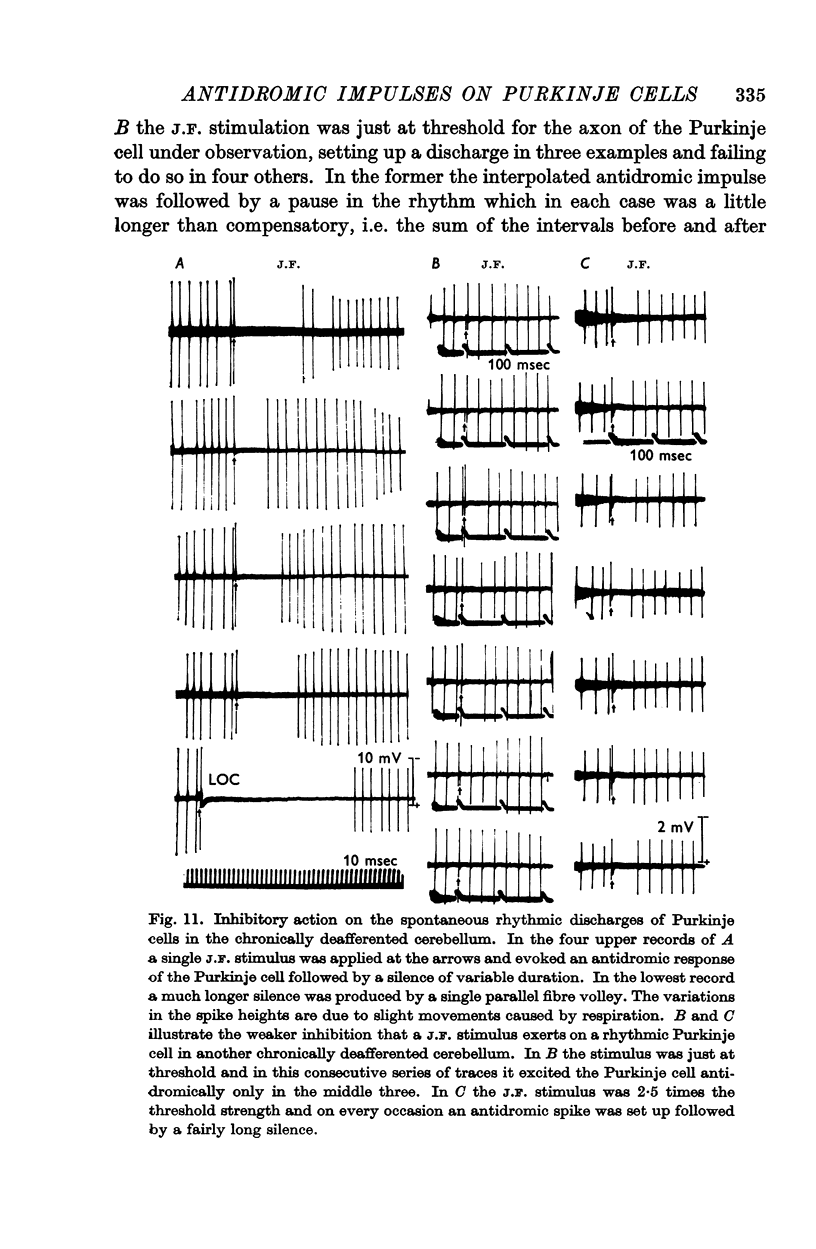
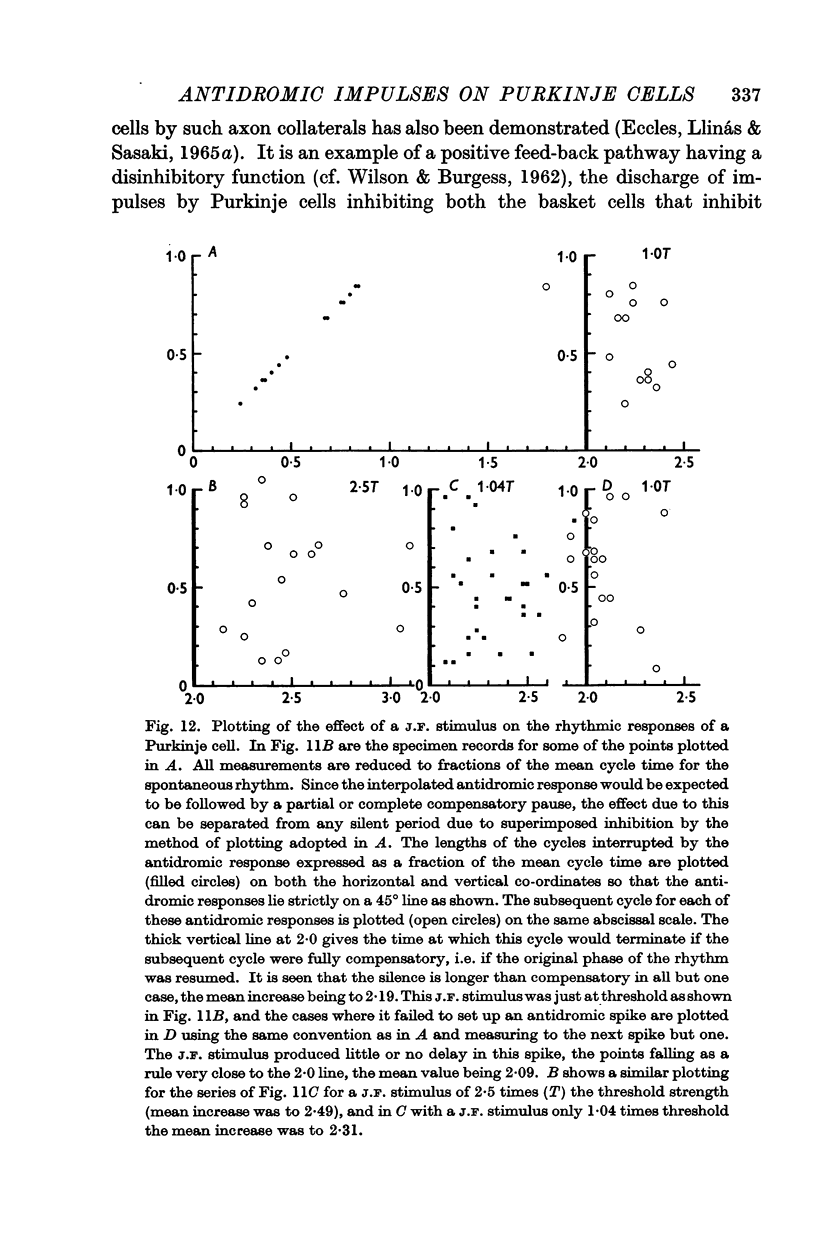
6. In the chronically deafferented cerebellum an antidromic volley in the Purkinje cell axons caused a brief inhibitory silence of rhythmically discharging Purkinje cells. It is suggested that this is a direct inhibitory action of the Purkinje axon collaterals, that parallels their direct inhibitory action that has been demonstrated by Ito and collaborators (1964) on the intracerebellar nuclei and Deiters nucleus.
7. In the chronically deafferented cerebellum an antidromic volley in the Purkinje axons produced not only the large negative spike potential indicative of antidromic soma-dendritic invasion, but also a later small and slow positive wave that appeared to be closely linked with the negative spike. It is shown how this would arise by current flow into the dendrites that had been depolarized but not excited by the initial antidromic invasion.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., VOORHOEVE P. E. POSTSYNAPTIC INHIBITION OF CEREBELLAR PURKINJE CELLS. J Neurophysiol. 1964 Nov;27:1138–1153. doi: 10.1152/jn.1964.27.6.1138. [DOI] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The electrical constants of the motoneurone membrane. J Physiol. 1959 Mar 12;145(3):505–528. doi: 10.1113/jphysiol.1959.sp006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The interpretation of spike potentials of motoneurones. J Physiol. 1957 Dec 3;139(2):198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREUTZFELDT O. D., LUX H. D., NACIMIENTO A. C. INTRACELLULAERE REIZUNG CORTICALER NERVENZELLEN. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Oct 5;281:129–151. [PubMed] [Google Scholar]
- ECCLES J., LLINAS R., SASAKI K. EXCITATION OF CEREBELLAR PURKINJE CELLS BY THE CLIMBING FIBRES. Nature. 1964 Jul 18;203:245–246. doi: 10.1038/203245a0. [DOI] [PubMed] [Google Scholar]
- ECCLES J., LLINAS R., SASAKI K. GOLGI CELL INHIBITION IN THE CEREBELLAR CORTEX. Nature. 1964 Dec 26;204:1265–1266. doi: 10.1038/2041265a0. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX C. A., BARNARD J. W. A quantitative study of the Purkinje cell dendritic branchlets and their relationship to afferent fibres. J Anat. 1957 Jul;91(3):299–313. [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Effects on Purkinje cells of surface stimulation of the cerebellum. J Physiol. 1957 Jan 23;135(1):73–92. doi: 10.1113/jphysiol.1957.sp005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO M., OSHIMA T. THE EXTRUSION OF SODIUM FROM CAT SPINAL MOTONEURONS. Proc R Soc Lond B Biol Sci. 1964 Nov 17;161:109–131. doi: 10.1098/rspb.1964.0083. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K. Monosynaptic inhibition of the intracerebellar nuclei induced rom the cerebellar cortex. Experientia. 1964 Oct 15;20(10):575–576. doi: 10.1007/BF02150304. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M. The cerebellar-evoked monosynaptic inhibition of Deiters' neurones. Experientia. 1964 Sep 15;20(9):515–516. doi: 10.1007/BF02154085. [DOI] [PubMed] [Google Scholar]
- TERZUOLO C. A., ARAKI T. An analysis of intra- versus extracellular potential changes associated with activity of single spinal motoneurons. Ann N Y Acad Sci. 1961 Sep 6;94:547–558. doi: 10.1111/j.1749-6632.1961.tb35558.x. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., BURGESS P. R. Disinhibition in the cat spinal cord. J Neurophysiol. 1962 May;25:392–404. doi: 10.1152/jn.1962.25.3.392. [DOI] [PubMed] [Google Scholar]


