Abstract
1. Membrane potential changes of smooth muscle cells were recorded during stimulation of the intramural inhibitory nerves to the taenia coli.
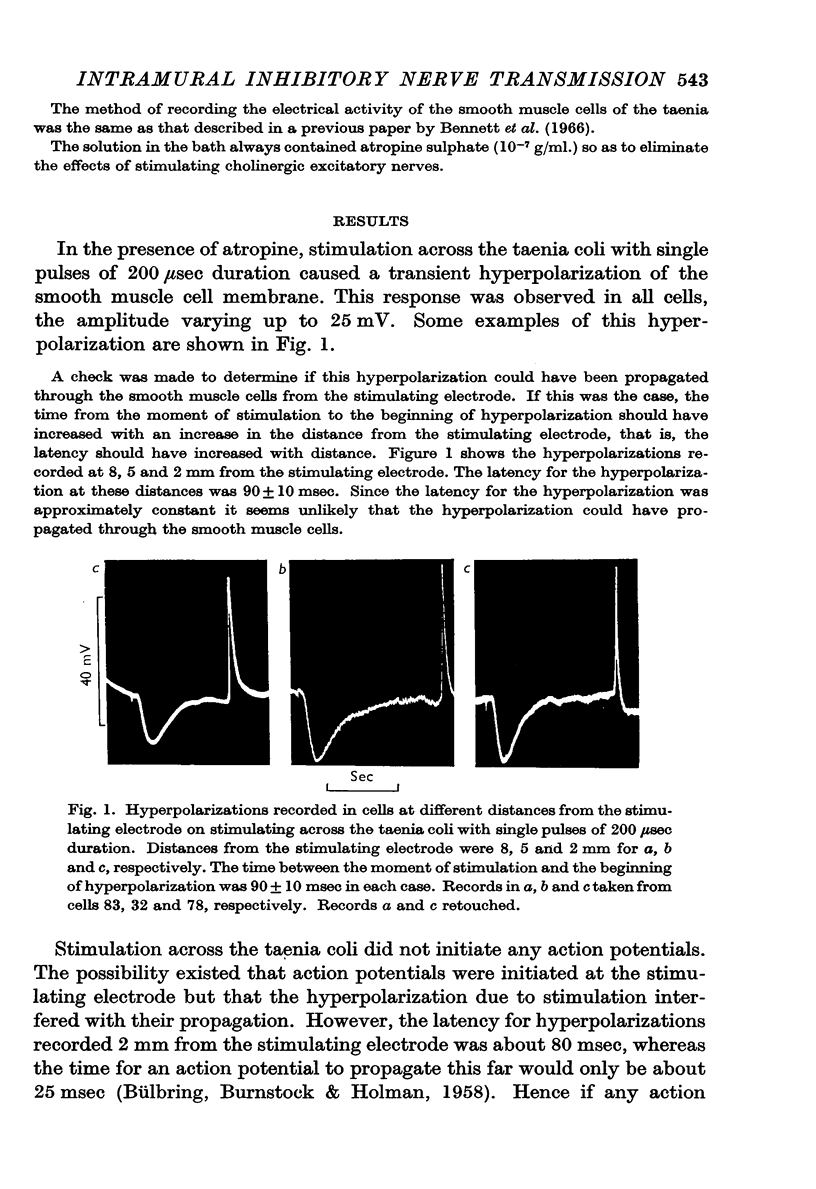
2. Stimulation across the taenia coli with single pulses of 200 μsec duration excites the intramural nerves and not the muscle directly.
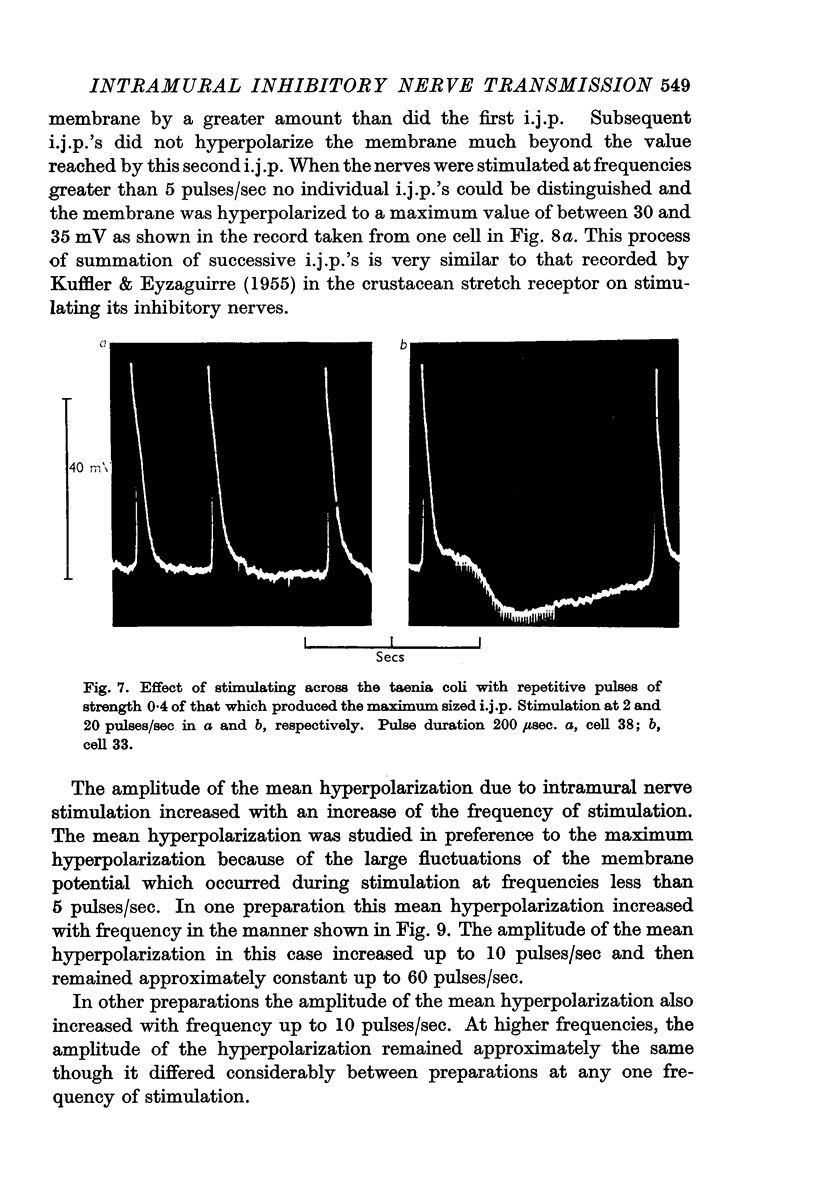
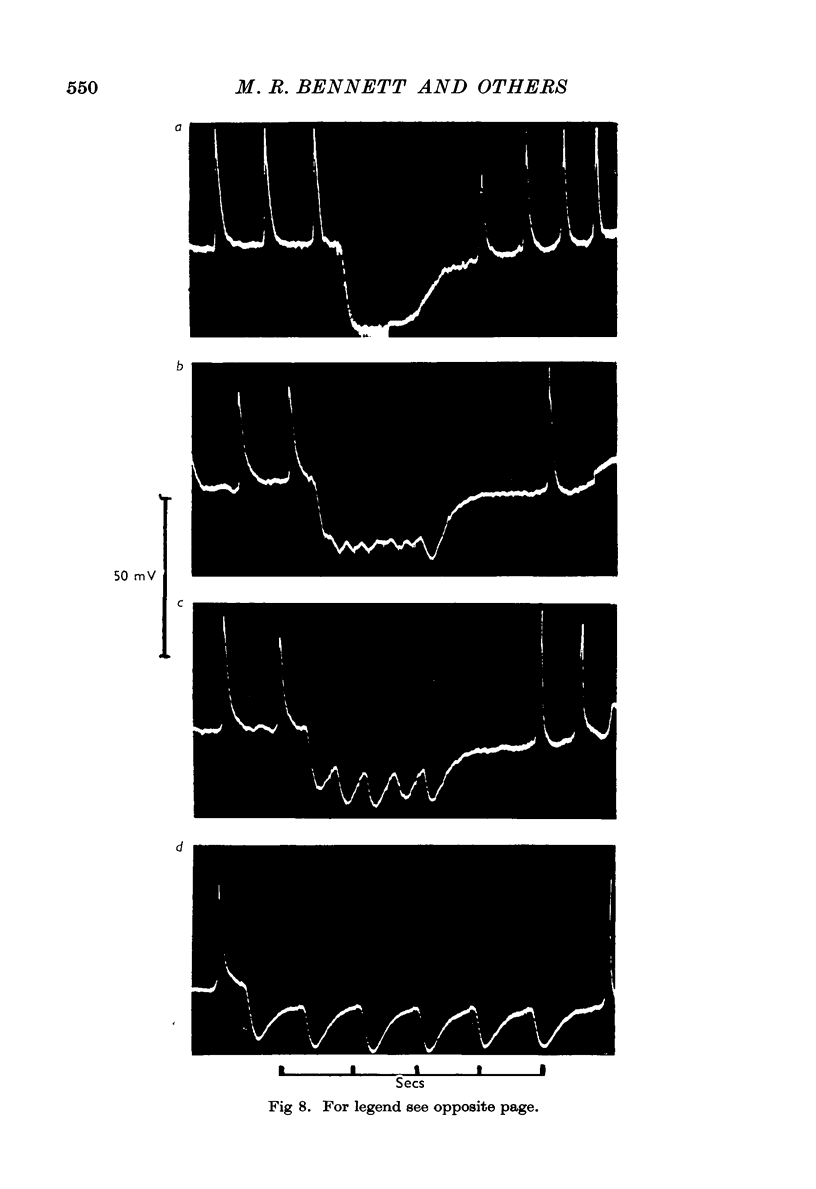
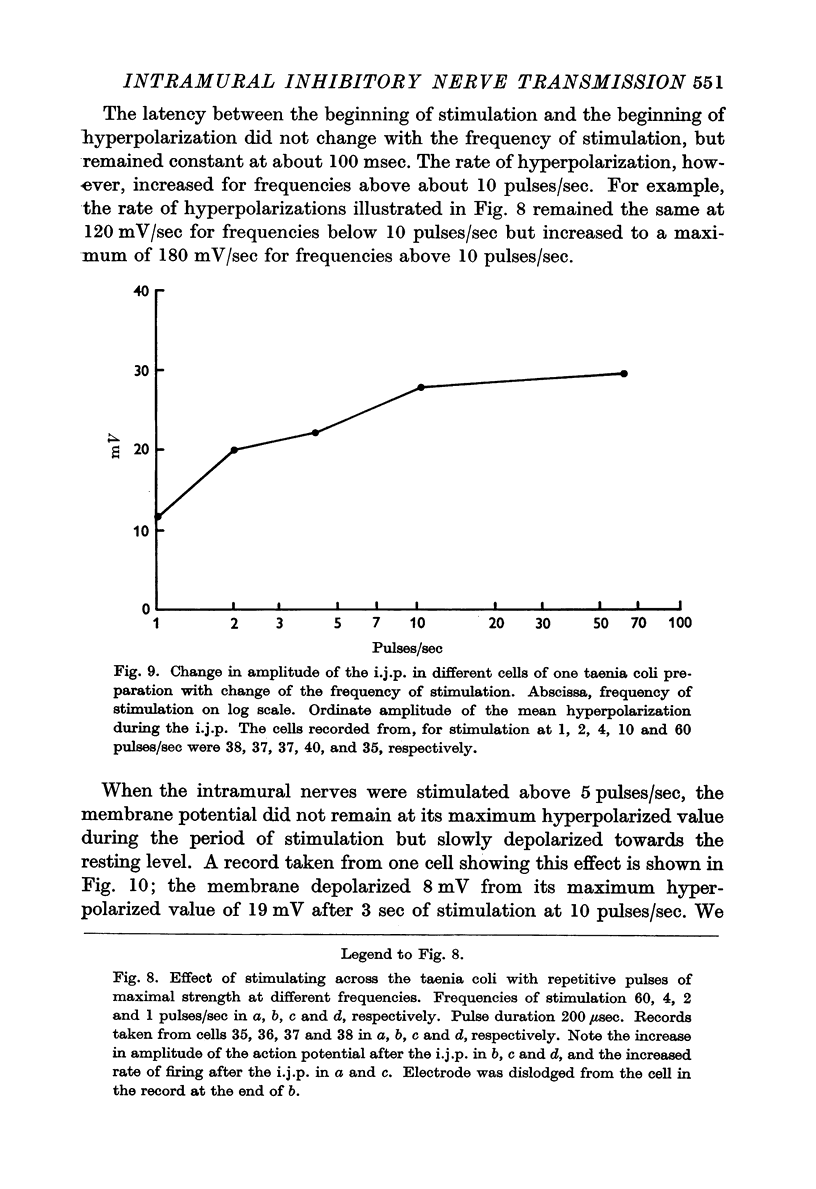
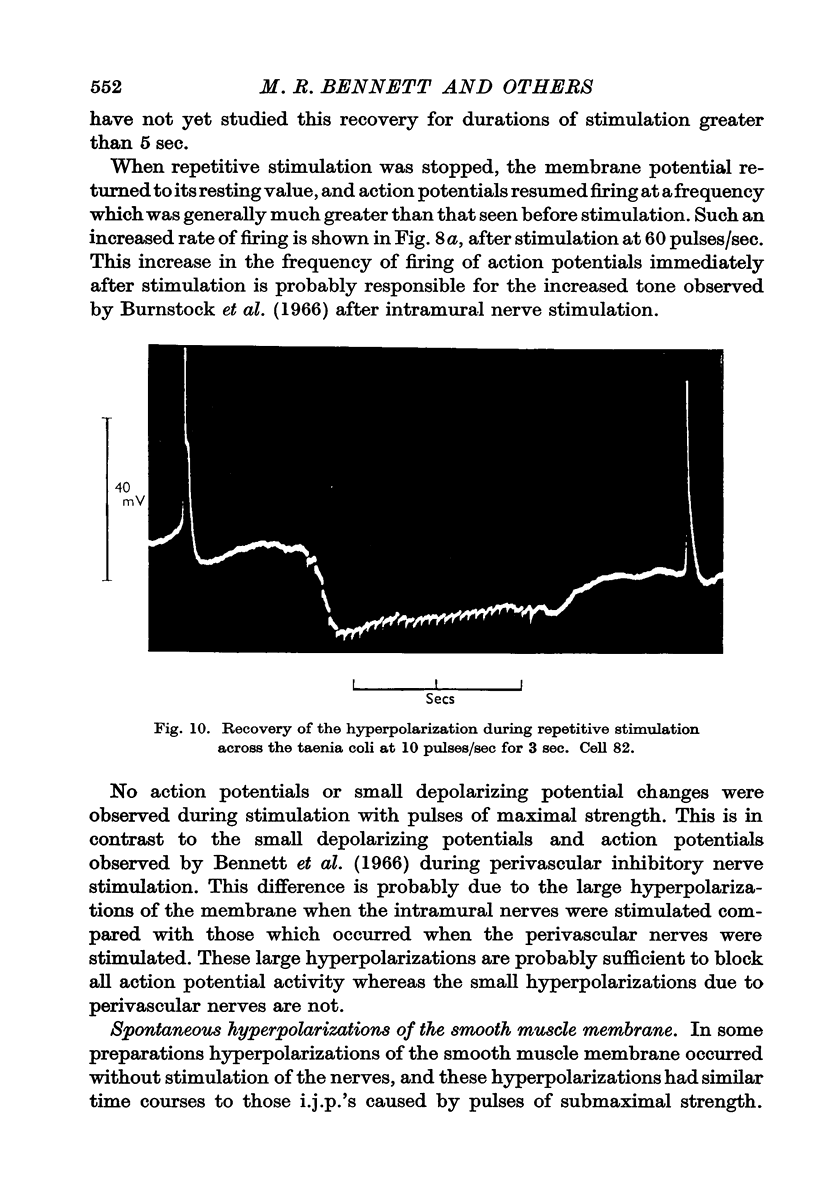
3. The membrane potential changes due to stimulation of the intramural inhibitory nerves were different from those produced by perivascular inhibitory nerve stimulation in the following ways: hyperpolarizations (i.j.p.'s) of up to 25 mV were produced in response to single pulses; the latency, i.e. the time taken for the membrane to hyperpolarize after a stimulus of maximal strength, was as short as 80 msec; when the nerves were stimulated repetitively the membrane was hyperpolarized by up to 35 mV and all spontaneous activity was abolished; the mean hyperpolarization due to repetitive stimulation increased with the frequency of stimulation up to 10 pulses/sec and then remained constant; the hyperpolarization due to stimulation at frequencies greater than 5 pulses/sec was not maintained but decreased after 3-5 sec of stimulation; and finally when stimulation had ceased action potentials commenced firing at frequencies greater than normal.
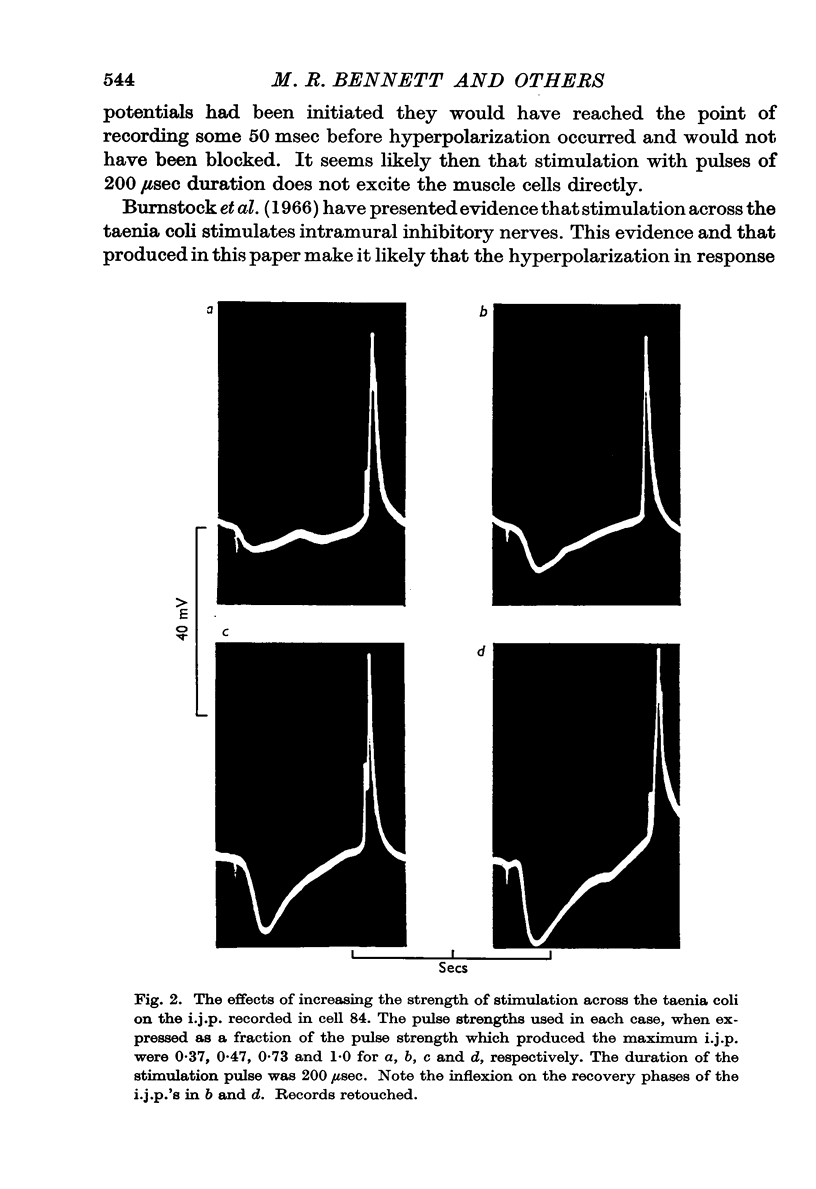
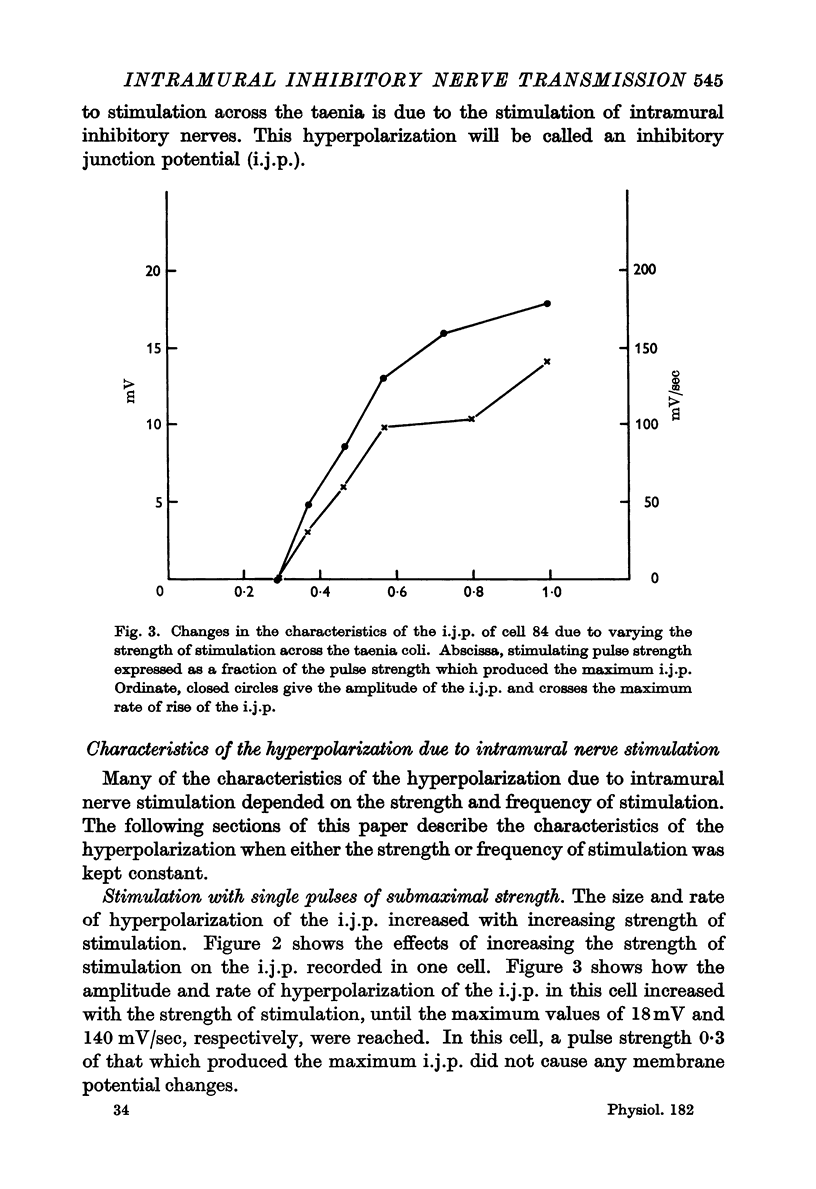
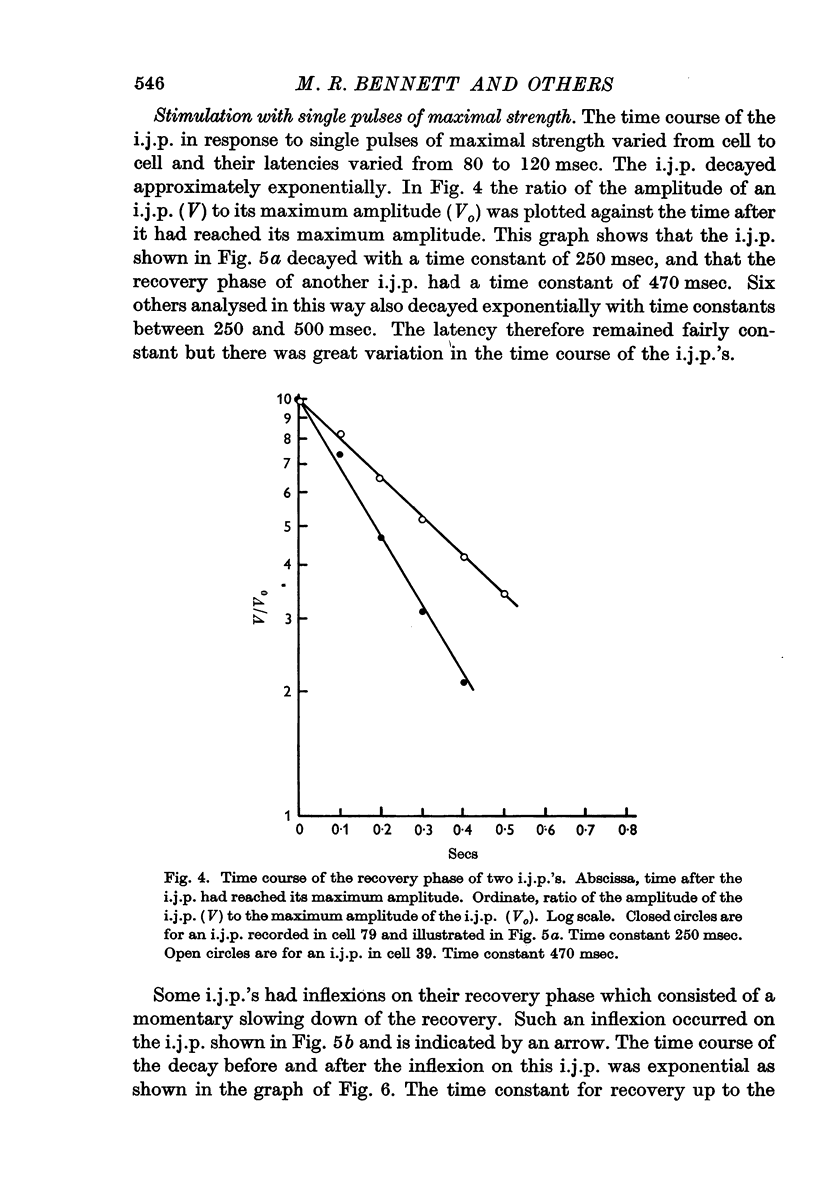
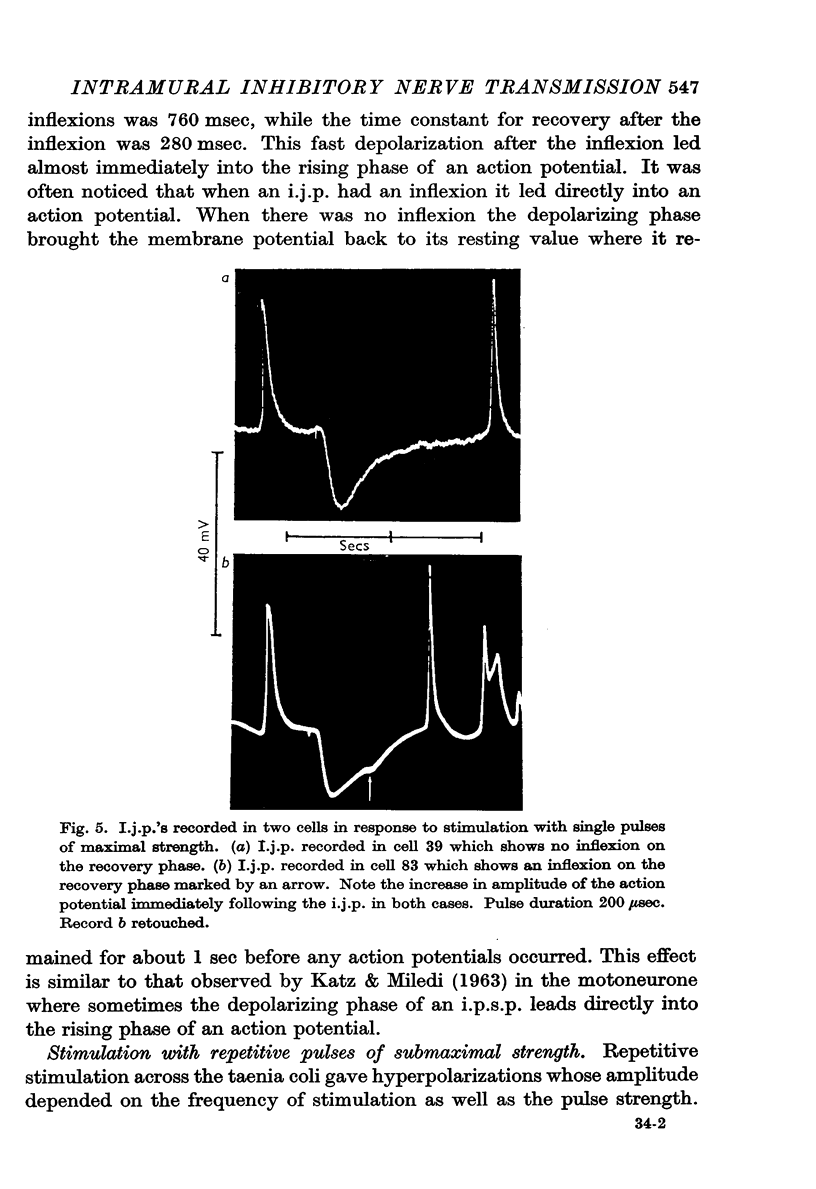
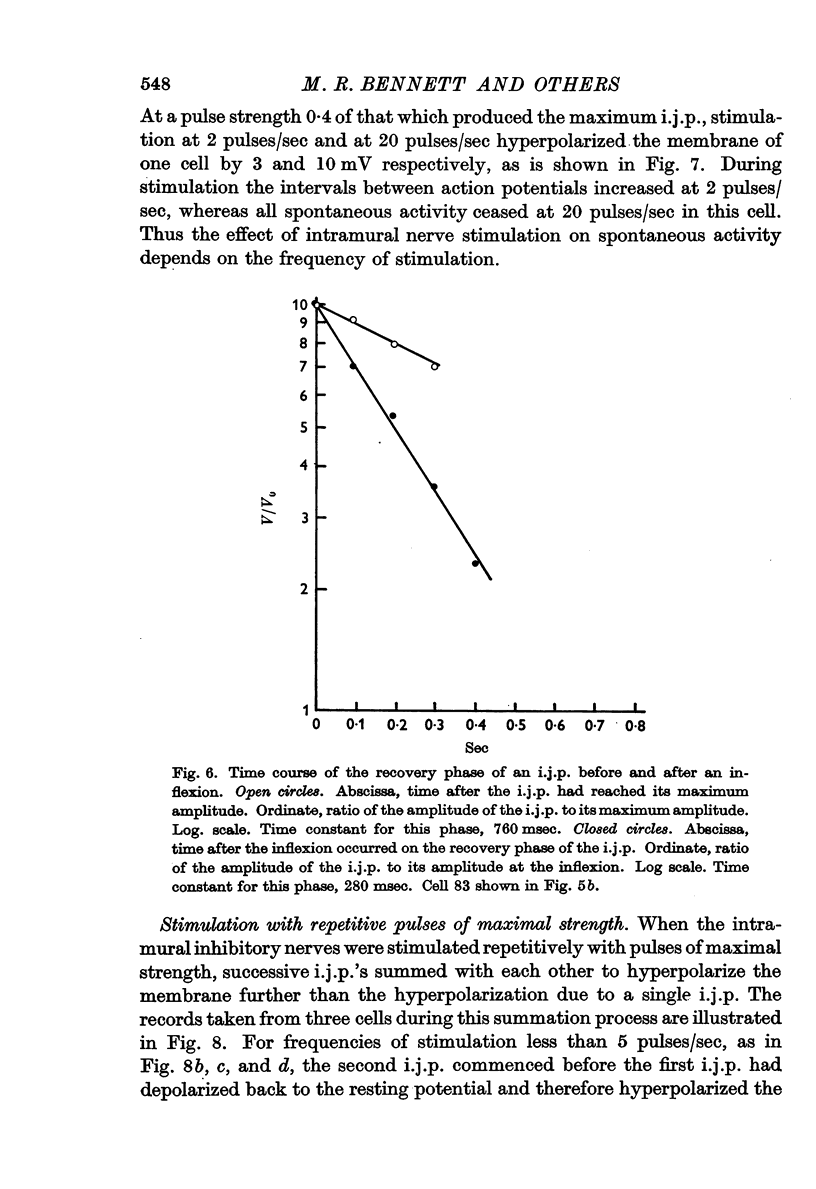
4. The amplitude and rate of hyperpolarization of the i.j.p. increased with increasing strength of stimulation until a maximum amplitude and rate of hyperpolarization was reached. The recovery or depolarizing phase of the i.j.p. was exponential with a time constant which varied from about 250 msec to 500 msec and could not therefore be due to the discharge of the membrane capacitance. In some cases there was an inflexion on this depolarizing phase and in these cases recovery led directly into an action potential.
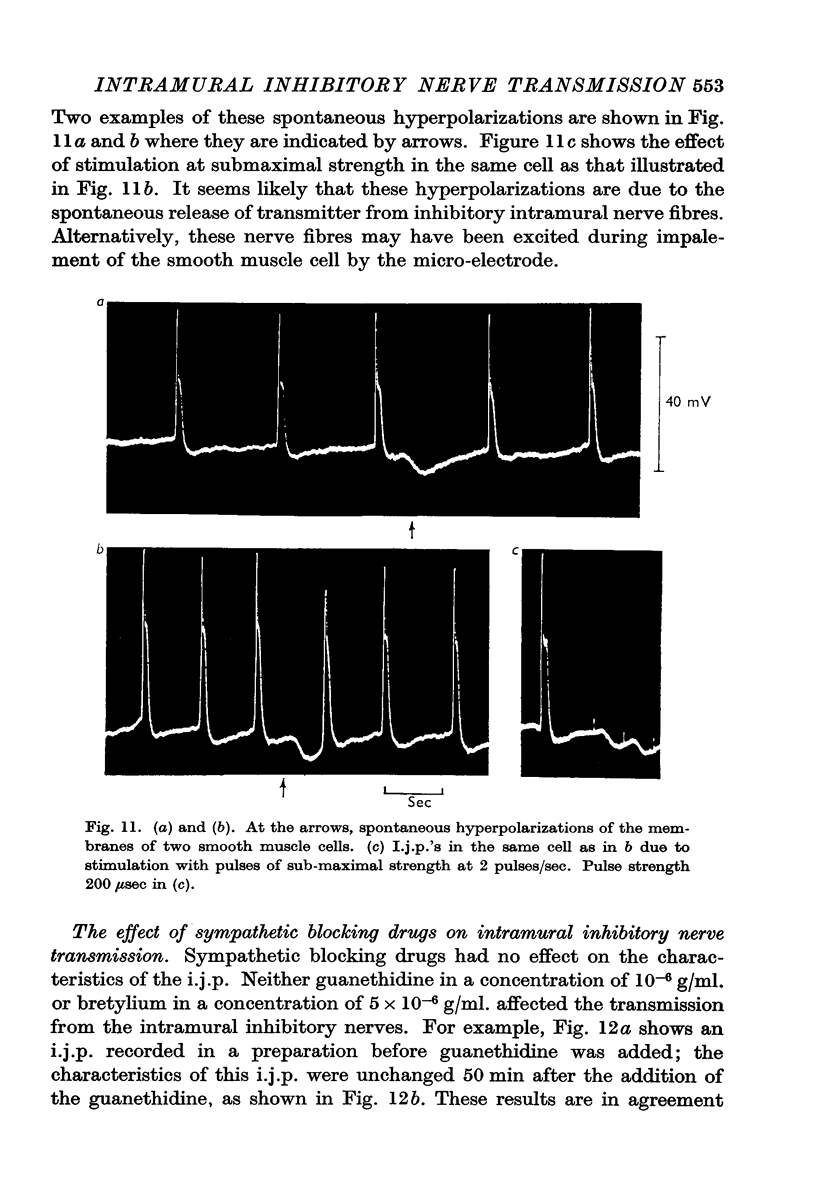
5. Spontaneous hyperpolarizations of the membrane were seen in some cells, and these hyperpolarizations were similar to those recorded on submaximal stimulation of the intramural nerves.
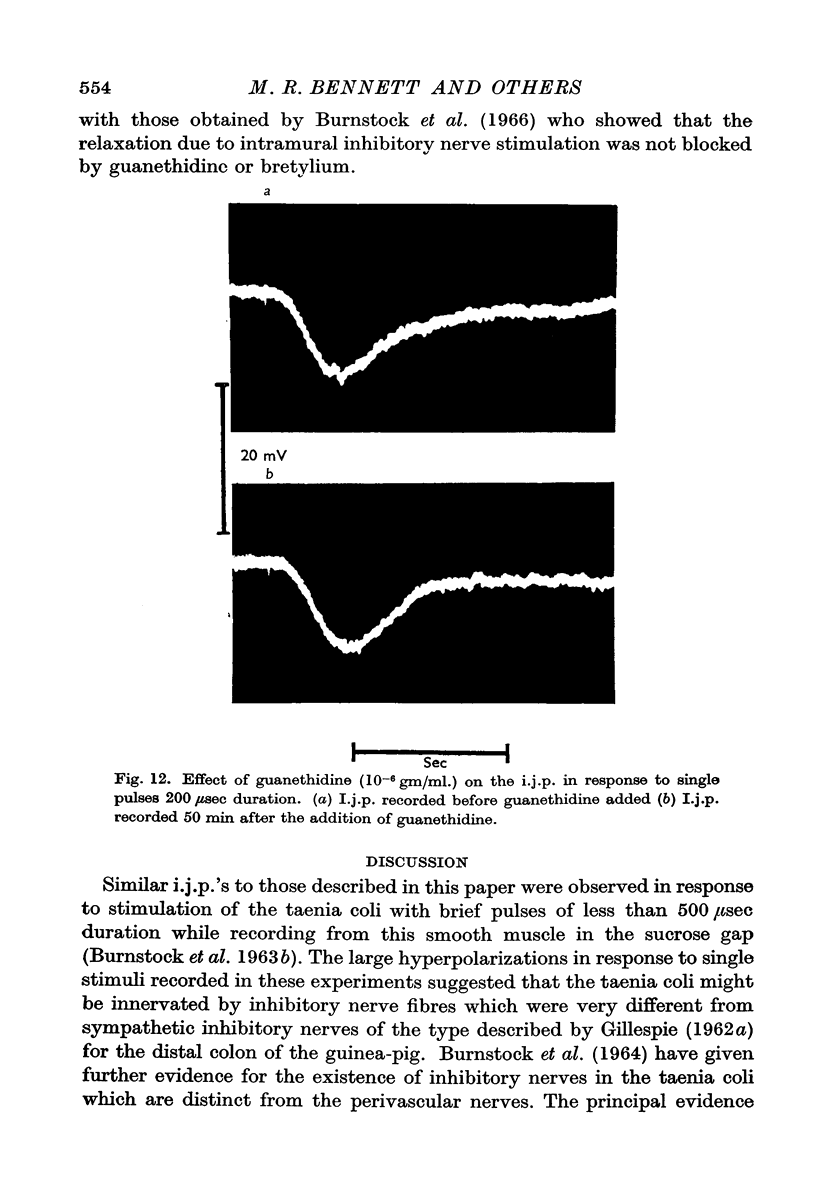
6. There were no changes in the characteristics of the i.j.p. in the presence of guanethidine or bretylium.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963 May 11;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- ARAKI T., TERZUOLO C. A. Membrane currents in spinal motoneurons associated with the action potential and synaptic activity. J Neurophysiol. 1962 Nov;25:772–789. doi: 10.1152/jn.1962.25.6.772. [DOI] [PubMed] [Google Scholar]
- BUELBRING E., KURIYAMA H. THE EFFECT OF ADRENALINE ON THE SMOOTH MUSCLE OF GUINEA-PIG TAENIA COLI IN RELATION TO THE DEGREE OF STRETCH. J Physiol. 1963 Nov;169:198–212. doi: 10.1113/jphysiol.1963.sp007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., BURNSTOCK G., HOLMAN M. E. Excitation and conduction in the smooth muscle of the isolated taenia coli of the guinea-pig. J Physiol. 1958 Aug 6;142(3):420–437. doi: 10.1113/jphysiol.1958.sp006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Measurements of oxygen consumption in smooth muscle. J Physiol. 1953 Oct;122(1):111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Membrane potentials of smooth muscle fibres of the taenia coli of the guinea-pig. J Physiol. 1954 Aug 27;125(2):302–315. doi: 10.1113/jphysiol.1954.sp005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., BENNETT M., HOLMAN M. E. INHIBITION OF THE SMOOTH MUSCLE ON THE TAENIA COLI. Nature. 1963 Nov 9;200:581–582. doi: 10.1038/200581a0. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., BENNETT M., HOLMAN M. E. INNERVATION OF THE GUINEA-PIG TAENIA COLI: ARE THERE INTRINSIC INHIBITORY NERVES WHICH ARE DISTINCT FROM SYMPATHETIC NERVES? Int J Neuropharmacol. 1964 May;3:163–166. doi: 10.1016/0028-3908(64)90003-6. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. E. Transmission from perivascular inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):527–540. doi: 10.1113/jphysiol.1966.sp007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Rand M. J. The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol. 1966 Feb;182(3):504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C. Membrane time constants of cat motoneurons and time courses of synptic action. Exp Neurol. 1961 Jul;4:1–22. doi: 10.1016/0014-4886(61)90074-7. [DOI] [PubMed] [Google Scholar]
- GASSER H. S. Unmedullated fibers originating in dorsal root ganglia. J Gen Physiol. 1950 Jul 20;33(6):651–690. doi: 10.1085/jgp.33.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. S. Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic-nerve stimulation. J Physiol. 1962 Jun;162:54–75. doi: 10.1113/jphysiol.1962.sp006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. S. The electrical and mechanical responses of intestinal smooth muscle cells to stimulation of their extrinsic parasympathetic nerves. J Physiol. 1962 Jun;162:76–92. doi: 10.1113/jphysiol.1962.sp006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., WATANABE A., SAITO N. Potential changes in syncytial neurons of lobster cardiac ganglion. J Neurophysiol. 1959 Sep;22:554–572. doi: 10.1152/jn.1959.22.5.554. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. A STUDY OF SPONTANEOUS MINIATURE POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1963 Sep;168:389–422. doi: 10.1113/jphysiol.1963.sp007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., EYZAGUIRRE C. Synaptic inhibition in an isolated nerve cell. J Gen Physiol. 1955 Sep 20;39(1):155–184. doi: 10.1085/jgp.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- SPEDEN R. N. ELECTRICAL ACTIVITY OF SINGLE SMOOTH MUSCLE CELLS OF THE MESENTERIC ARTERY PRODUCED BY SPLANCHNIC NERVE STIMULATION IN THE GUINEA PIG. Nature. 1964 Apr 11;202:193–194. doi: 10.1038/202193a0. [DOI] [PubMed] [Google Scholar]
- URSILLO R. C. Electrical activity of the isolated nerve-urinary bladder strip preparation of the rabbit. Am J Physiol. 1961 Sep;201:408–412. doi: 10.1152/ajplegacy.1961.201.3.408. [DOI] [PubMed] [Google Scholar]


