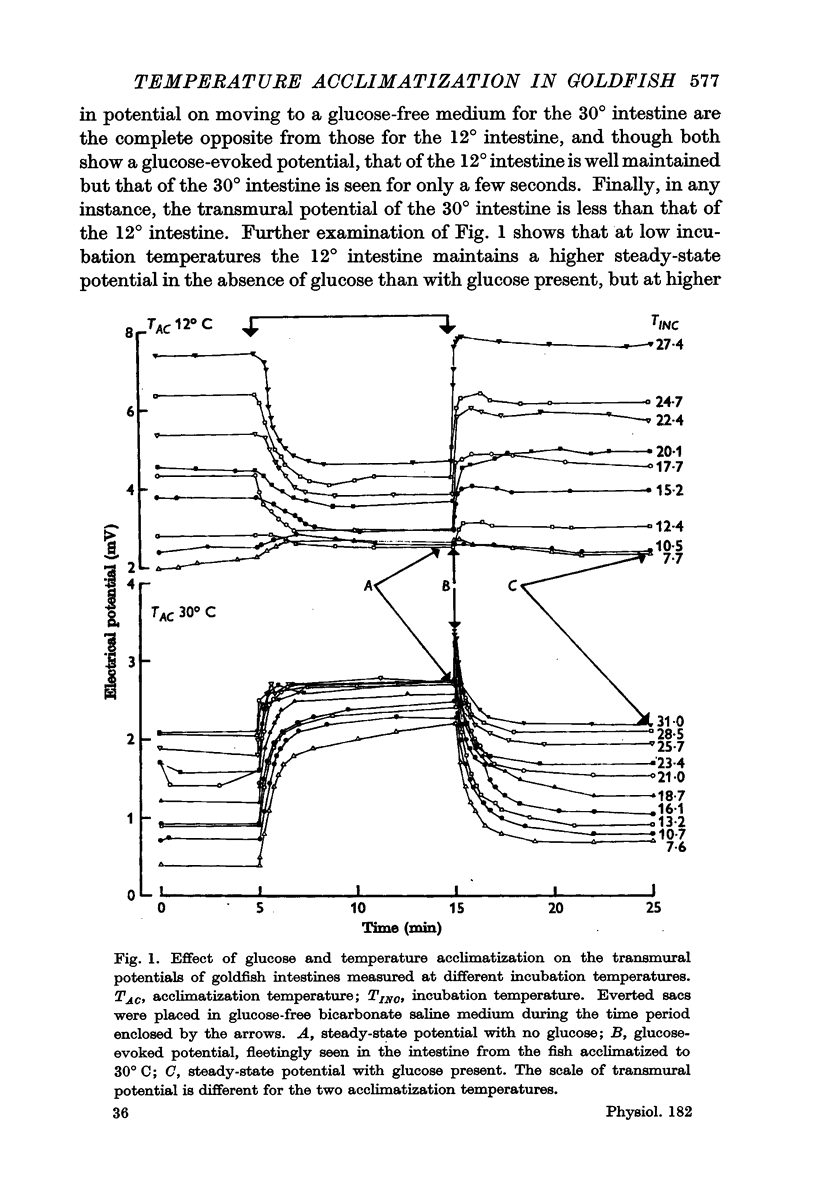
Abstract
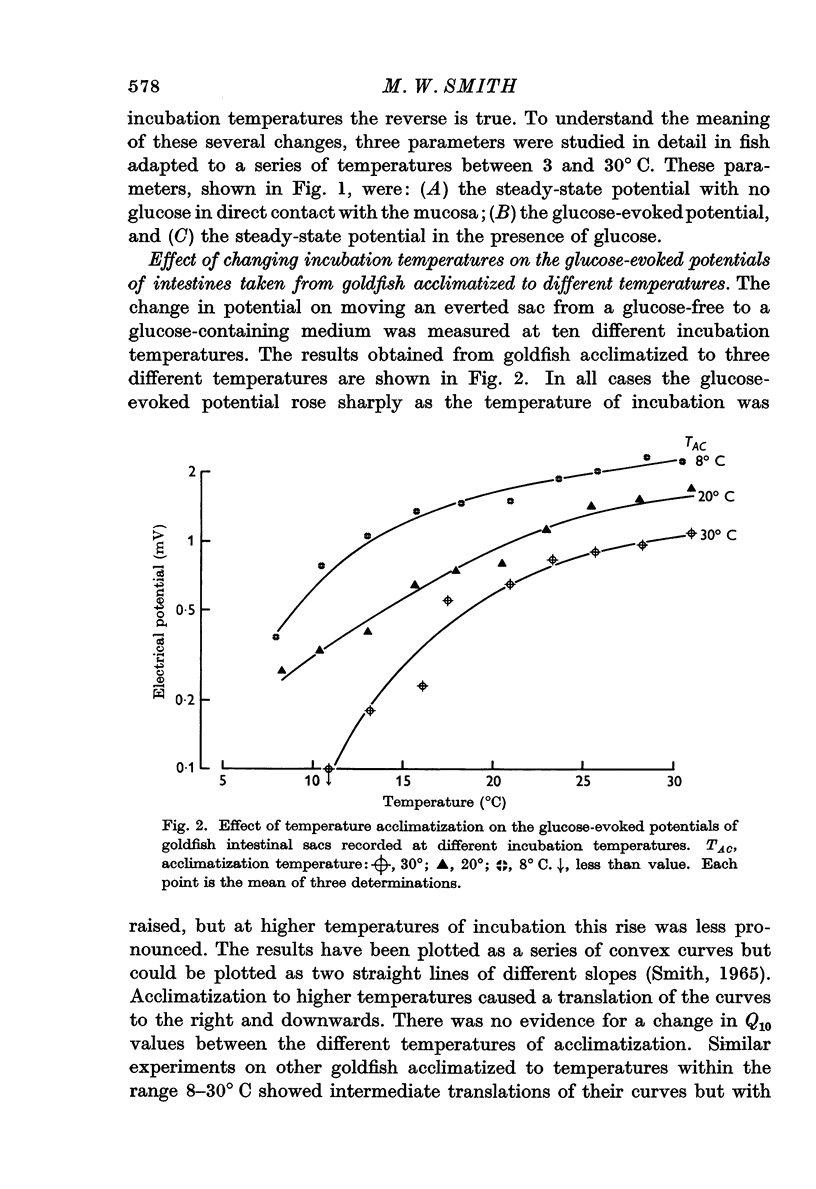
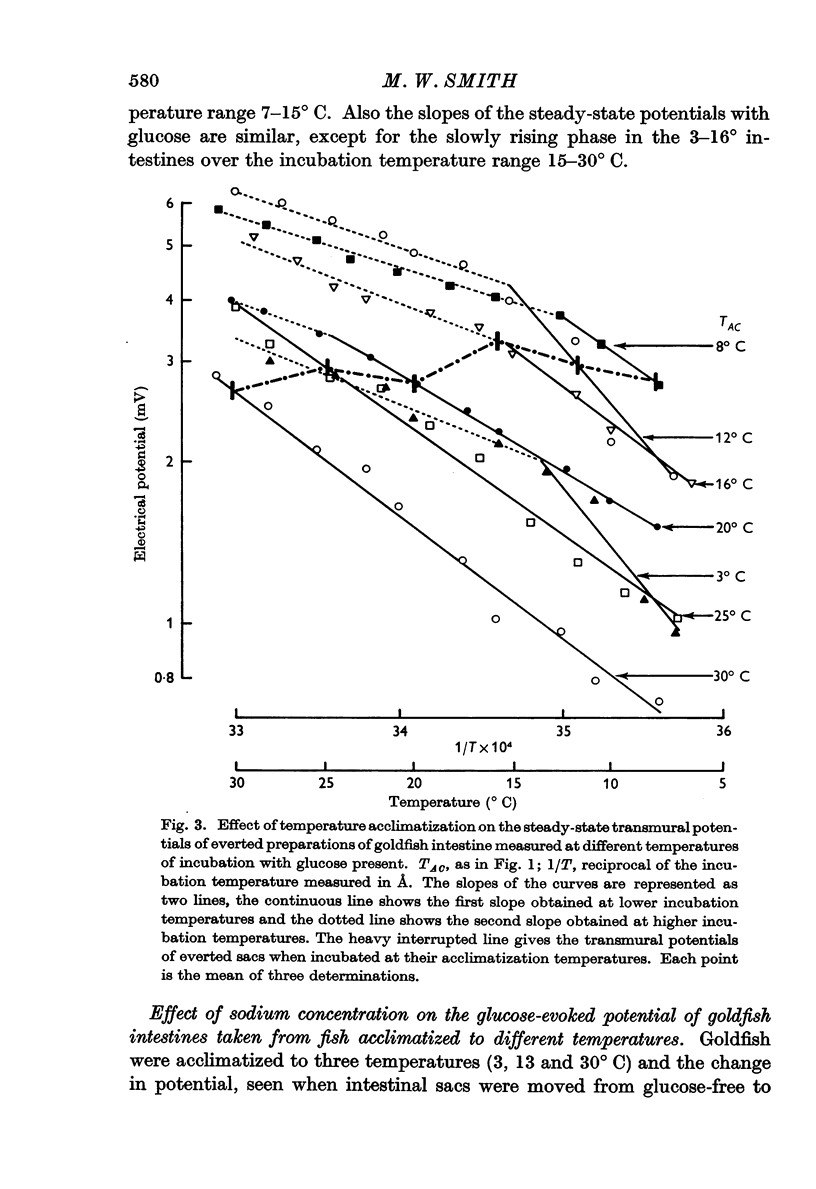
1. Transmural potentials across goldfish intestines in vitro were found to depend on the acclimatization temperature of the fish. At any incubation temperature potentials were lower in fish kept previously at a high temperature, and if the transmural potentials were recorded at incubation temperatures equal to the previous acclimatization temperatures the values remained constant from 8 to 30° C. The glucose-evoked potential was also reduced by previous acclimatization of the fish to a high temperature.
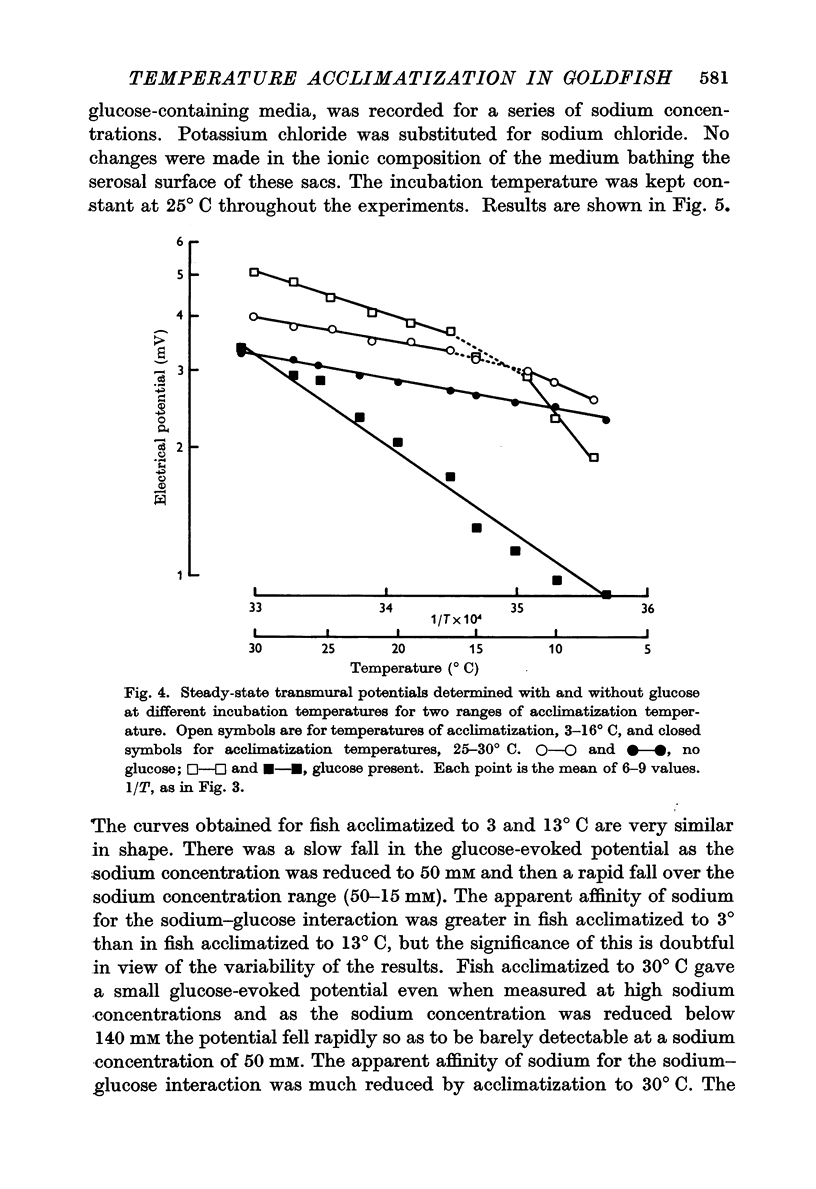
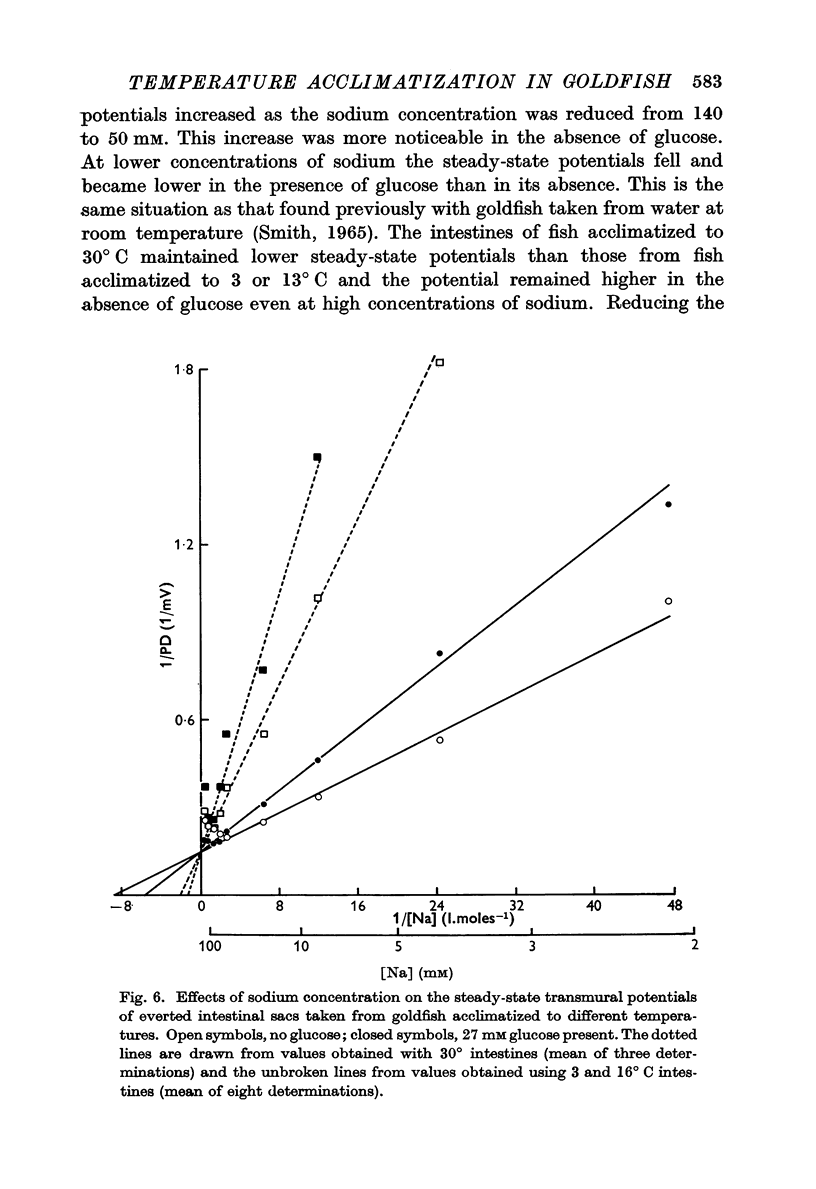
2. As the sodium concentration was reduced the steady transmural potential increased and later fell in proportion to the low external sodium concentration, but the glucose-evoked potential fell as soon as the sodium concentration was reduced below 140 mM. Similar changes were seen with intestines taken from fish acclimatized to a high temperature but both the steady-state potential and the transitory glucose-evoked potential were more dependent on the external sodium concentration.
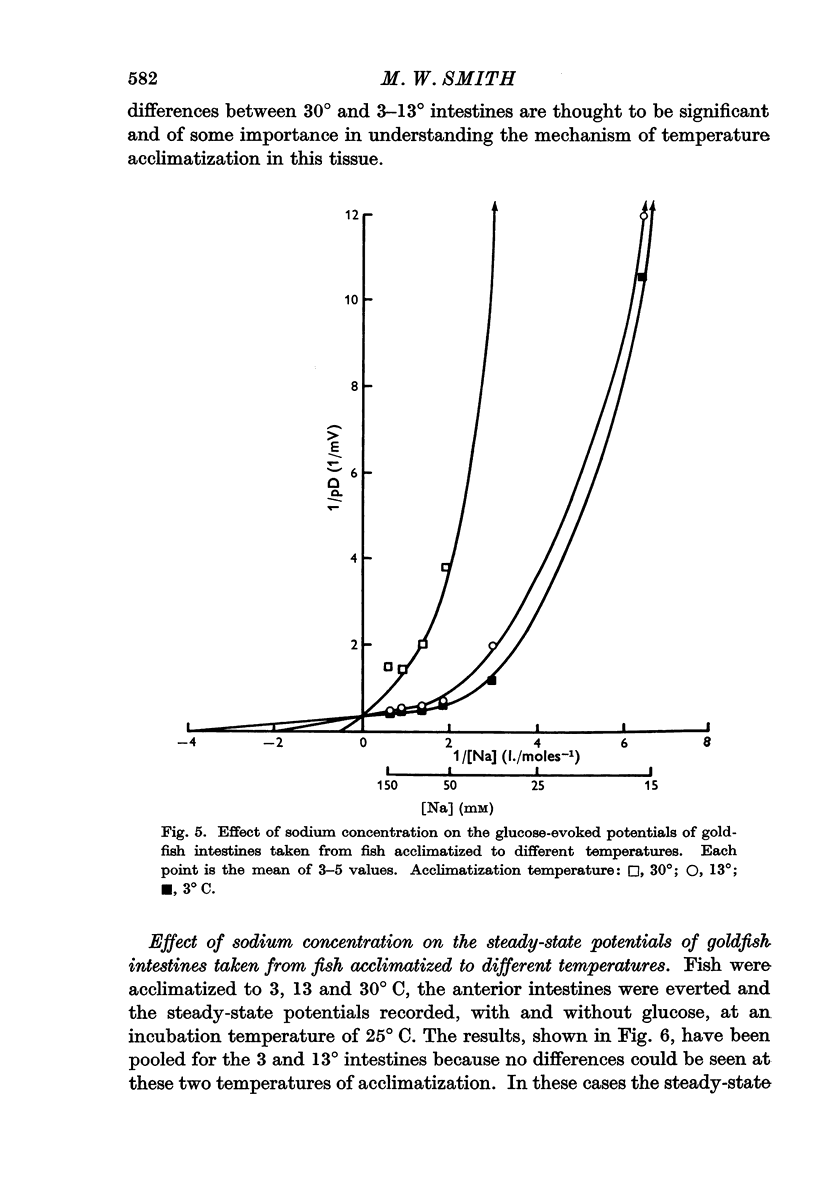
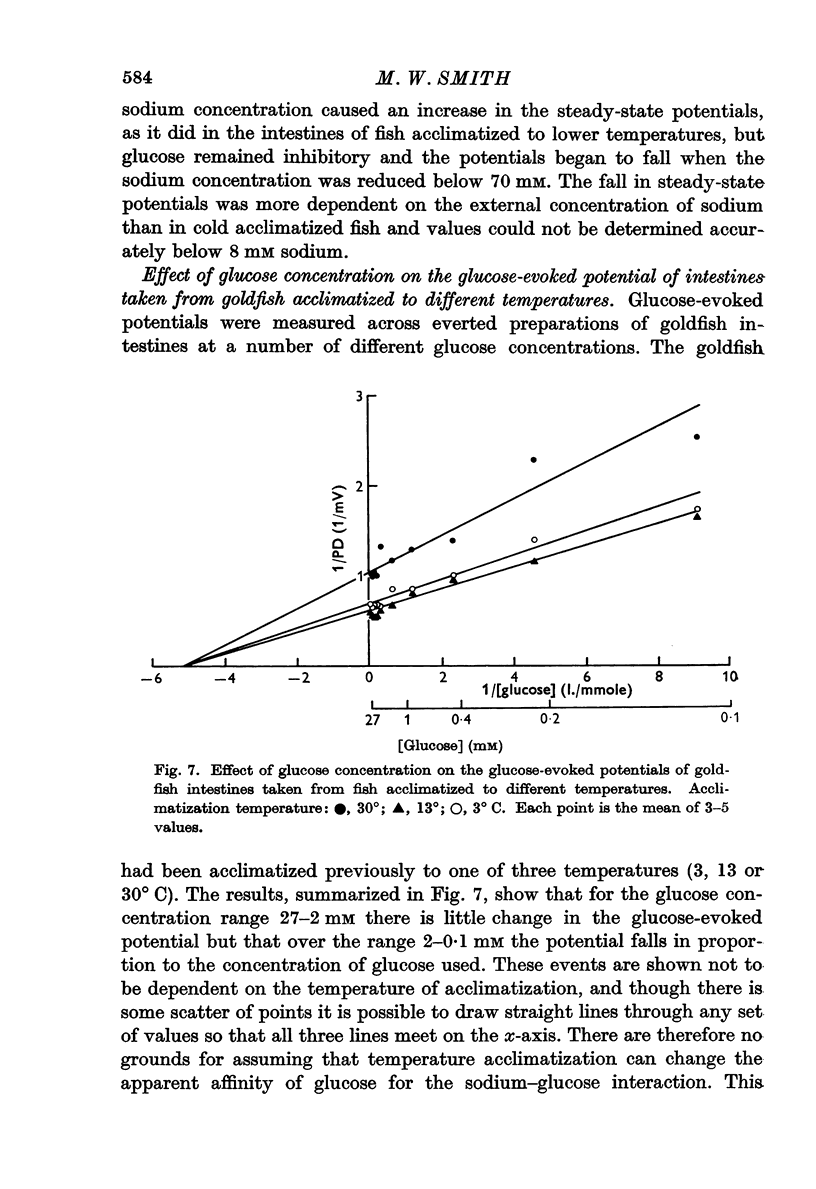
3. The maximum glucose-evoked potential depended on the concentration of glucose used and temperature acclimatization had no significant effect on this relation. The steady potential was lower in the presence of glucose at low incubation temperatures but higher at higher incubation temperatures, and the temperature at which glucose ceased to inhibit depended on the previous acclimatization temperature. Glucose also lowered the steady potential, whatever the previous acclimatization temperature, when the external sodium concentration was low.
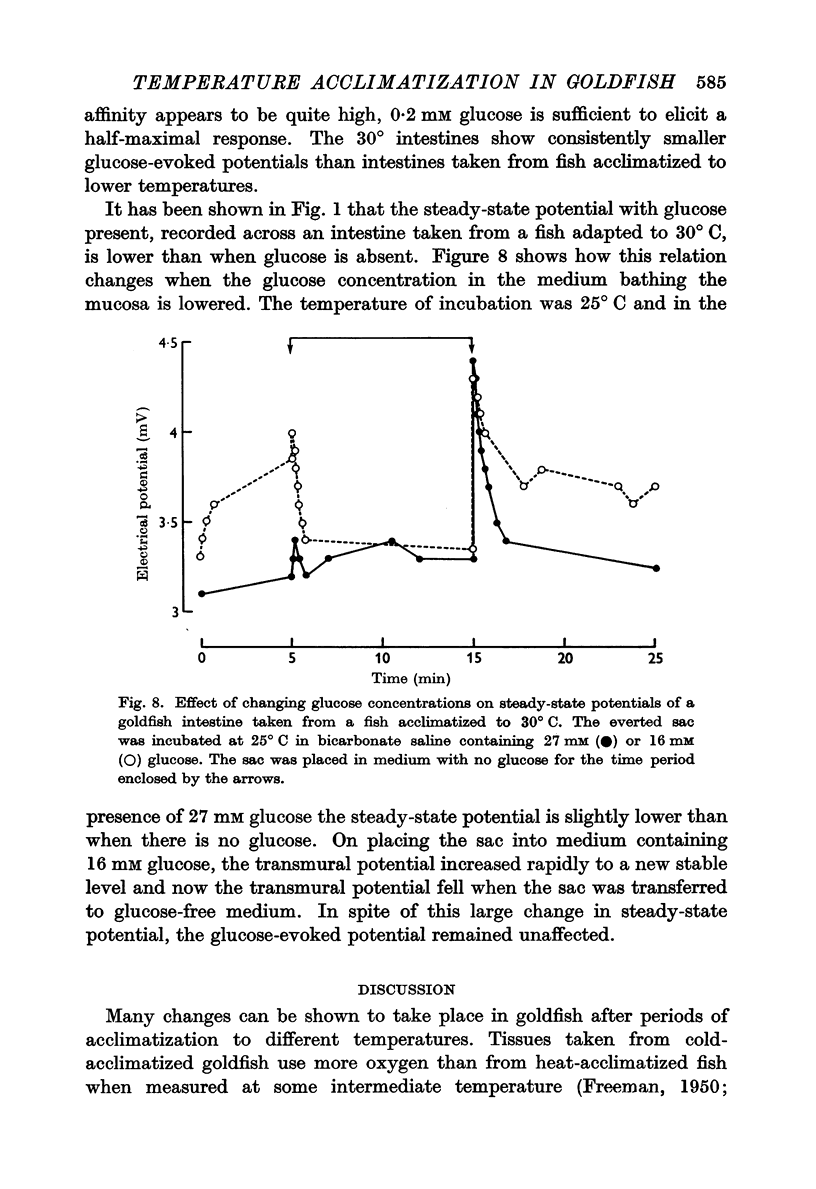
4. The inhibitory effect of glucose on the steady potential of an intestine taken from a 30°-acclimatized fish could be abolished by lowering the external concentration of glucose from 27 to 16 mM.
5. Intestines taken from fish acclimatized to 3° C gave variable results.
6. It is concluded that sodium moves across the luminal membrane of the goldfish mucosa attached to a carrier which can exist in one of two forms. It is changes in this postulated carrier which serve to stabilize sodium transport at different acclimatization temperatures. Changes in the concentration of this postulated carrier may also occur and function in the regulation of sodium transport, particularly at acclimatization temperatures below 15° C, where the switching of the carrier does not operate.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CEREIJIDO M., HERRERA F. C., FLANIGAN W. J., CURRAN P. F. THE INFLUENCE OF NA CONCENTRATION ON NA TRANSPORT ACROSS FROG SKIN. J Gen Physiol. 1964 May;47:879–893. doi: 10.1085/jgp.47.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER K. C., ELSON P. F. The selected temperature of Atlantic salmon and speckled trout and the effect of temperature on the response to an electrical stimulus. Physiol Zool. 1950 Jan;23(1):27–34. doi: 10.1086/physzool.23.1.30084896. [DOI] [PubMed] [Google Scholar]
- FRAZIER H. S., DEMPSEY E. F., LEAF A. Movement of sodium across the mucosal surface of the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 Jan;45:529–543. doi: 10.1085/jgp.45.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN J. A. Oxygen consumption, brain metabolism and respiratory movements of goldfish during temperature acclimatization, with special reference to lowered temperatures. Biol Bull. 1950 Dec;99(3):416–424. doi: 10.2307/1538472. [DOI] [PubMed] [Google Scholar]
- HOLLANDS B. C., SMITH M. W. PHOSPHATASES OF THE GOLDFISH INTESTINE. J Physiol. 1964 Dec;175:31–37. doi: 10.1113/jphysiol.1964.sp007501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER D. K., WESTFALL B. A., PLATNER W. S. Water and electrolyte balance of goldfish under conditions of anoxia, cold and inanition. Am J Physiol. 1956 Mar;184(3):553–556. doi: 10.1152/ajplegacy.1956.184.3.553. [DOI] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. INTERACTIONS BETWEEN ACTIVE SODIUM TRANSPORT AND ACTIVE AMINO-ACID TRANSPORT IN ISOLATED RABBIT ILEUM. Nature. 1965 Jan 16;205:292–294. doi: 10.1038/205292a0. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. The interaction between active sodium transport and active sugar transport in the isolated rabbit ileum. Biochim Biophys Acta. 1963 May 14;71:503–505. doi: 10.1016/0006-3002(63)91121-1. [DOI] [PubMed] [Google Scholar]
- Smith M. W. Electrical properties and glucose transfer in the goldfish intestine. Experientia. 1964 Nov 15;20(11):613–614. doi: 10.1007/BF02144815. [DOI] [PubMed] [Google Scholar]
- Smith M. W. Sodium-glucose interactions in the goldfish intestine. J Physiol. 1966 Feb;182(3):559–573. doi: 10.1113/jphysiol.1966.sp007837. [DOI] [PMC free article] [PubMed] [Google Scholar]


