Abstract
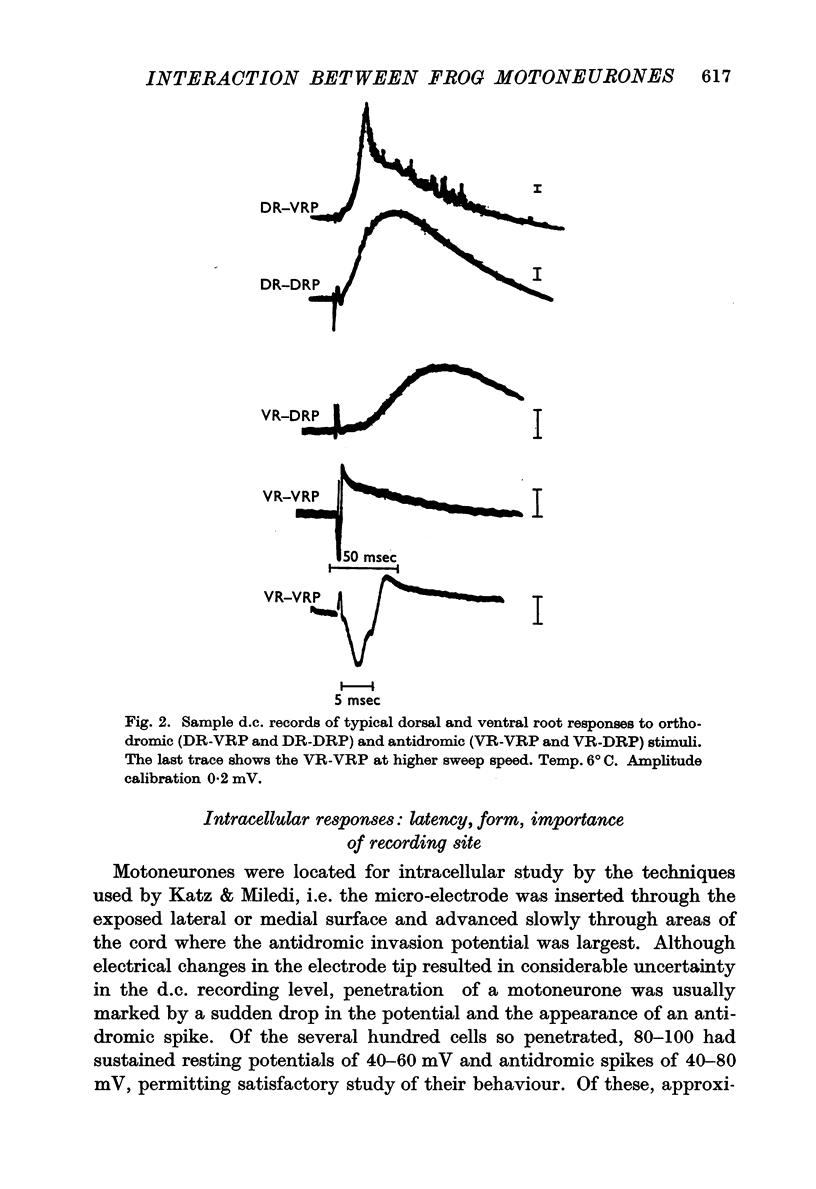
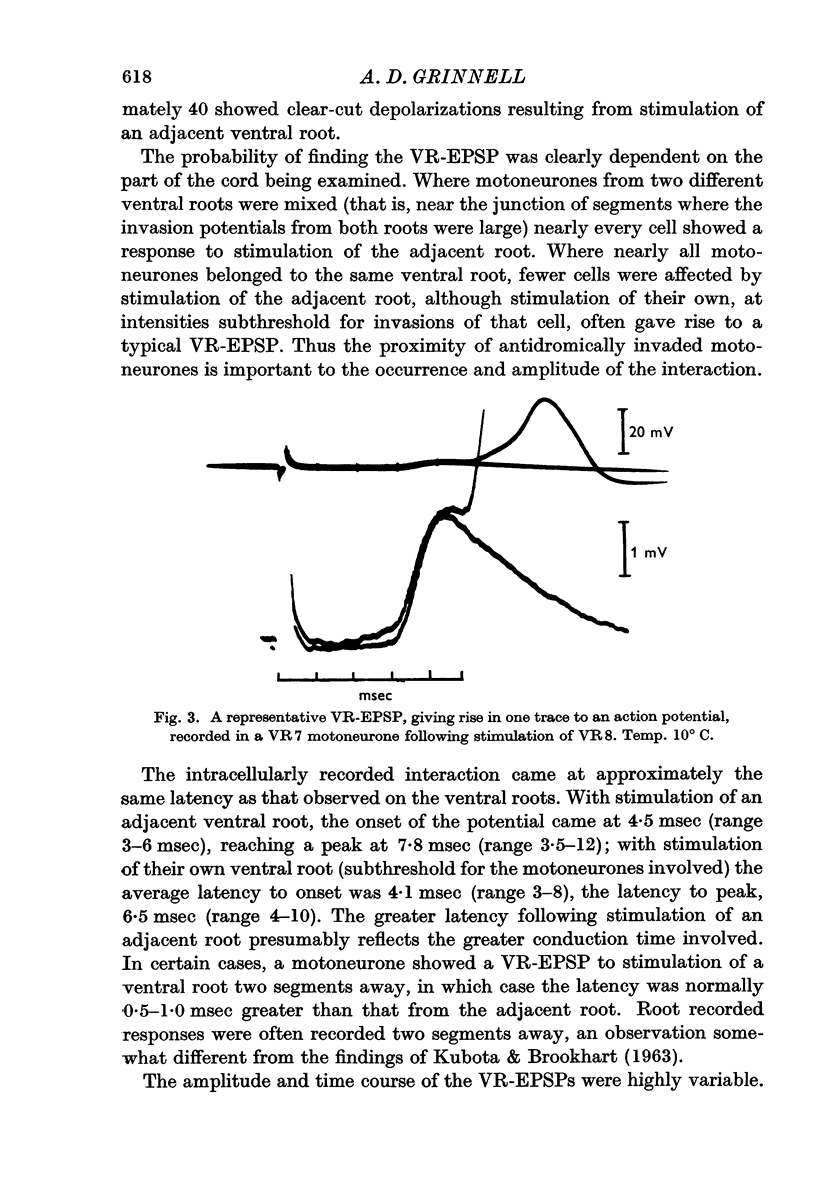
1. A short-latency interaction between motoneurones has been studied with intracellular and root potential recordings from the isolated spinal cord of the frog. Antidromic stimulation of one ventral root causes brief depolarization (VR-EPSP) of the motoneurones of adjacent, non-excited motoneurones. The summed activity of many such VR-EPSPs can be seen as a brief depolarization (VR-VRP) passing out an adjacent ventral root.
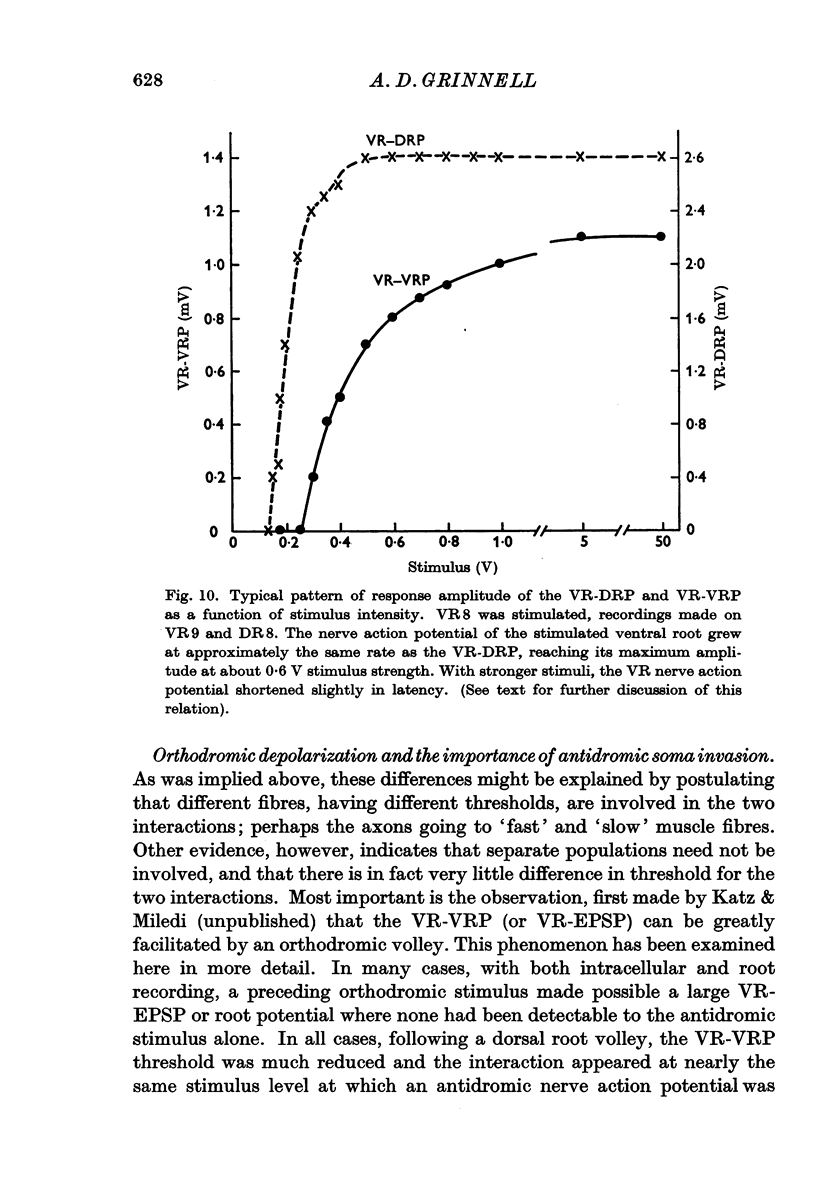
2. Both intracellular and root-recorded signs of this interaction are graded in amplitude.
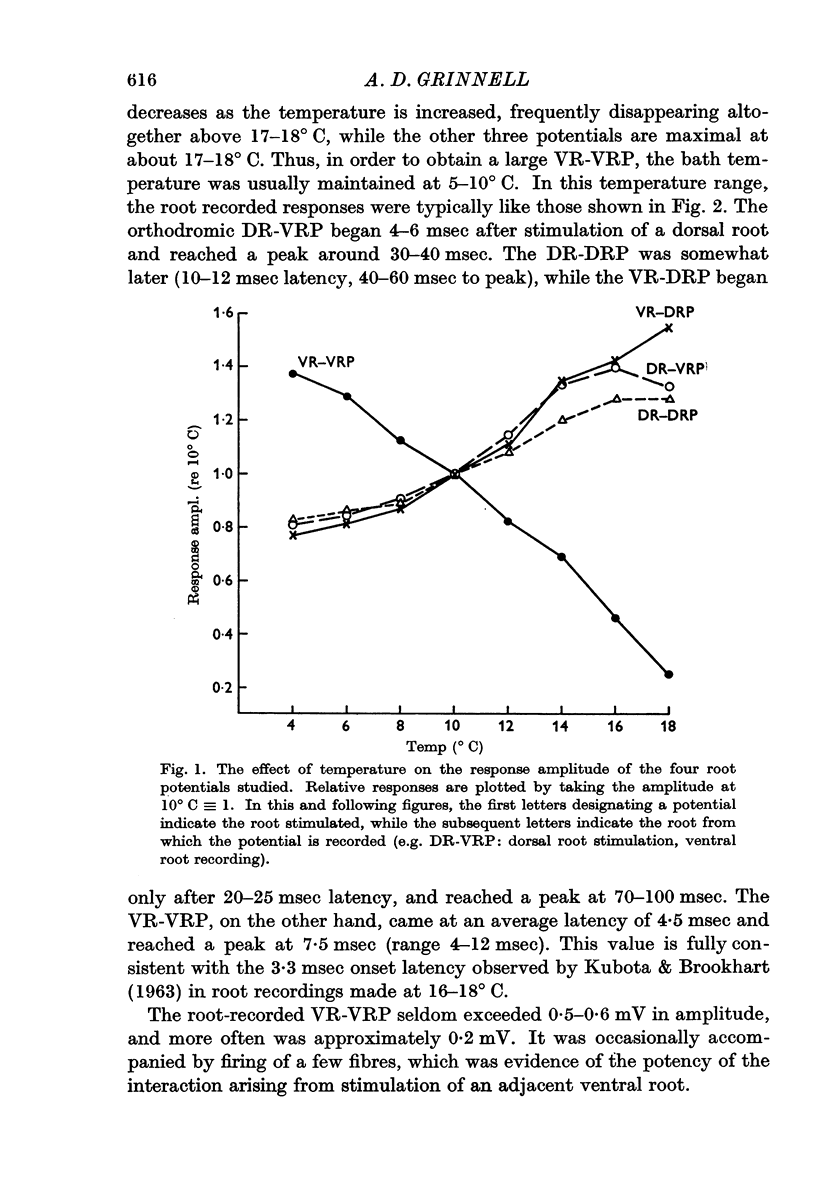
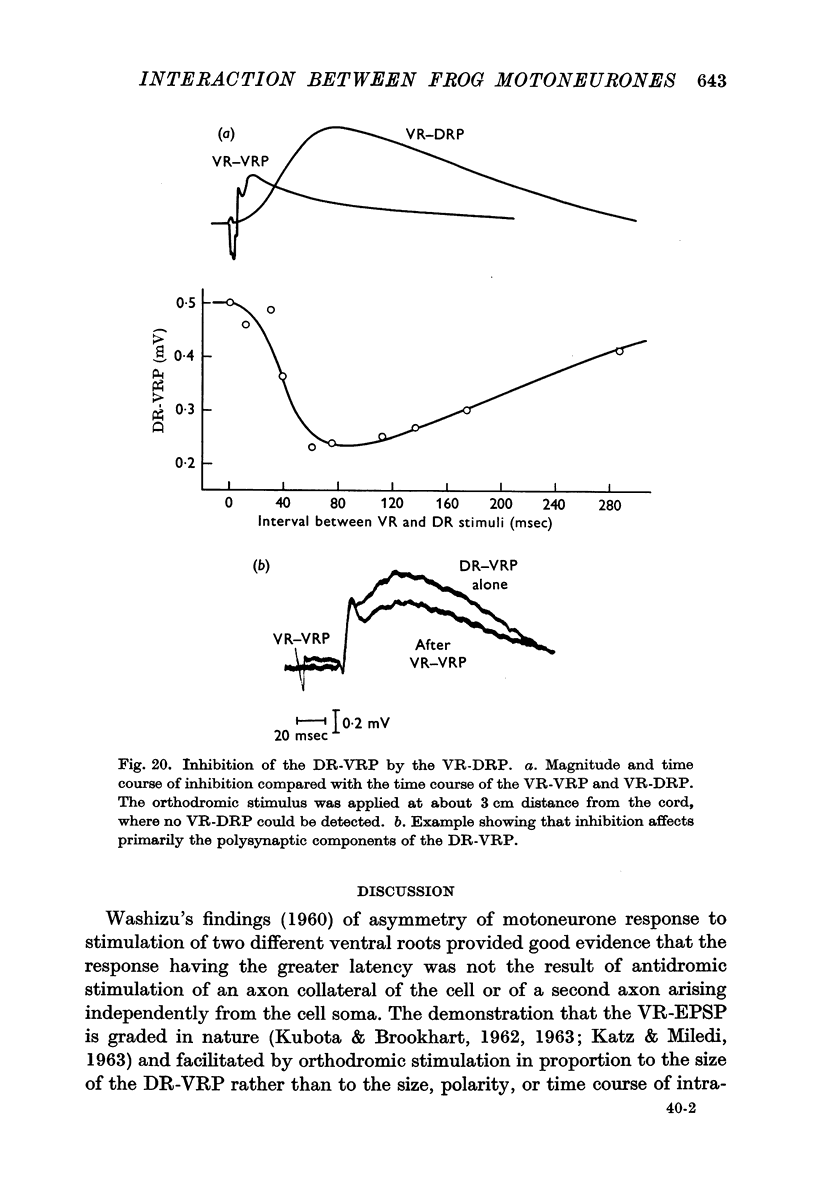
3. It was found that this interaction decreased with increasing temperature. This is in contrast to the behaviour of the ventral root potential resulting from dorsal root stimulation (DR-VRP) or the dorsal root potentials resulting from either dorsal root (DR-DRP) or ventral root (VR-DRP) stimulation, all of which increased in amplitude from below 10 to about 17° C.
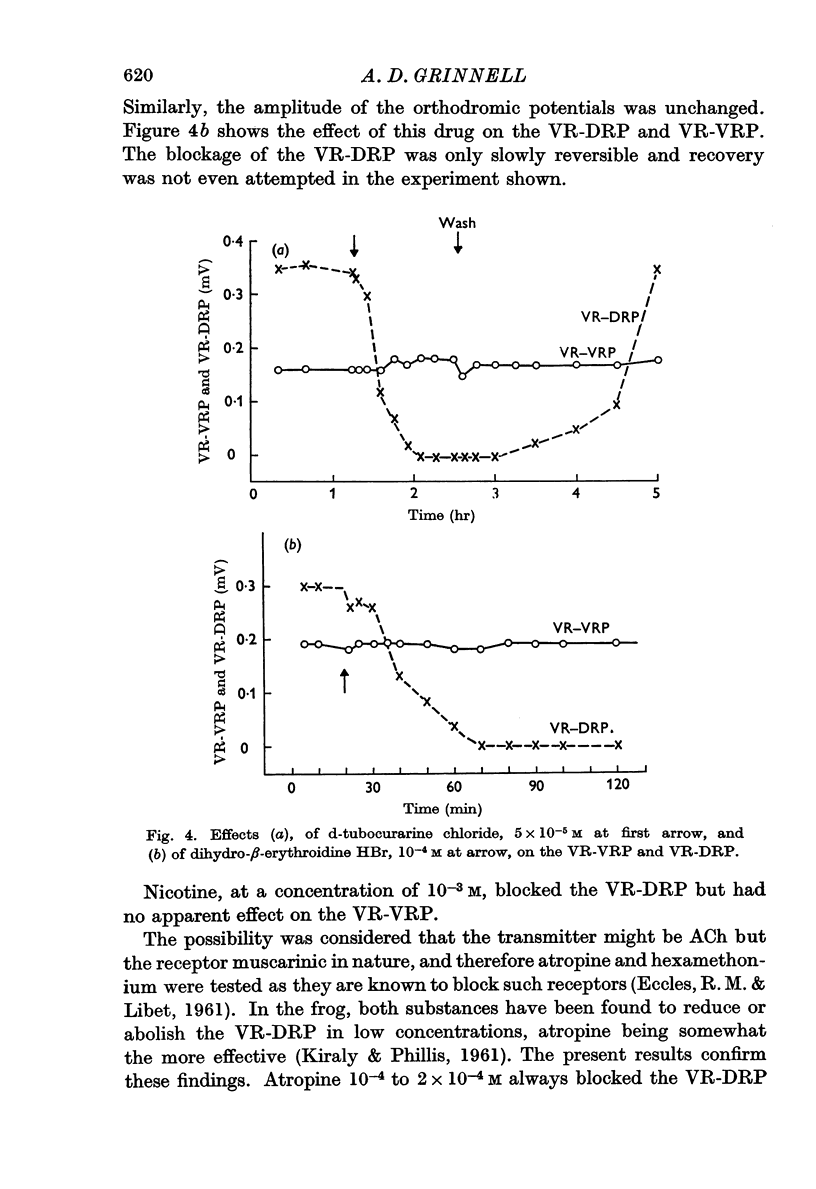
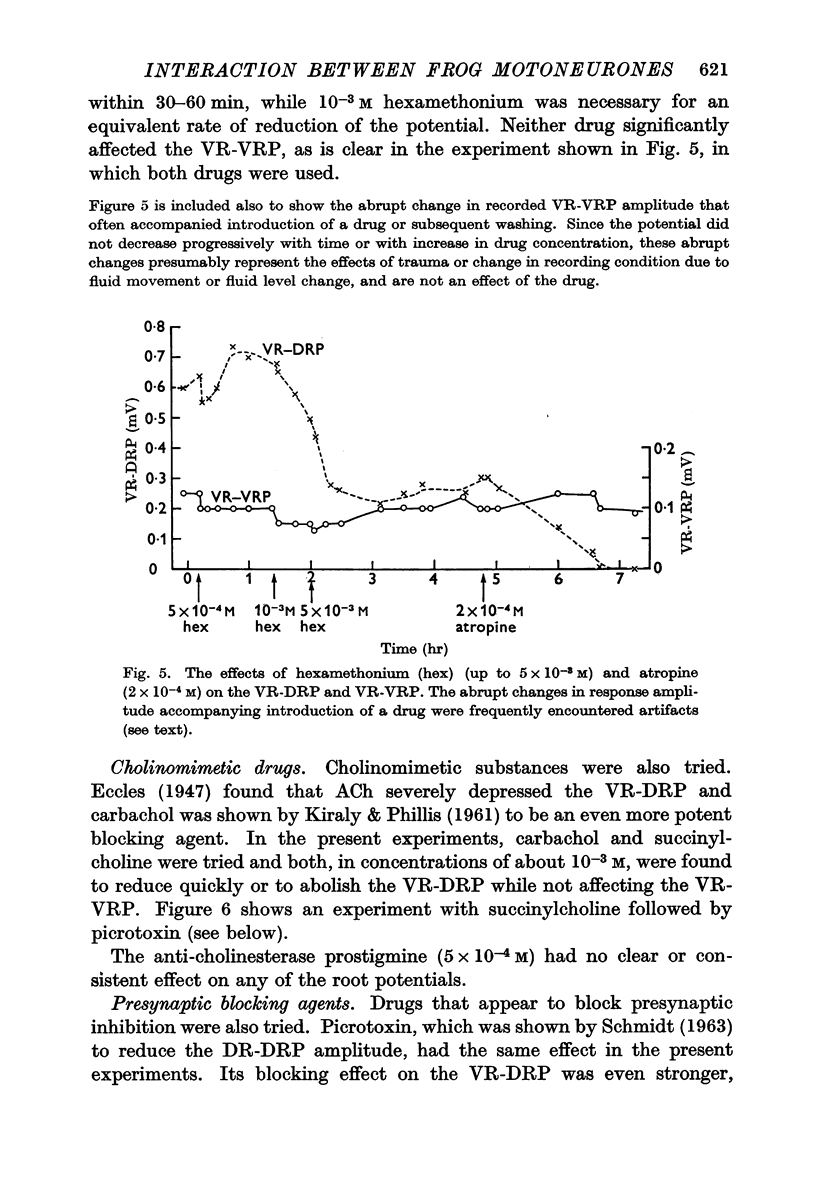
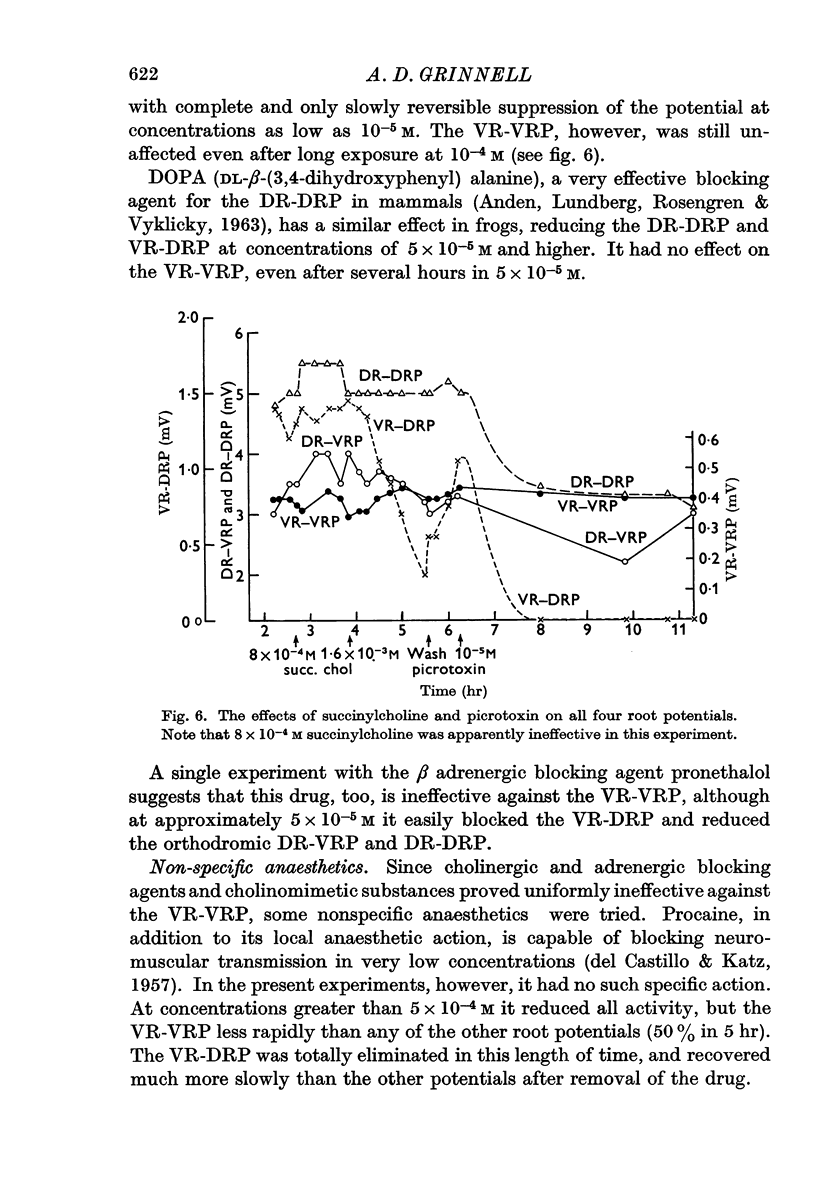
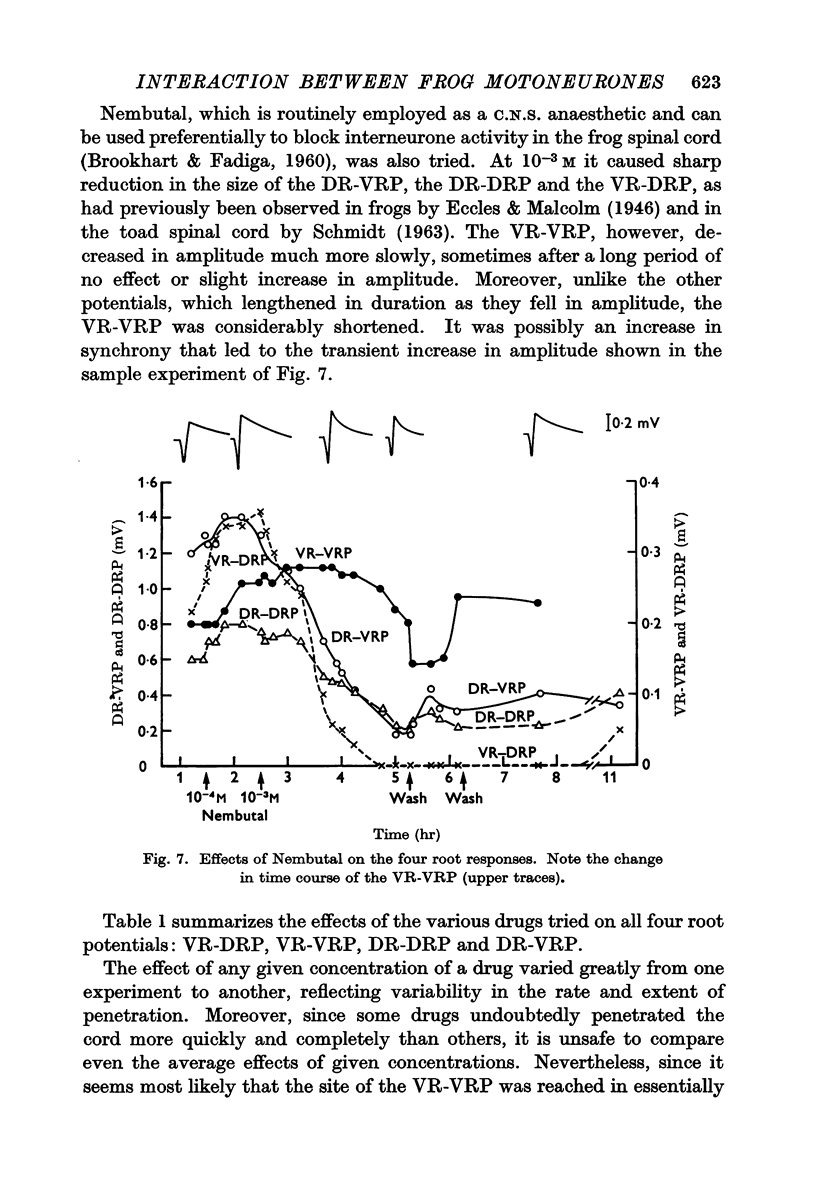
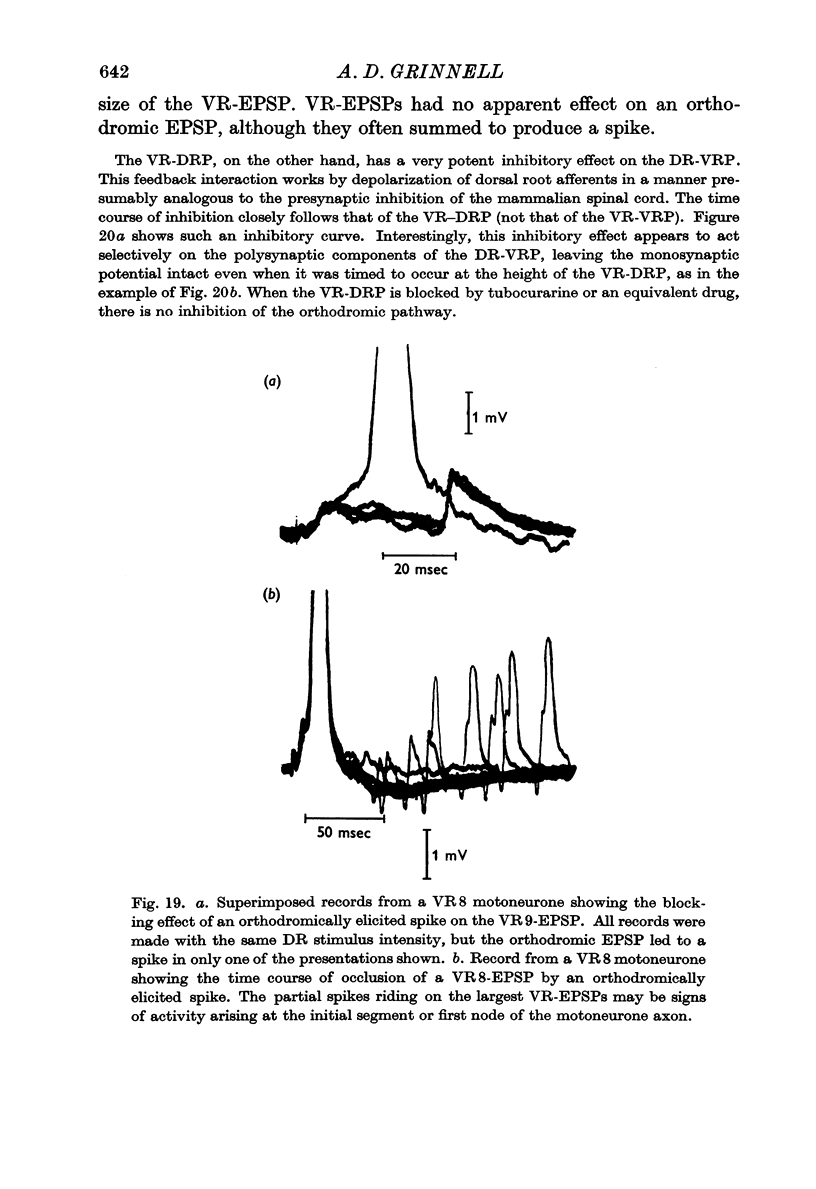
4. Pharmacological evidence suggests that the interaction between motoneurones is not chemically mediated. The VR-VRP was not affected by a large variety of transmitter blocking agents, including curare, dihydro-β-erythroidine, atropine, succinylcholine, hexamethonium and DOPA, while the VR-DRP, which probably originates with the release of ACh from an axon collateral, was consistently blocked.
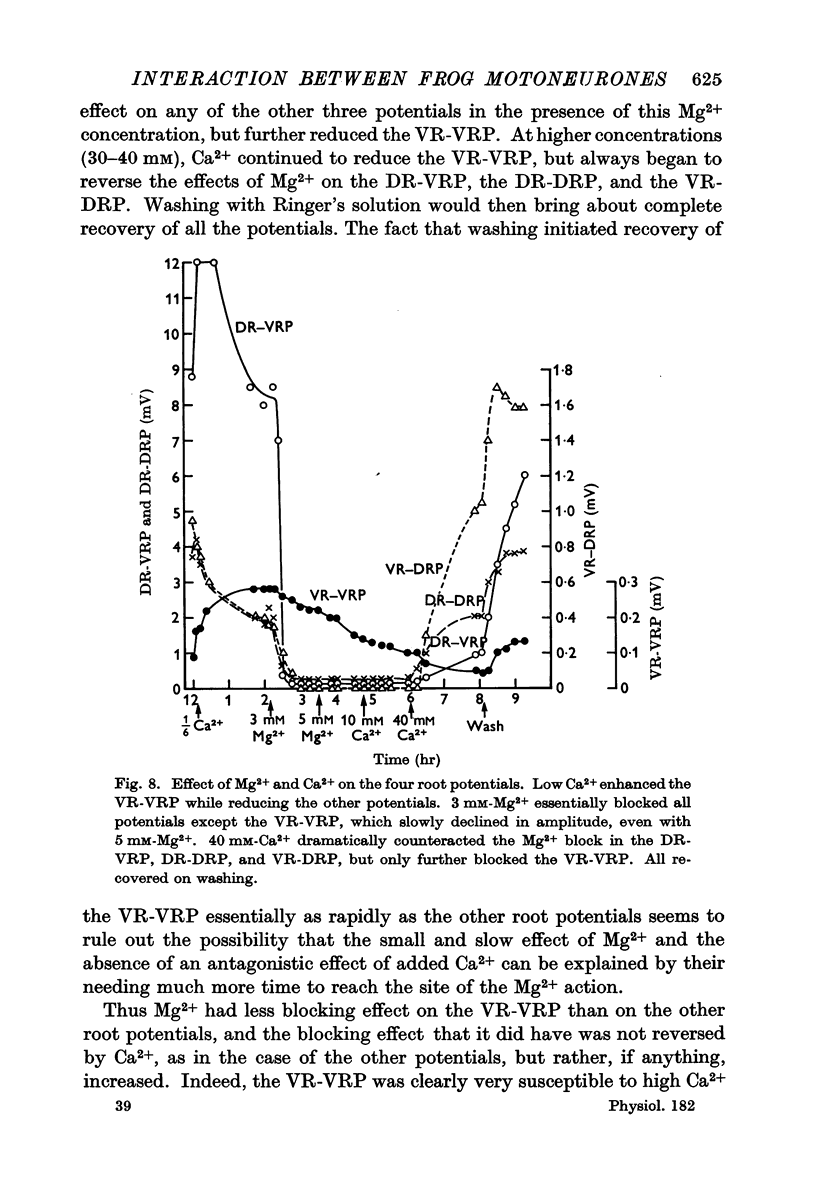
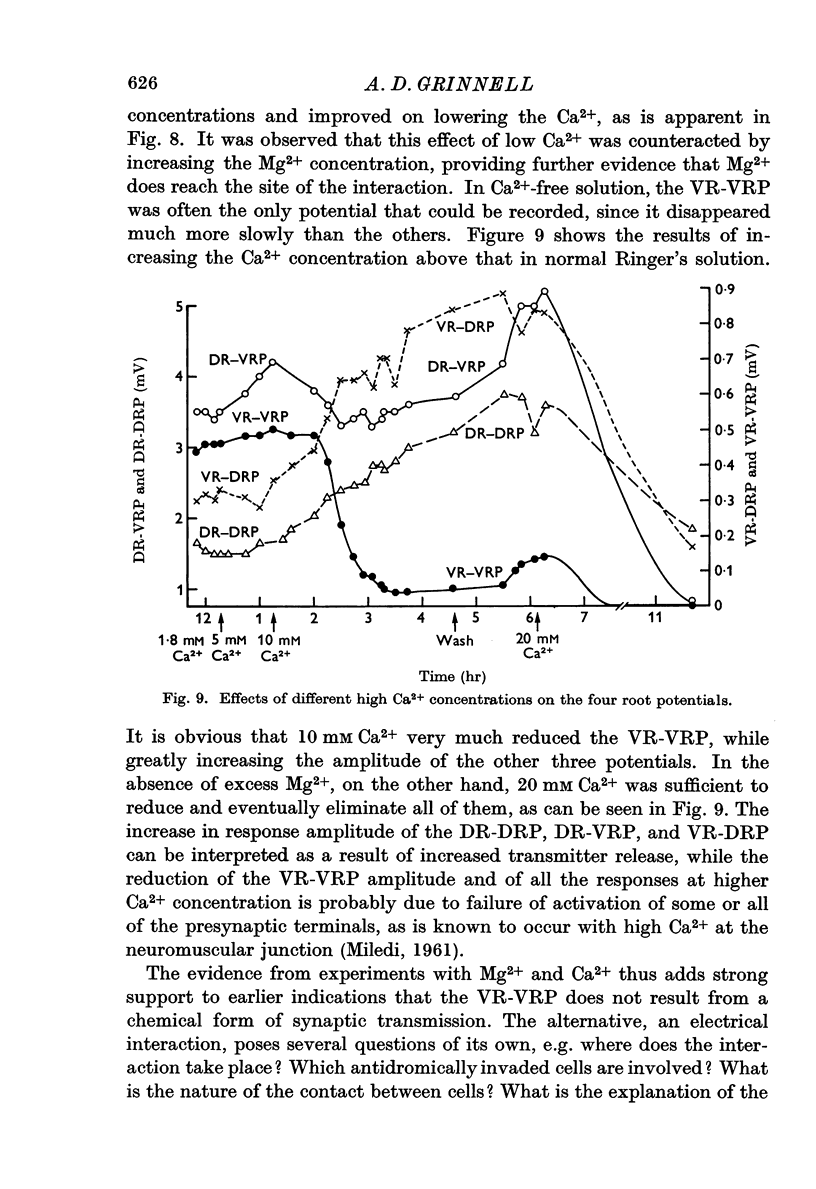
5. Mg2+ suppressed the VR-VRP more slowly than the other potentials, and this suppression was increased by adding Ca2+, rather than reversed, as in the case of the other root potentials, which are presumably mediated by chemical transmission.
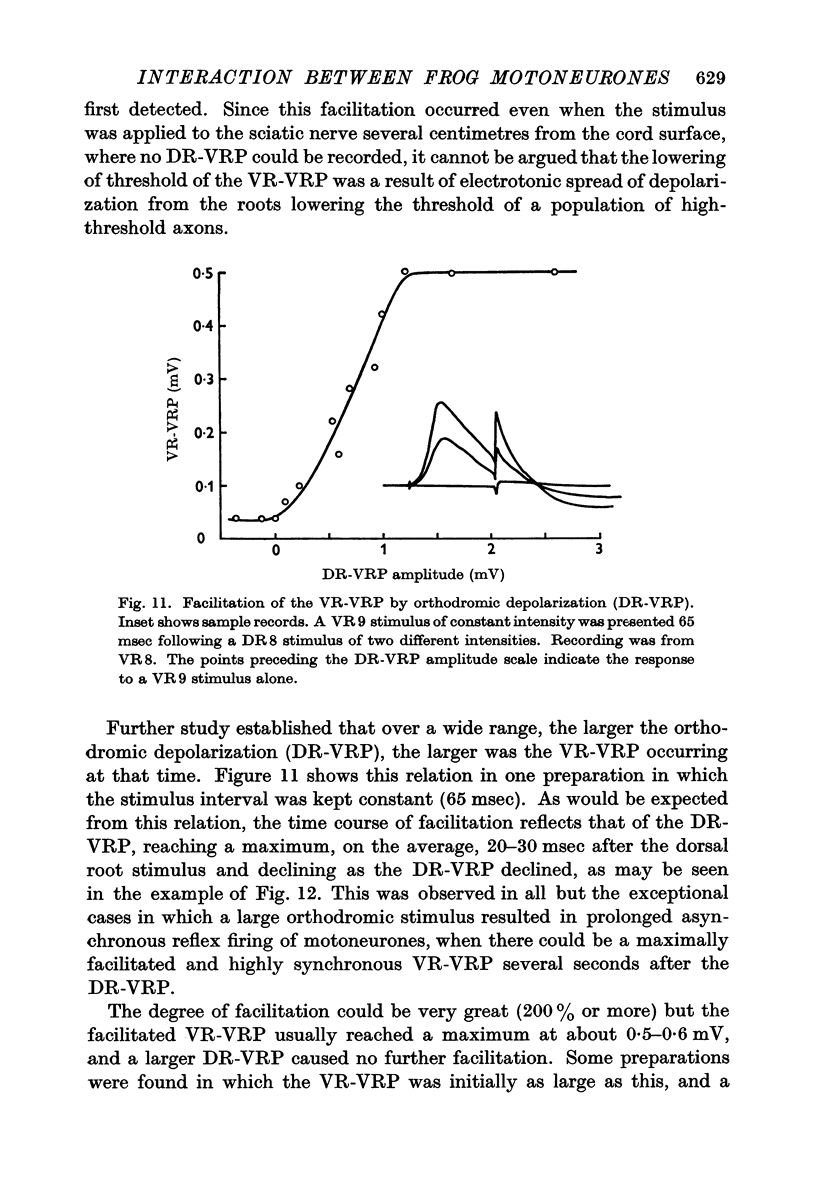
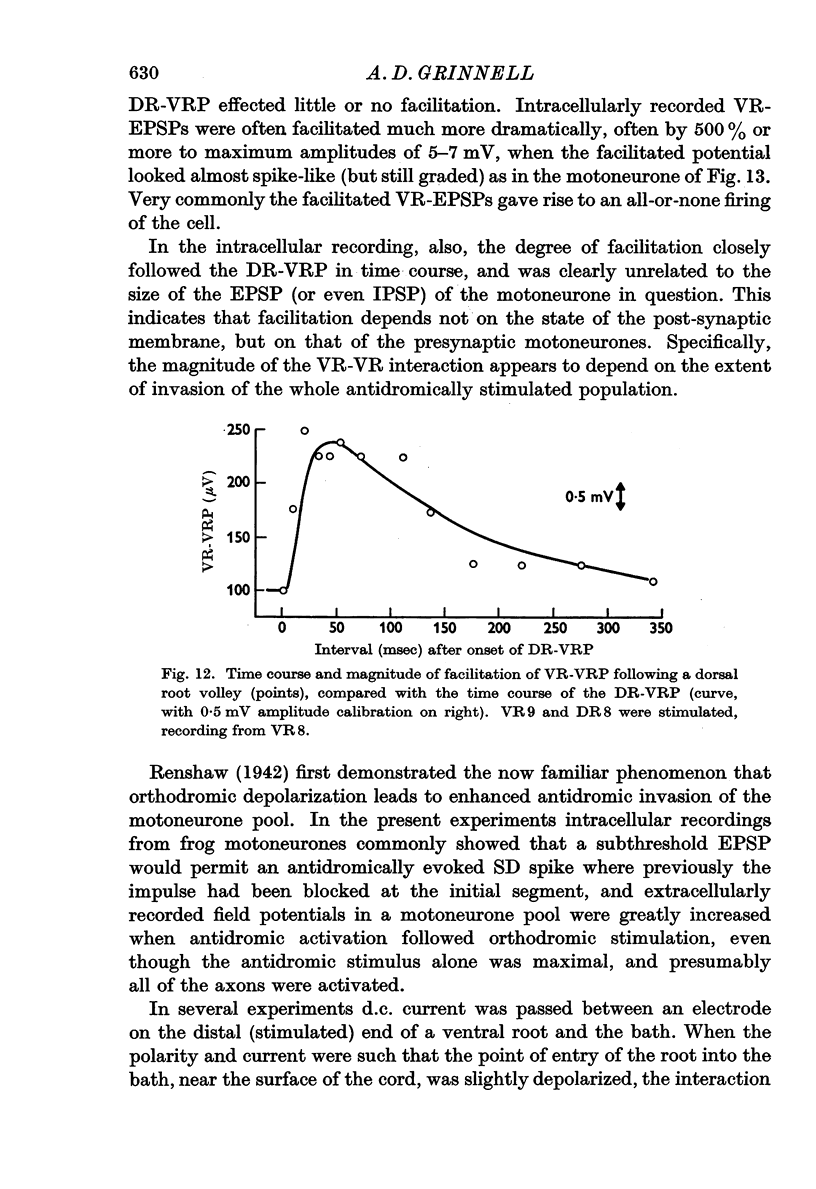
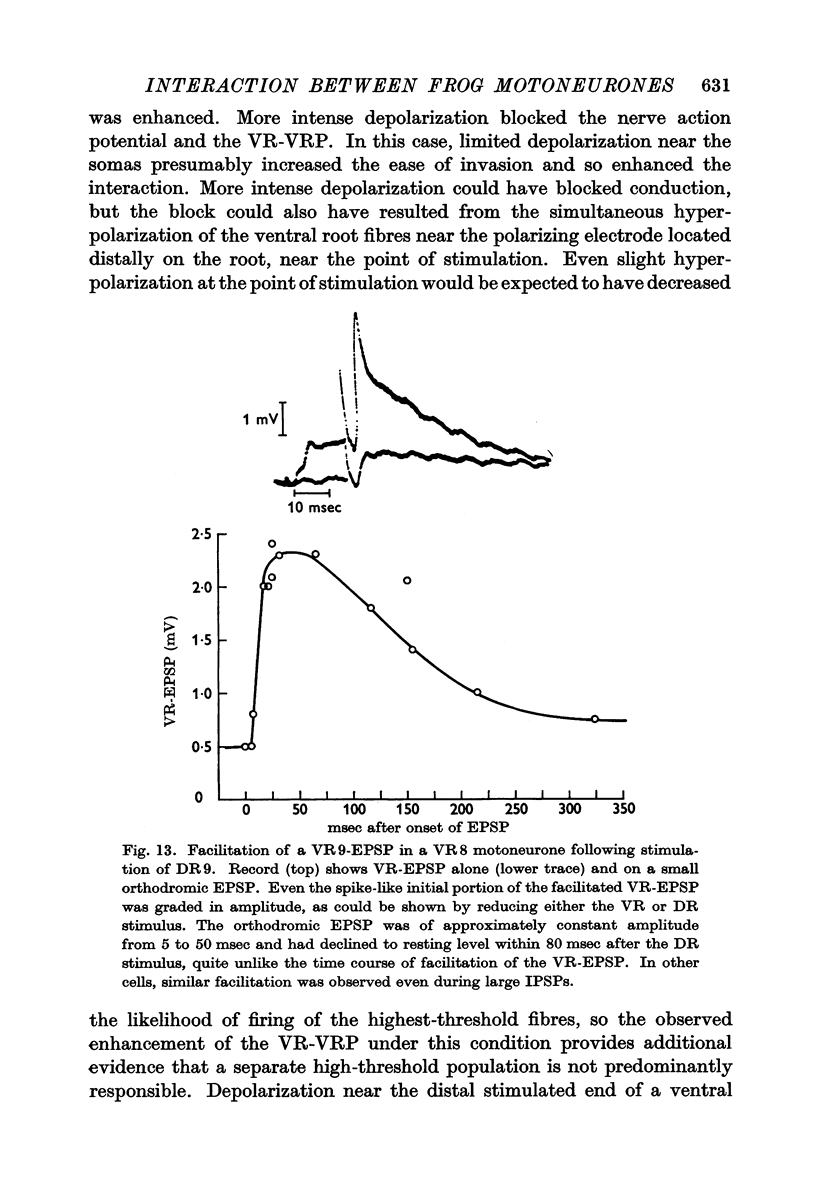
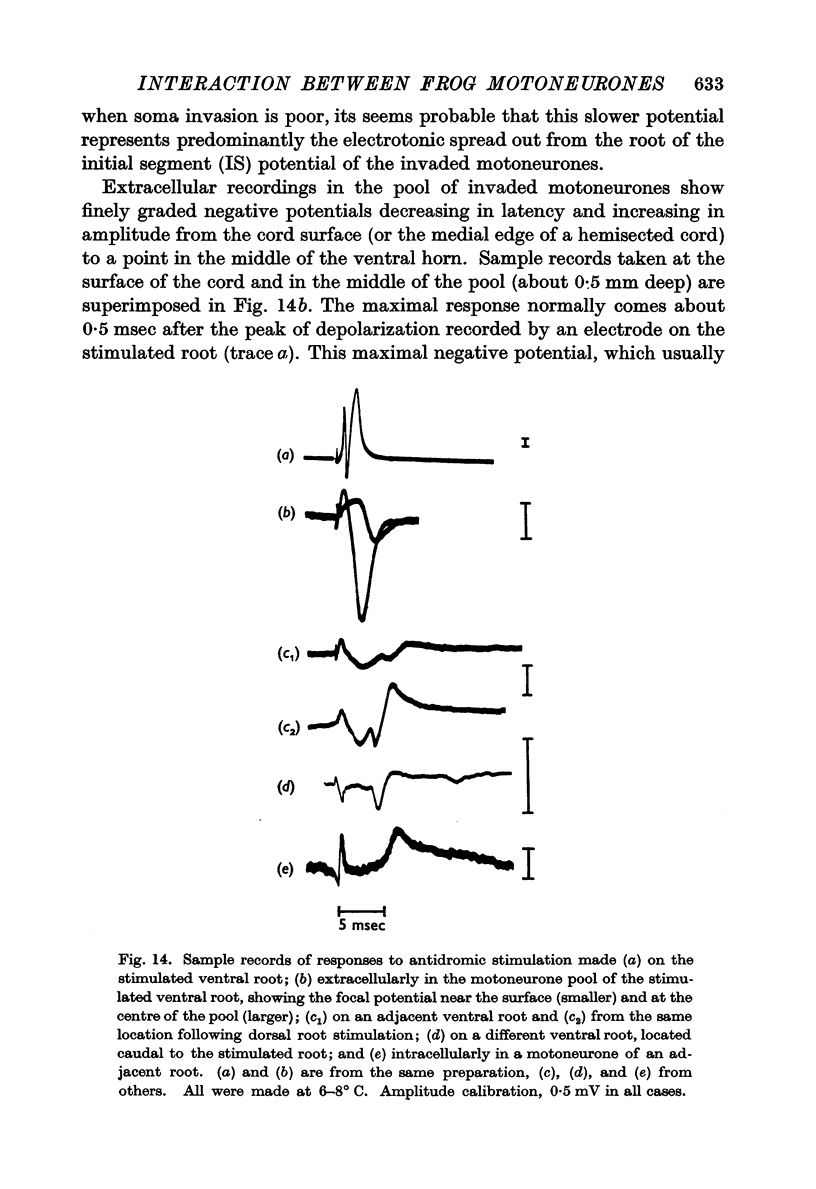
6. The interaction between motoneurones is strongly facilitated by orthodromic depolarization of the motoneurones being antidromically stimulated. Extracellular recordings within the cord support the conclusion that this facilitation is a result of the enhancement of antidromic invasion, perhaps especially of the dendrites, by slight depolarization.
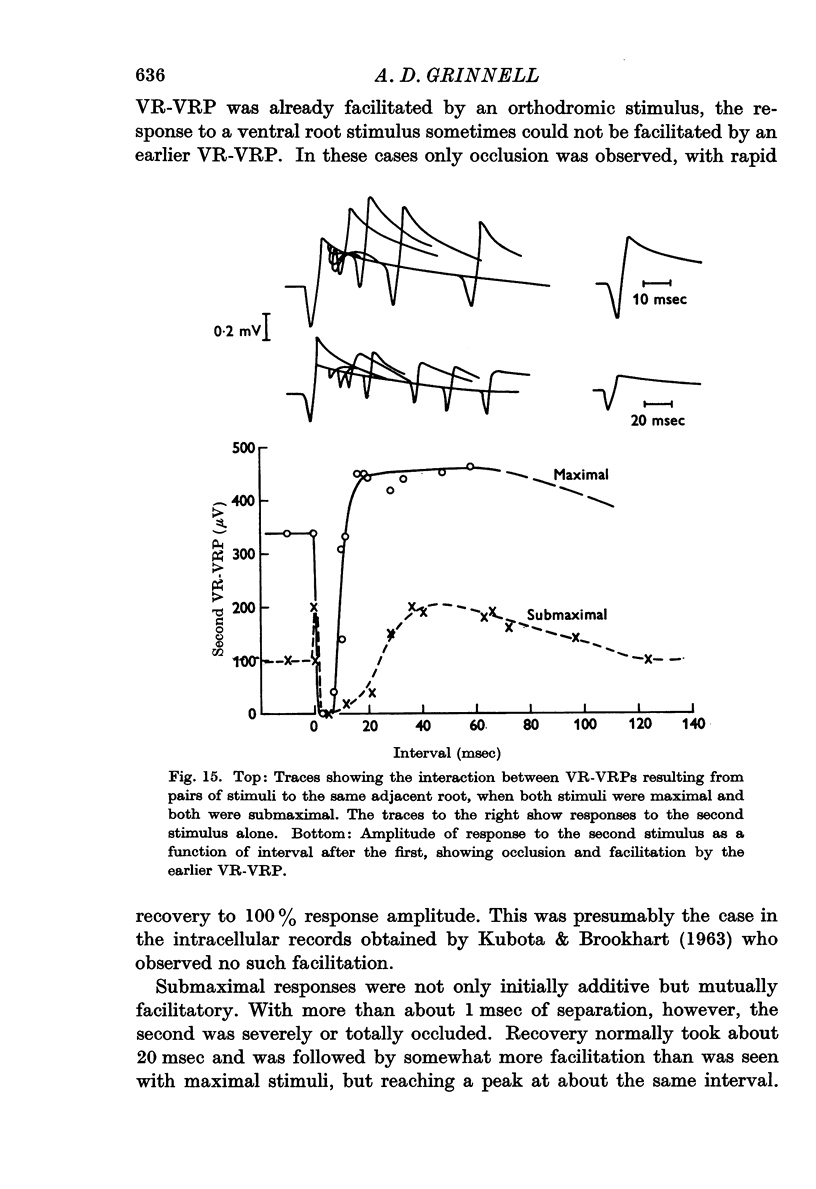
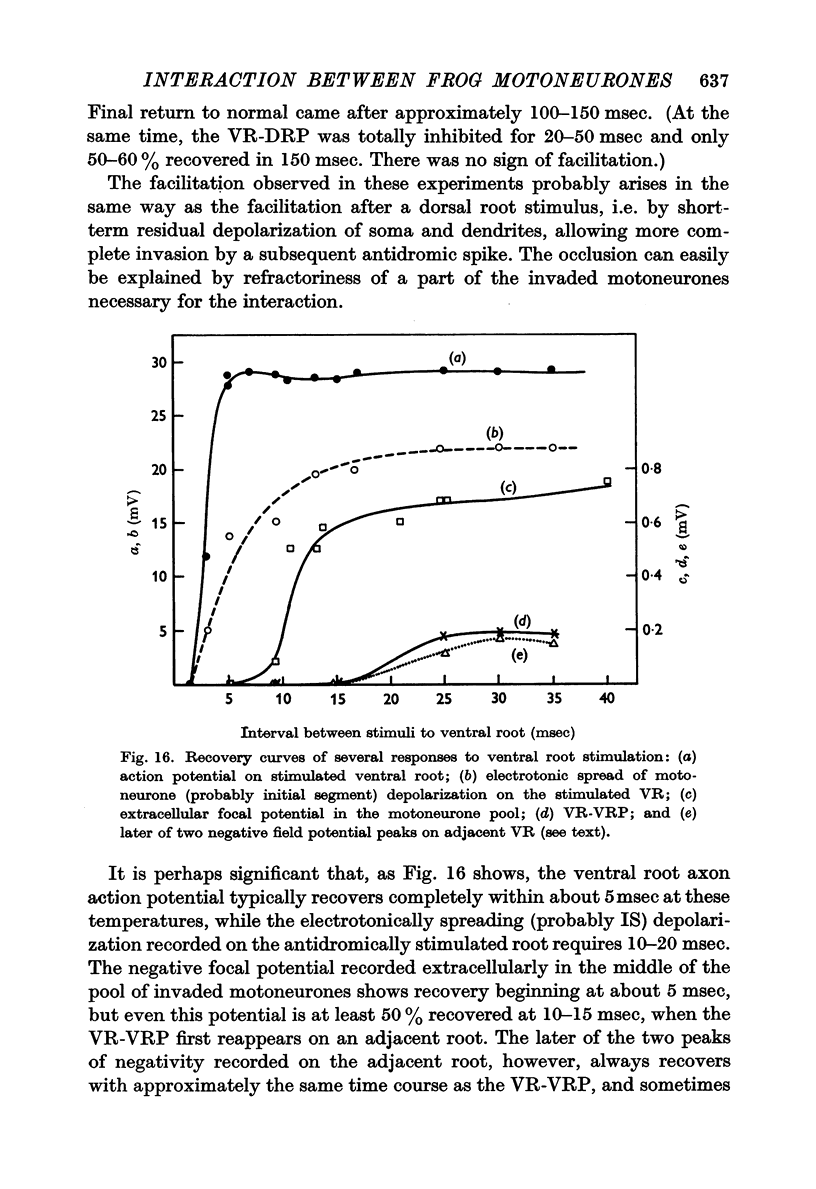
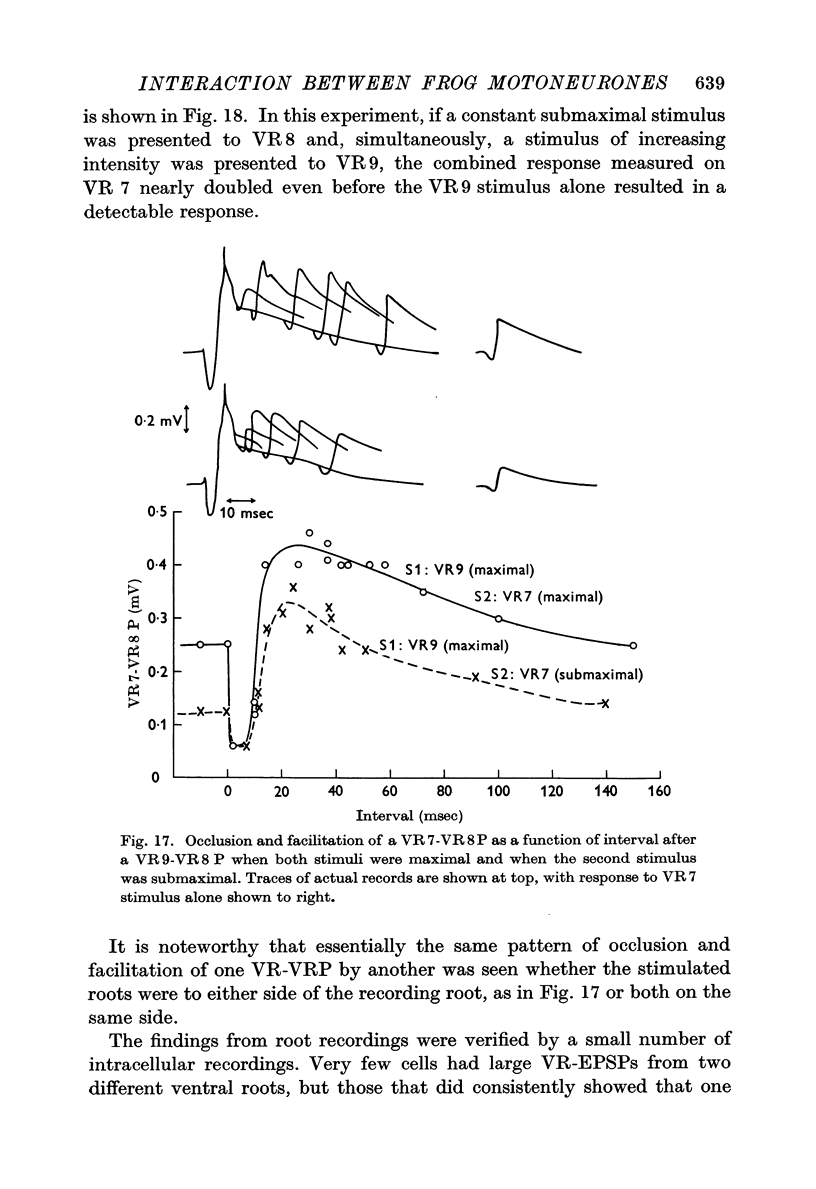
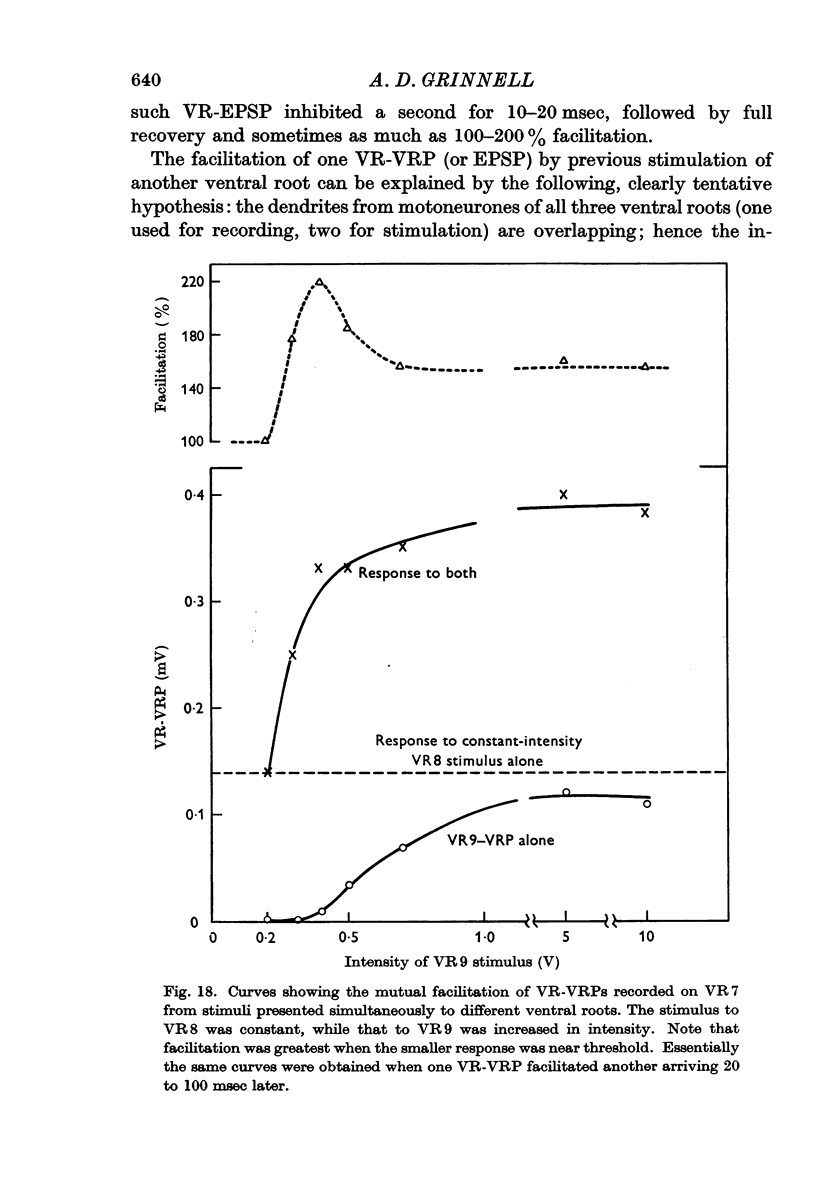
7. One VR-VRP (or VR-EPSP) first suppresses response to another (for about 10 msec), then facilitates response to the second, with maximum effect around 20-40 msec. This is the case whether both stimuli go to the same or to different ventral roots, although occlusion is less and facilitation greater in the latter case. Occlusion of the VR-EPSP also results from full excitation of the cell in which recording is being done.
8. The mechanism of this interaction remains uncertain, but it would seem likely that overlapping dendrites of adjacent motoneurones interact with each other electrically through close apposition or specialized contacts. Occlusion would result from the refractoriness of strongly depolarized dendrites, facilitation from the enhancement of invasion of antidromically stimulated motoneurones by the weaker (or residual) depolarization occurring after earlier activity of motoneurones or their dendrites.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., LUNDBERG A., ROSENGREN E., VYKLICKY L. THE EFFECT OF DOPA ON SPINAL REFLEXES FROM THE FRA (FLEXOR REFLEX AFFERENTS). Experientia. 1963 Dec 15;19:654–655. doi: 10.1007/BF02151304. [DOI] [PubMed] [Google Scholar]
- BENNETT M. V., ALJURE E., NAKAJIMA Y., PAPPAS G. D. Electrotonic junctions between teleost spinal neurons: electrophysiology and ultrastructure. Science. 1963 Jul 19;141(3577):262–264. doi: 10.1126/science.141.3577.262. [DOI] [PubMed] [Google Scholar]
- BROOKHART J. M., FADIGA E. Potential field initiated during monosynaptic activation of frog motoneurones. J Physiol. 1960 Mar;150:633–655. doi: 10.1113/jphysiol.1960.sp006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. The interpretation of potential changes in the spinal cord. J Physiol. 1938 Apr 14;92(3):276–321. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock T. H. Neurophysiology: United States-Japan Joint Symposium. Science. 1964 Jun 12;144(3624):1361–1364. doi: 10.1126/science.144.3624.1361. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES R. M. The effect of diffusional barriers upon the pharmacology of cells within the central nervous system. J Physiol. 1958 May 28;141(3):446–463. doi: 10.1113/jphysiol.1958.sp005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LORENZO A. J. The fine structure of synapses in the ciliary ganglion of the chick. J Biophys Biochem Cytol. 1960 Feb;7:31–36. doi: 10.1083/jcb.7.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO L., KATZ B. A study of curare action with an electrical micromethod. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):339–356. doi: 10.1098/rspb.1957.0015. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LIBET B. Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J Physiol. 1961 Aug;157:484–503. doi: 10.1113/jphysiol.1961.sp006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. "ELECTRICAL TRANSMISSION" AT AN EXCITATORY SYNAPSE IN A VERTEBRATE BRAIN. Science. 1964 May 15;144(3620):878–880. doi: 10.1126/science.144.3620.878. [DOI] [PubMed] [Google Scholar]
- HAMA K. Some observations on the fine structure of the giant nerve fibers of the earthworm, Eisenia foetida. J Biophys Biochem Cytol. 1959 Aug;6(1):61–66. doi: 10.1083/jcb.6.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., KOSTIAL K. Effect of magnesium and calcium ions on the release of acetylcholine. J Physiol. 1954 May 28;124(2):234–241. doi: 10.1113/jphysiol.1954.sp005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. A STUDY OF SPONTANEOUS MINIATURE POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1963 Sep;168:389–422. doi: 10.1113/jphysiol.1963.sp007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRALY J. K., PHILLIS J. W. Action of some drugs on the dorsal root potentials of the isolated toad spinal cord. Br J Pharmacol Chemother. 1961 Oct;17:224–231. doi: 10.1111/j.1476-5381.1961.tb01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K. Intracellular slow potential of dorsal root fibers. Am J Physiol. 1956 Feb;184(2):338–344. doi: 10.1152/ajplegacy.1956.184.2.338. [DOI] [PubMed] [Google Scholar]
- KUBOTA K., BROOKHART J. M. RECURRENT FACILITATION OF FROG MOTONEURONS. J Neurophysiol. 1963 Nov;26:877–893. doi: 10.1152/jn.1963.26.6.877. [DOI] [PubMed] [Google Scholar]
- KUSANO K., HAGIWARA S. On the integrative synaptic potentials of Onchidium nerve cell. Jpn J Physiol. 1961 Feb 15;11:96–101. doi: 10.2170/jjphysiol.11.96. [DOI] [PubMed] [Google Scholar]
- LLOYD D. P. C. Electrical signs of impulse conduction in spinal motoneurons. J Gen Physiol. 1951 Nov;35(2):255–288. doi: 10.1085/jgp.35.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D. P. TEMPERATURE AND DENDRITIC RESPONSE OF SPINAL MOTONEURONS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):589–592. doi: 10.1073/pnas.45.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. TRANSMISSION THROUGH THE CILIARY GANGLION OF THE CHICK. J Physiol. 1963 Sep;168:464–475. doi: 10.1113/jphysiol.1963.sp007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON P. G., FRANK K. ORTHODROMICALLY PRODUCED CHANGES IN MOTONEURONAL EXTRACELLULAR FIELDS. J Neurophysiol. 1964 Sep;27:928–941. doi: 10.1152/jn.1964.27.5.928. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. D. Recent electron microscope observations on the ultrastructure of the crayfish median-to-motor giant synapse. Exp Cell Res. 1955 Feb;8(1):226–229. doi: 10.1016/0014-4827(55)90058-6. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R. F. PHARMACOLOGICAL STUDIES ON THE PRIMARY AFFERENT DEPOLARIZATION OF THE TOAD SPINAL CORD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASHIZU Y. Single spinal montoneurons excitable from two different antidromic pathways. Jpn J Physiol. 1960 Apr 29;10:121–131. doi: 10.2170/jjphysiol.10.121. [DOI] [PubMed] [Google Scholar]


