Abstract
1. Tracer movements and ionic composition have been observed in the smooth muscle of the guinea-pig taenia coli in vitro at 4, and 35° C. The muscle was quiescent at 4° C, and did not show spontaneous electrical nor mechanical activity for 25 min after re-warming. It was therefore possible to make observations on the quiescent tissue at 35° C.
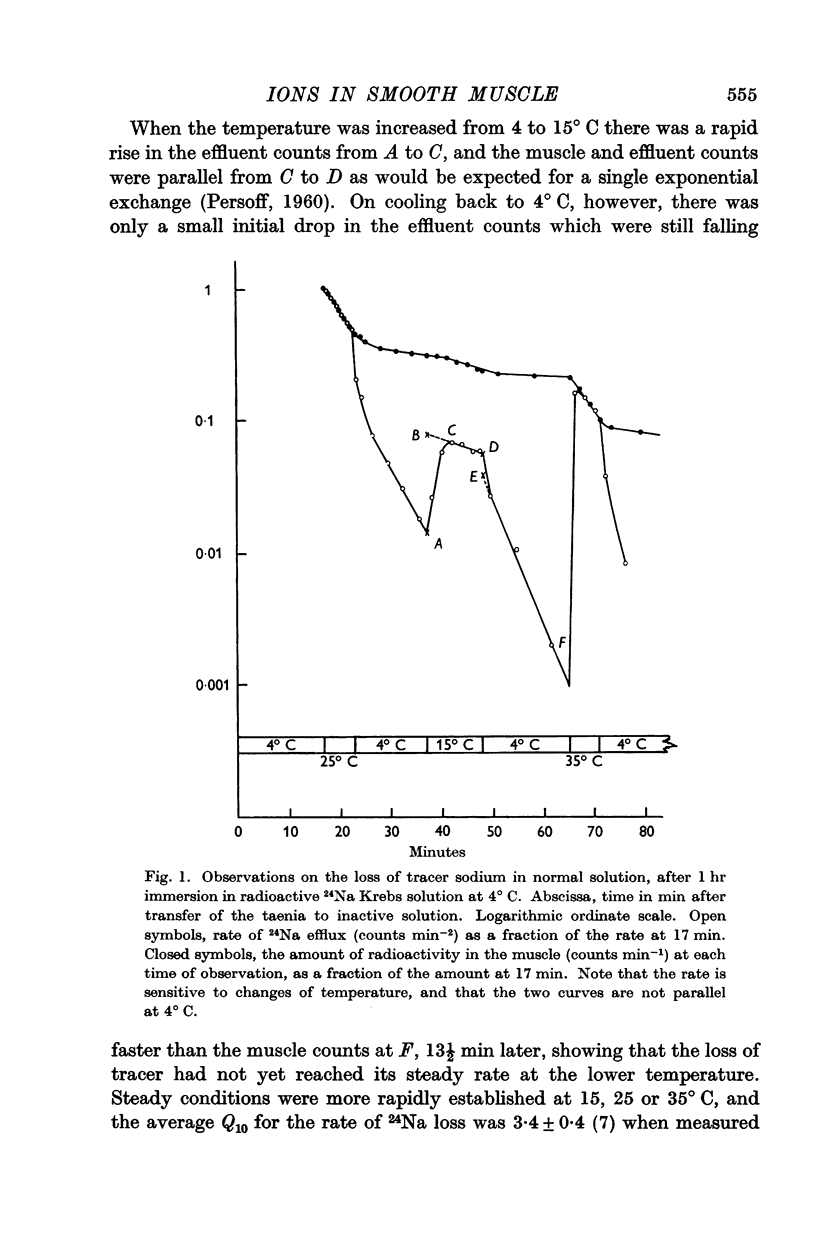
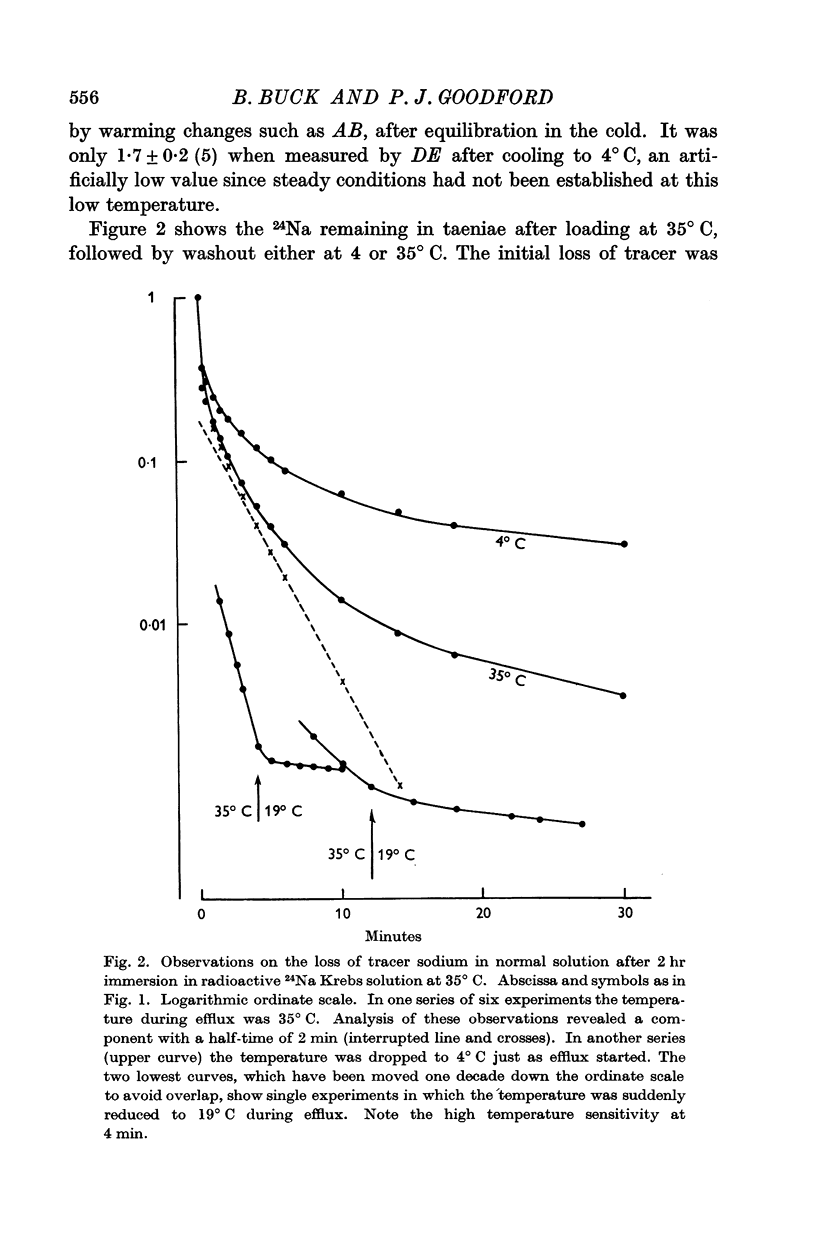
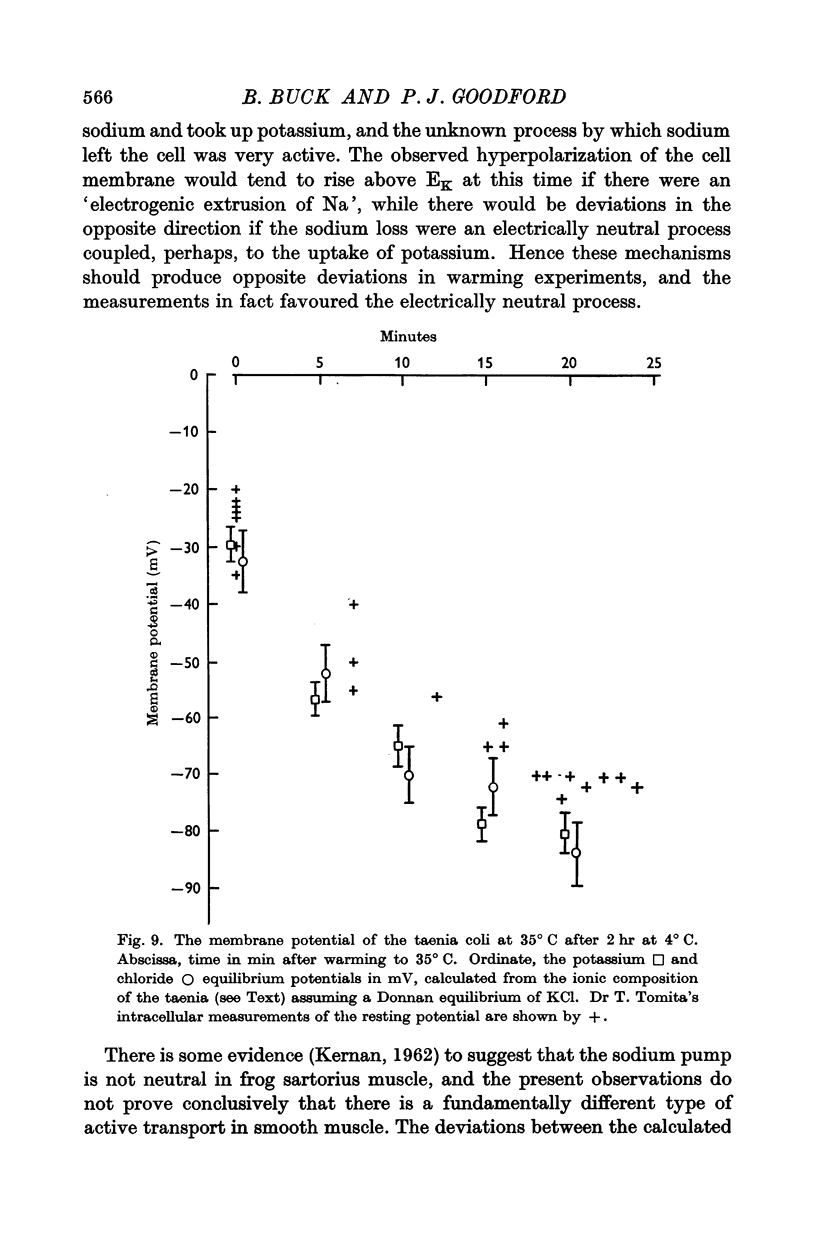
2. The rate of loss of tracer sodium was sensitive to changes of temperature, with an average Q10 of 3·4 ± 0·4 on warming in the range 4-35° C. Exponential analysis of 24Na efflux at 35° C showed an initial rapid phase, a second phase with t½ = 2 min, and a slowly exchanging phase. The second phase was most sensitive to changes of temperature and contained 15 m-mole Na/kg fresh wt. The corresponding transmembrane flux in spontaneously active muscle at 35° C would then be 30 p-mole cm-2 sec-1, and 20% of the available metabolic energy would be needed to maintain this exchange.
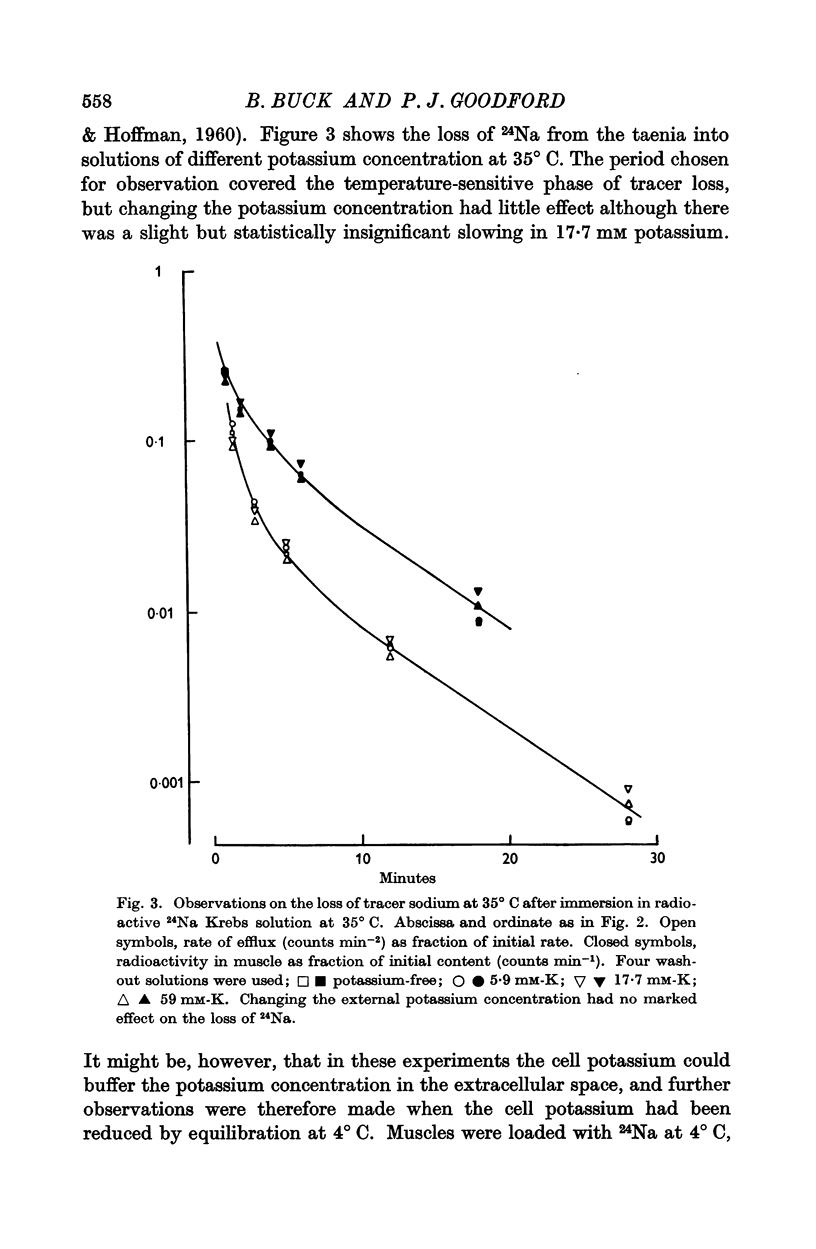
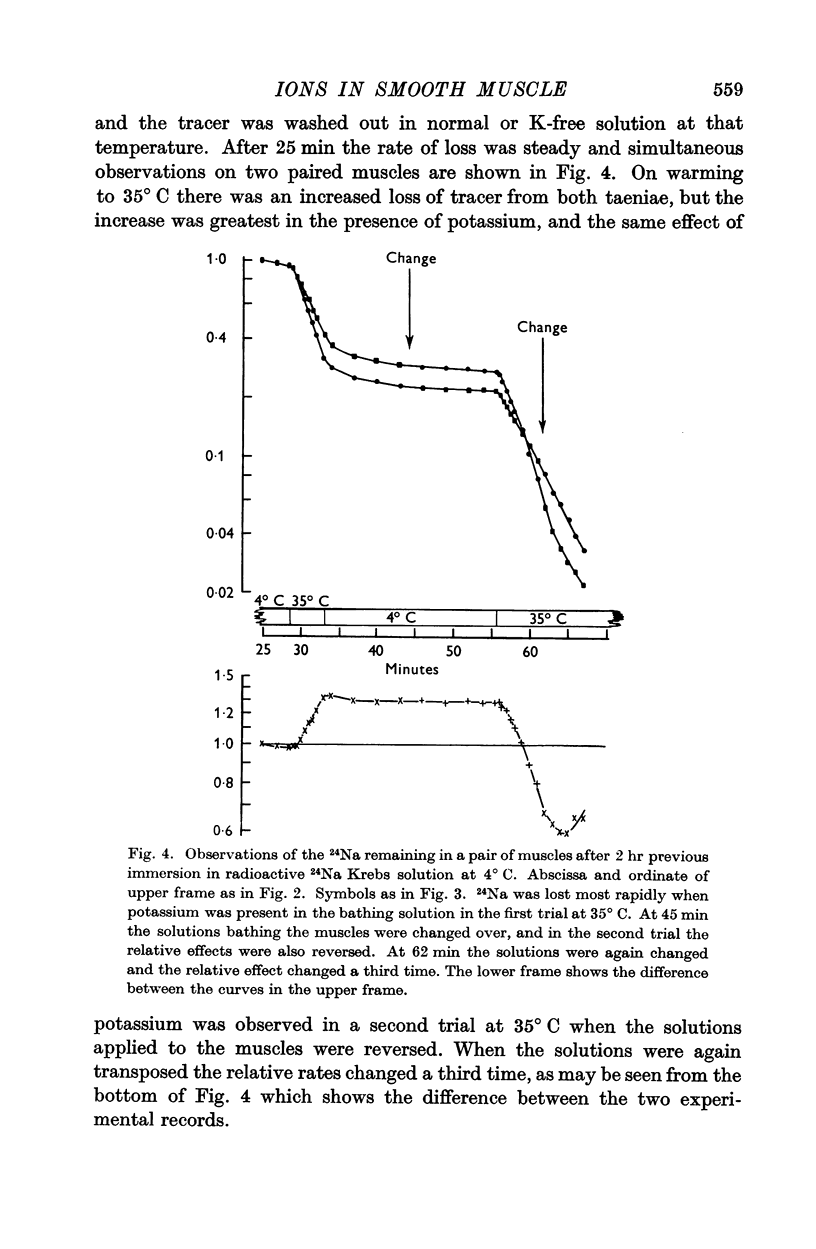
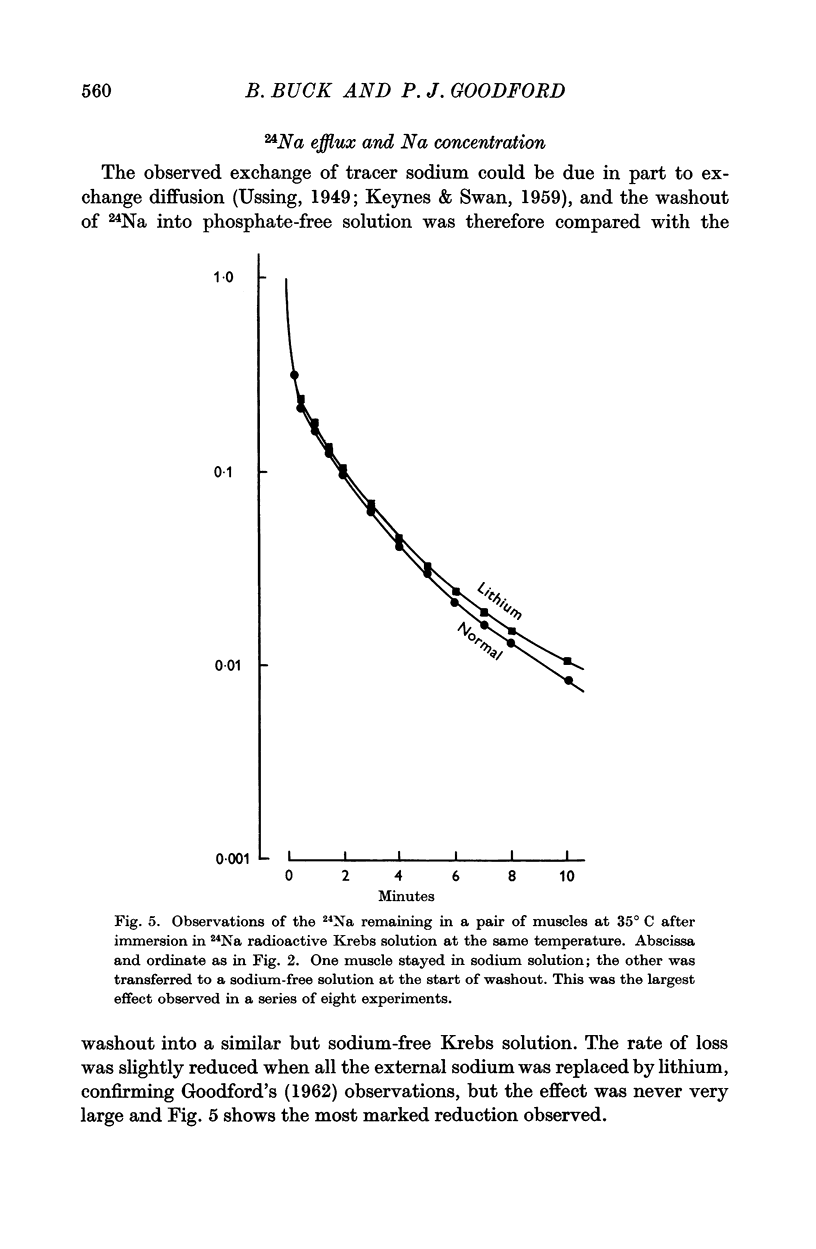
3. The rate of loss of tracer sodium was reduced in potassium-free or sodium-free solutions, but the effects were not impressive.
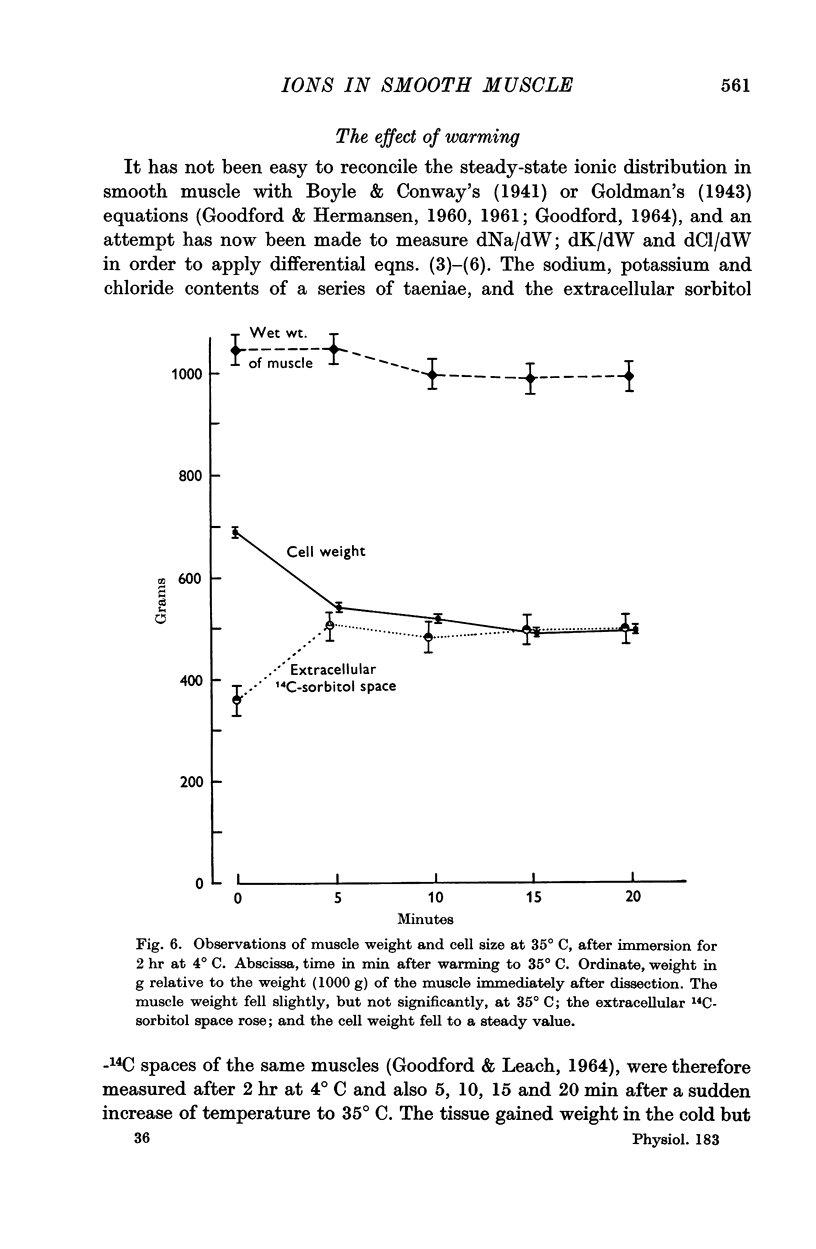
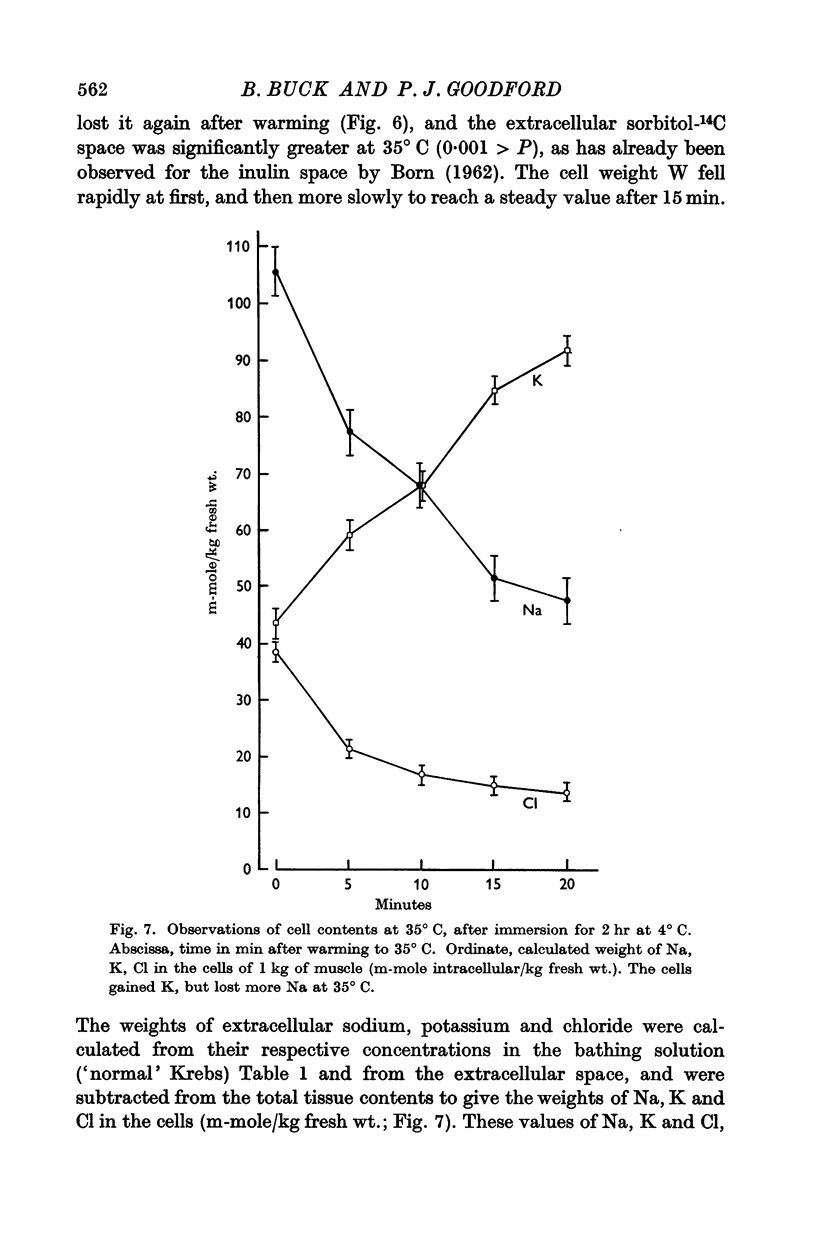
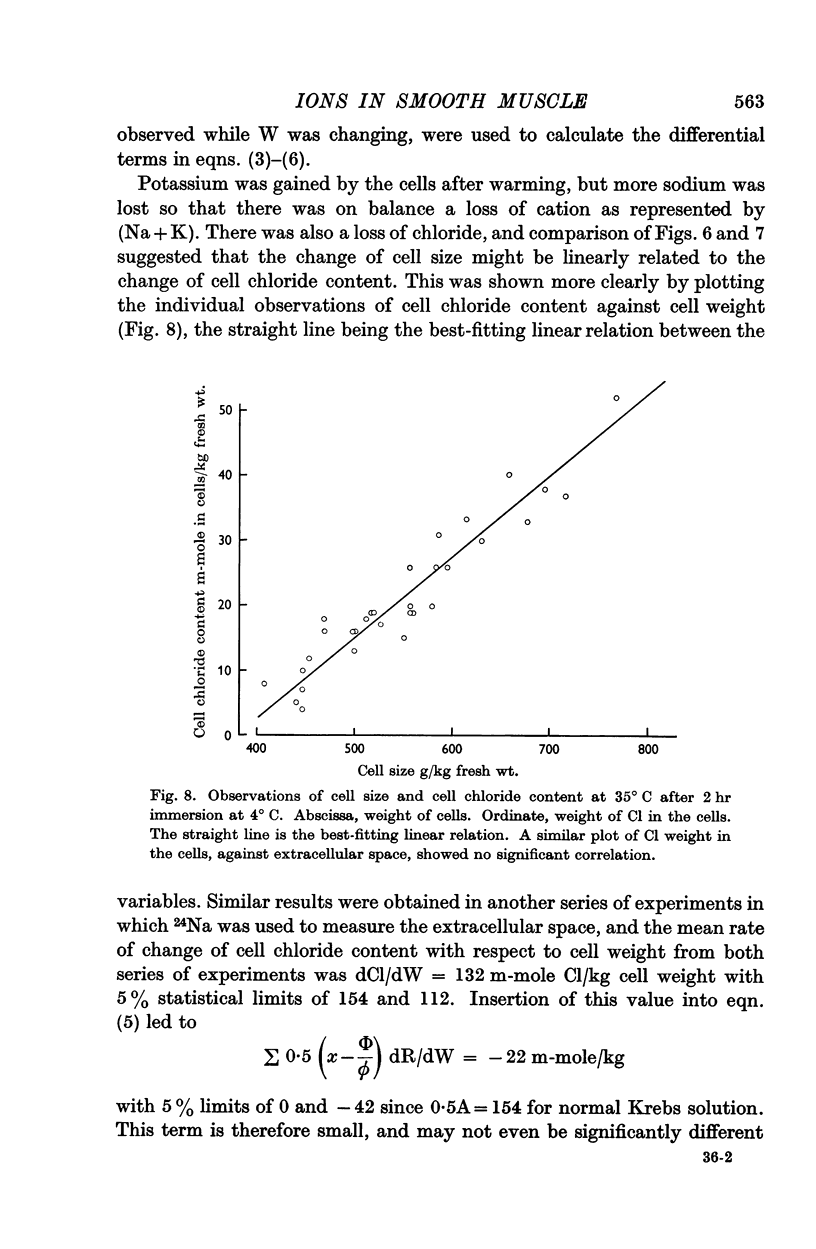
4. The cell size increased at 4° C, and fell again on warming while Na and Cl left the cell and K was taken up. Cell chloride content was linearly related to cell size.
5. The observations are interpreted on the assumption that the cytoplasm is osmotically isotonic and electrically neutral, and that some Na, K and Cl are sequestered elsewhere in the tissue. It is concluded that the quiescent smooth-muscle cell membrane is rather more permeable to sodium than the skeletal-muscle cell membrane.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., BULBRING E. Metabolic factors affecting the electrical activity of intestinal smooth muscle. J Physiol. 1961 Apr;156:344–356. doi: 10.1113/jphysiol.1961.sp006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHR D. F. ELECTROLYTES AND SMOOTH MUSCLE CONTRACTION. Pharmacol Rev. 1964 Mar;16:85–127. [PubMed] [Google Scholar]
- BORN G. V. The fate of 5-hydroxytryptamine in a smooth muscle and in connective tissue. J Physiol. 1962 Apr;161:160–174. doi: 10.1113/jphysiol.1962.sp006879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Measurements of oxygen consumption in smooth muscle. J Physiol. 1953 Oct;122(1):111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. The action of adrenaline on excitability and membrane potential in the taenia coli of the guinea-pig and the effect of DNP on this action and on the action of acetylcholine. J Physiol. 1958 Aug 29;143(1):183–194. doi: 10.1113/jphysiol.1958.sp006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAESAR R., EDWARDS G. A., RUSKA H. Architecture and nerve supply of mammalian smooth muscle tissue. J Biophys Biochem Cytol. 1957 Nov 25;3(6):867–878. doi: 10.1083/jcb.3.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTLOVE E., TRANTHAM H. V., BOWMAN R. L. An instrument and method for automatic, rapid, accurate, and sensitive titration of chloride in biologic samples. J Lab Clin Med. 1958 Mar;51(3):461–468. [PubMed] [Google Scholar]
- FREEMAN-NARROD M., GOODFORD P. J. Sodium and potassium content of the smooth muscle of the guinea-pig taenia coli at different temperatures and tensions. J Physiol. 1962 Oct;163:399–410. doi: 10.1113/jphysiol.1962.sp006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J. CHLORIDE CONTENT AND 36CL UPTAKE IN THE SMOOTH MUSCLE OF THE GUINEA-PIG TAENIA COLI. J Physiol. 1964 Mar;170:227–237. doi: 10.1113/jphysiol.1964.sp007326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J., HERMANSEN K. Sodium and potassium movements in the unstriated muscle of the guinea-pig taenia coli. J Physiol. 1961 Oct;158:426–448. doi: 10.1113/jphysiol.1961.sp006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J. THE LOSS OF RADIOACTIVE 45-CALCIUM FROM THE SMOOTH MUSCLE OF THE GUINEA-PIG TAENIA COLI. J Physiol. 1965 Jan;176:180–190. doi: 10.1113/jphysiol.1965.sp007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J. The sodium content of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1962 Oct;163:411–422. doi: 10.1113/jphysiol.1962.sp006986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. Ionic movements and electrical activity in giant nerve fibres. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):1–37. doi: 10.1098/rspb.1958.0001. [DOI] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., MAISEL G. W. The energy requirement for sodium extrusion from a frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):383–392. doi: 10.1098/rspb.1954.0031. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSOFF D. A. A comparison of methods for measuring efflux of labelled potassium from contracting rabbit atria. J Physiol. 1960 Jul;152:354–366. doi: 10.1113/jphysiol.1960.sp006492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH M. W. THE IN VITRO ABSORPTION OF WATER AND SOLUTES FROM THE INTESTINE OF GOLDFISH, CARASSIUS AURATUS. J Physiol. 1964 Dec;175:38–49. doi: 10.1113/jphysiol.1964.sp007502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]


