Abstract
1. Cats' adrenal glands were acutely denervated and perfused, in situ, with Locke's solution.
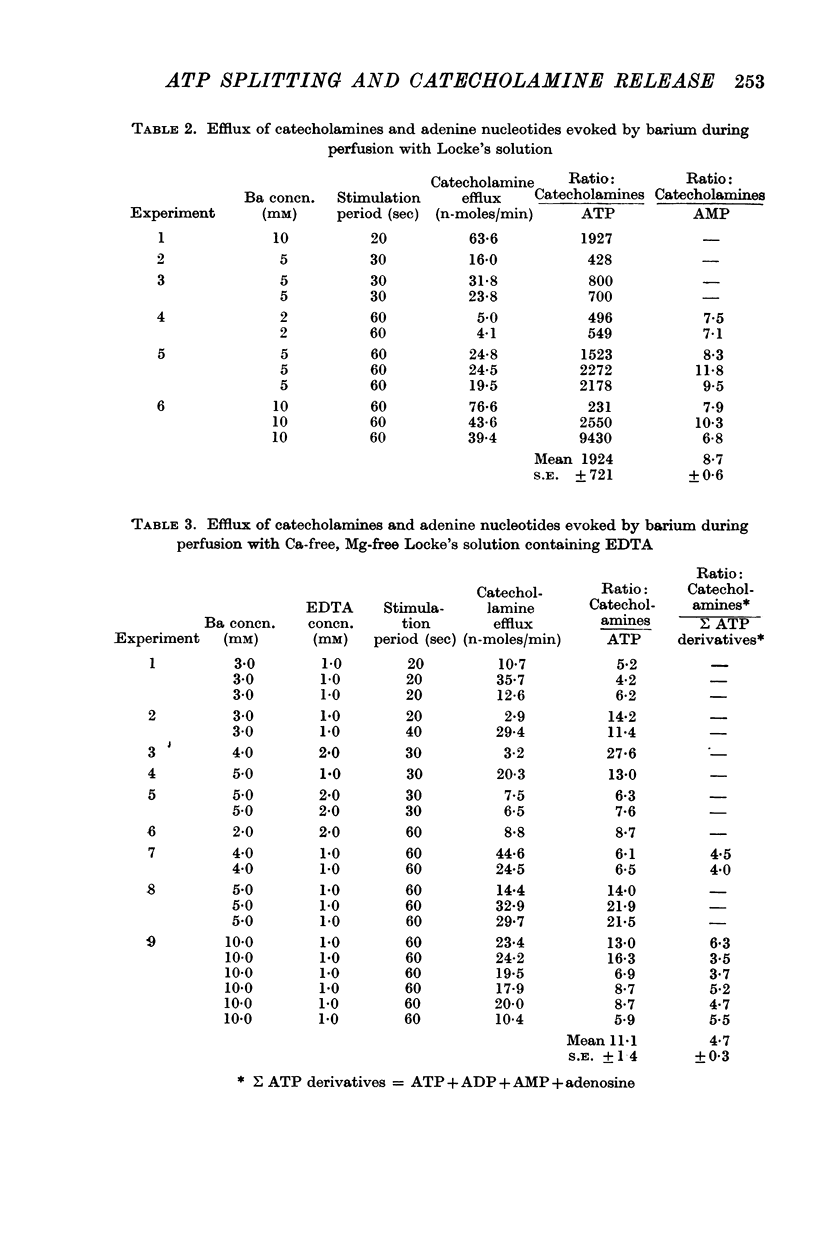
2. When the medullary secretogogue Ba (2-10 mM) was added to the perfusion medium, large amounts of AMP and traces of ATP were found in the venous effluent from the adrenal gland along with the catecholamines. In this respect Ba mimicked the physiological secretogogue, acetylcholine.
3. Control perfusions with known concentrations of ATP showed that under such conditions ATP was rapidly broken down within the adrenal vasculature.
4. When such intravascular hydrolysis of ATP was suppressed by perfusing with a Ca-free, Mg-free Locke's solution containing 1-2 mM EDTA, catecholamine secretion induced by Ba was accompanied by the discharge of large amounts of ATP but relatively little AMP.
5. The ATP that accompanied catecholamine secretion in such circumstances is assumed to derive from the `heavy' nucleotide-rich chromaffin granules and it is concluded that the mechanism for catecholamine secretion does not depend on hydrolysis of the ATP within these granules.
6. The release of ATP (unhydrolysed) supports the hypothesis that the chromaffin granules discharge their contents at the cell surface by the process of `reverse pinocytosis'.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANKS P. THE ADENOSINE-TRIPHOSPHATASE ACTIVITY OF ADRENAL CHROMAFFIN GRANULES. Biochem J. 1965 May;95:490–496. doi: 10.1042/bj0950490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLASCHKO H., BORN G. V., D'IORIO A., EADE N. R. Observations on the distribution of catechol amines and adenosinetriphosphate in the bovine adrenal medulla. J Physiol. 1956 Sep 27;133(3):548–557. doi: 10.1113/jphysiol.1956.sp005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'IORIO A. The release of catecholamines from the isolated chromaffine granules of the adrenal medulla using sulphydryl inhibitors. Can J Biochem Physiol. 1957 Jun;35(6):395–400. [PubMed] [Google Scholar]
- DE ROBERTIS E., VAZ FERREIRA A. Electron microscope study of the excretion of cathecol-containing droplets in the adrenal medulla. Exp Cell Res. 1957 Jun;12(3):568–574. doi: 10.1016/0014-4827(57)90172-6. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Evidence that the secreting adrenal chromaffin cell releases catecholamines directly from ATP-rich granules. J Physiol. 1966 Mar;183(1):236–248. doi: 10.1113/jphysiol.1966.sp007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M., Rubin R. P. Efflux of adenine nucleotides from perfused adrenal glands exposed to nicotine and other chromaffin cell stimulants. J Physiol. 1965 Jul;179(1):130–137. doi: 10.1113/jphysiol.1965.sp007652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARP N. A. Different pools of catecholamines stored in the adrenal medulla. Acta Physiol Scand. 1960 Sep 30;50:8–22. doi: 10.1111/j.1748-1716.1960.tb02068.x. [DOI] [PubMed] [Google Scholar]
- HILLARP N. A. Enzymic systems involving adenosinephosphates in the adrenaline and noradrenaline containing granules of the adrenal medulla. Acta Physiol Scand. 1958 Feb 4;42(2):144–165. doi: 10.1111/j.1748-1716.1958.tb01548.x. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. Studies on the Na ion and K ion activated ATP hydrolysing enzyme system. The role of SH groups. Biochem Biophys Res Commun. 1963 Jan 18;10:79–84. doi: 10.1016/0006-291x(63)90272-9. [DOI] [PubMed] [Google Scholar]


