Abstract
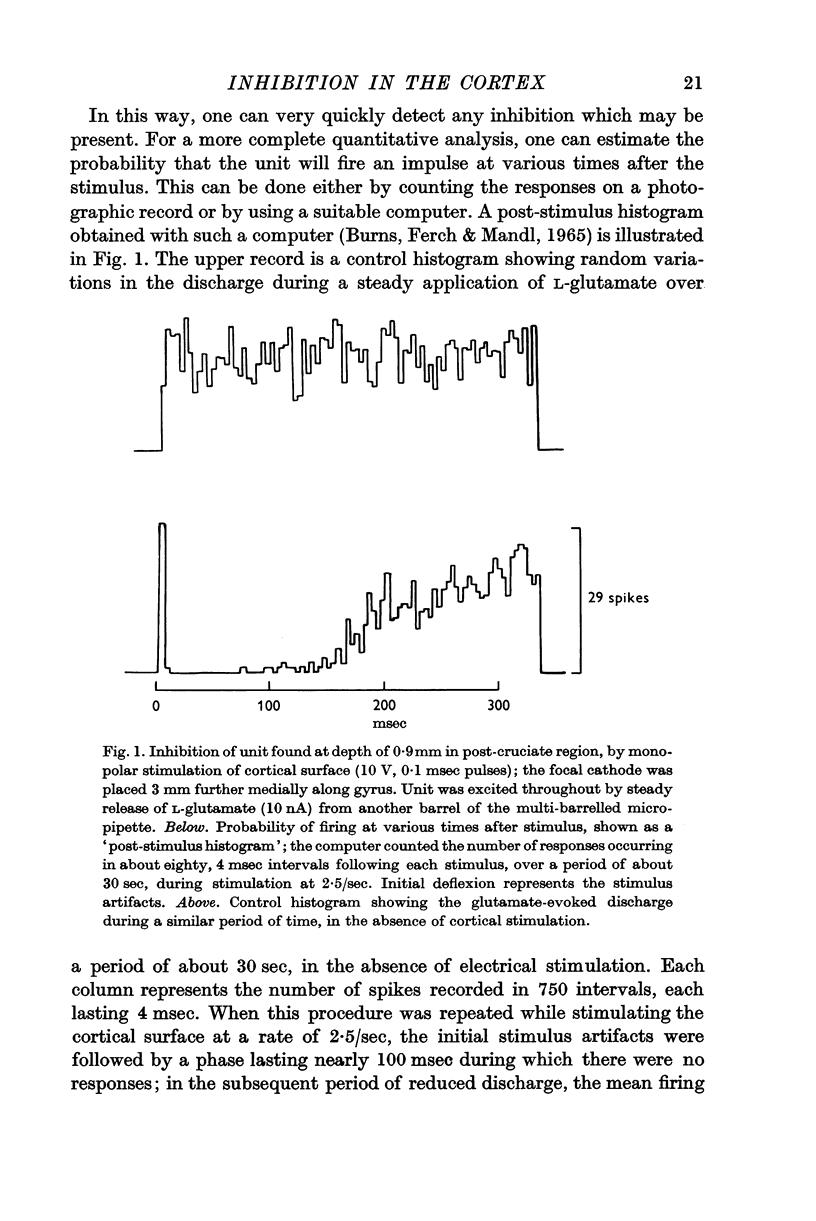
1. In cats, rabbits and monkeys, single cortical shocks can reduce the excitability of cortical neurones for 100-300 msec; the inhibitory effect is readily demonstrated, even in previously quiescent cells, against a background of activity evoked with small amounts of L-glutamate, released from an extracellular recording micropipette by iontophoresis.
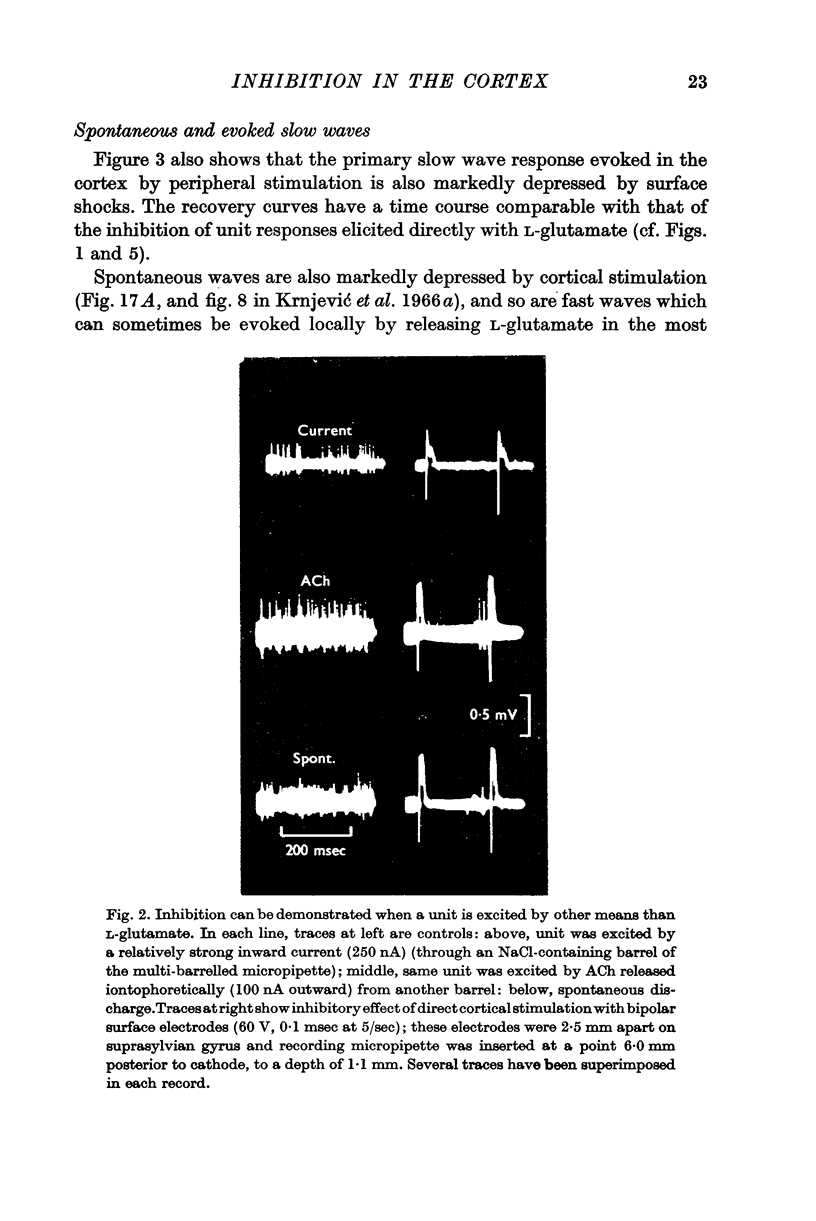
2. Other forms of cortical activity are also inhibited in a similar way by direct or indirect cortical stimulation; they include single unit discharges produced by iontophoretic applications of ACh or by a cathodal current, spontaneous discharges, and slow wave activity, both spontaneous and evoked.
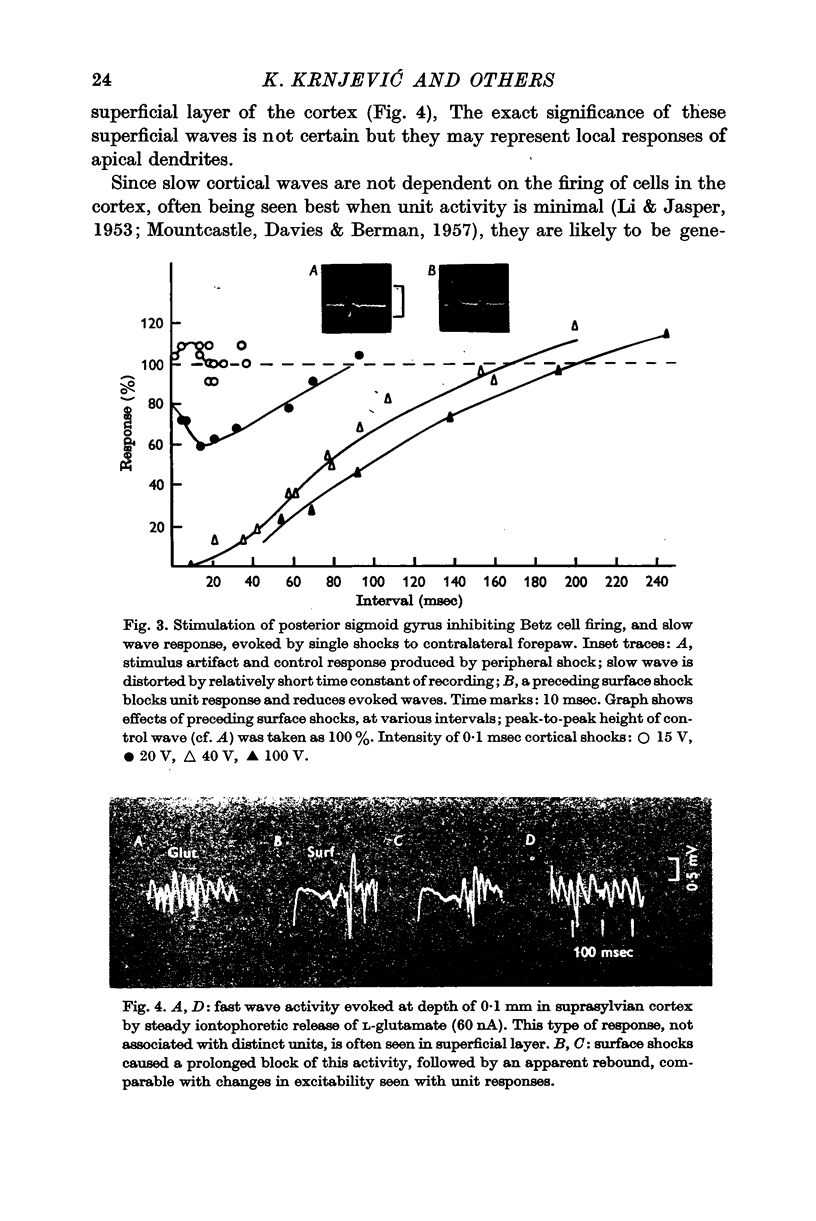
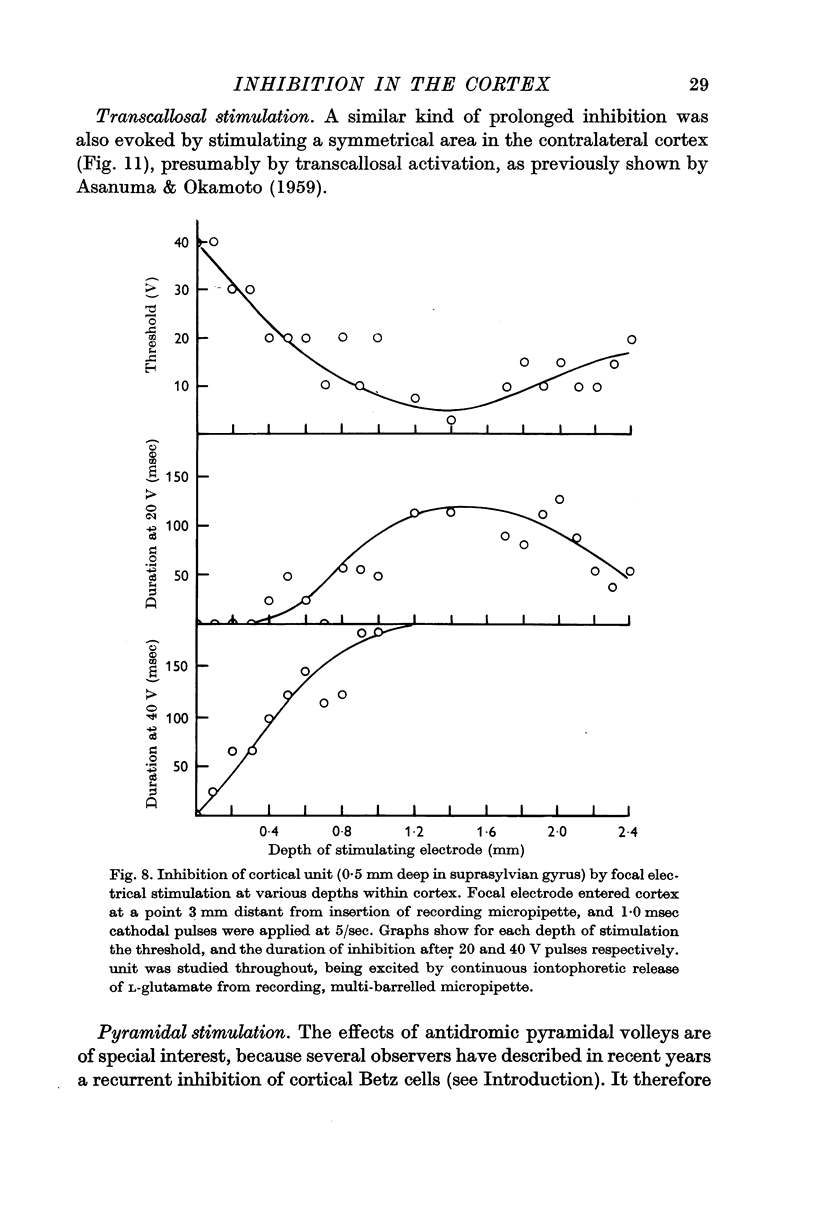
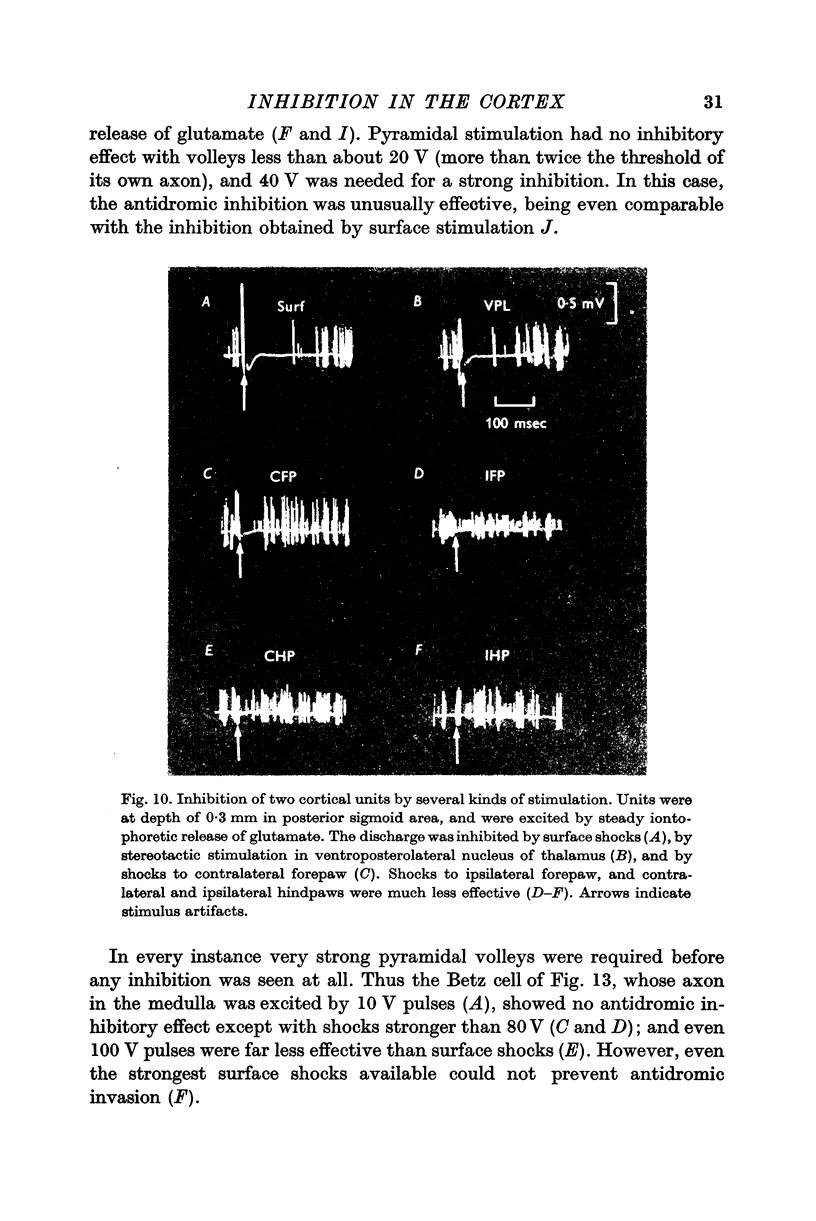
3. Most stimuli which elicit cortical activity also evoke some inhibition in the cortex, for instance, transcallosal volleys, and thalamic or peripheral shocks. In each case, a characteristic, prolonged depression is produced by single shocks.
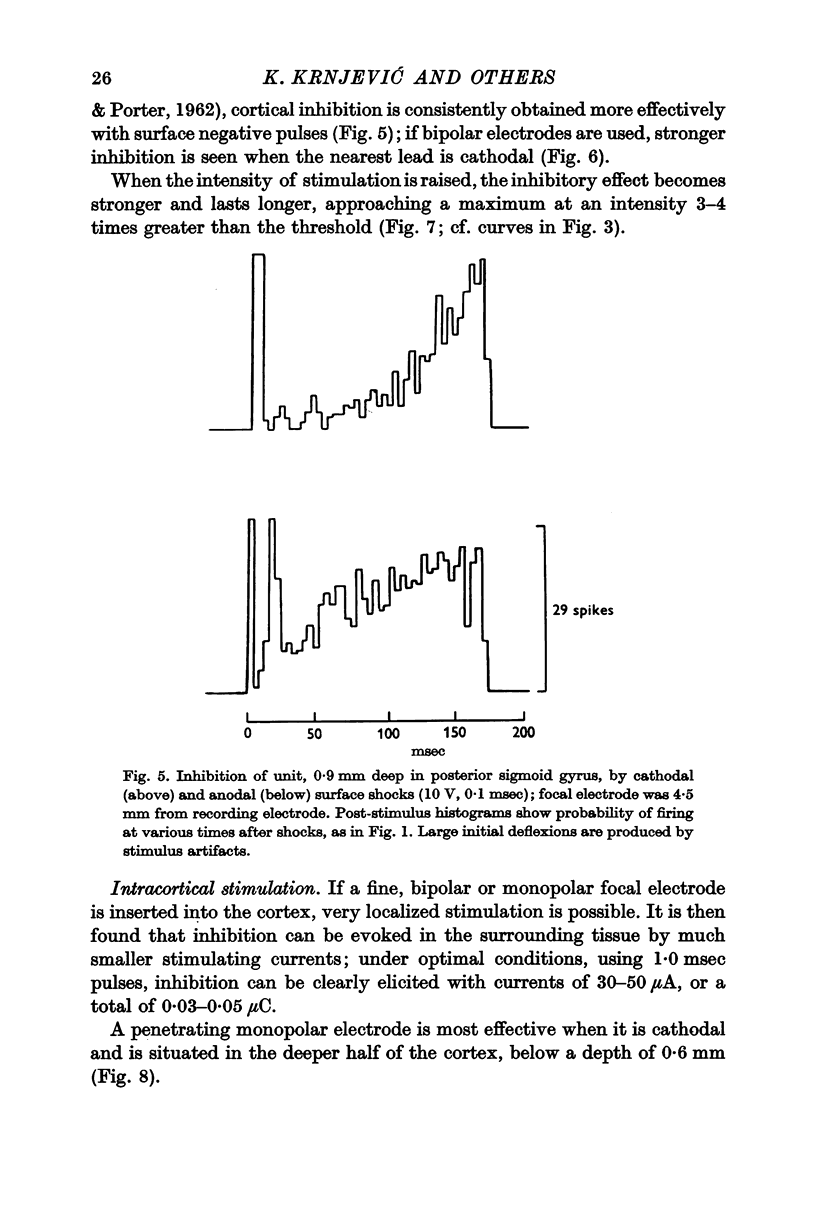
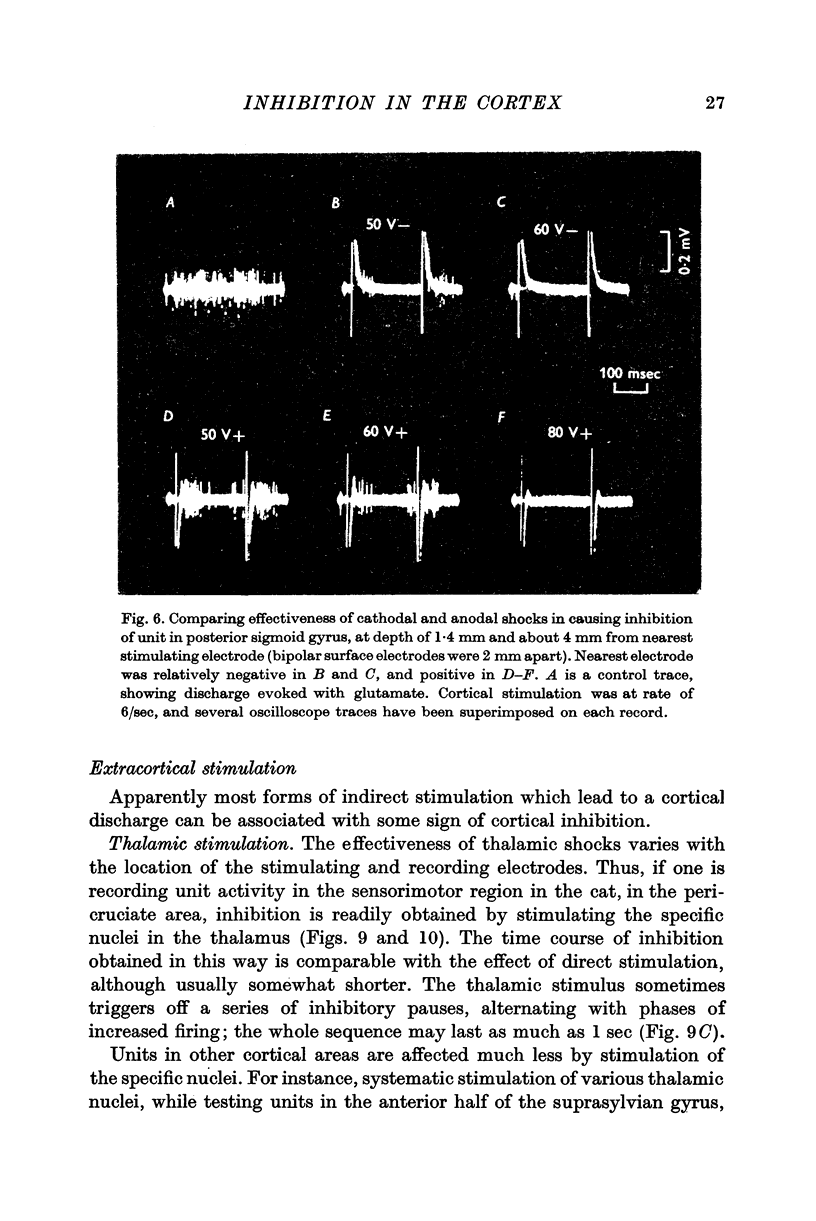
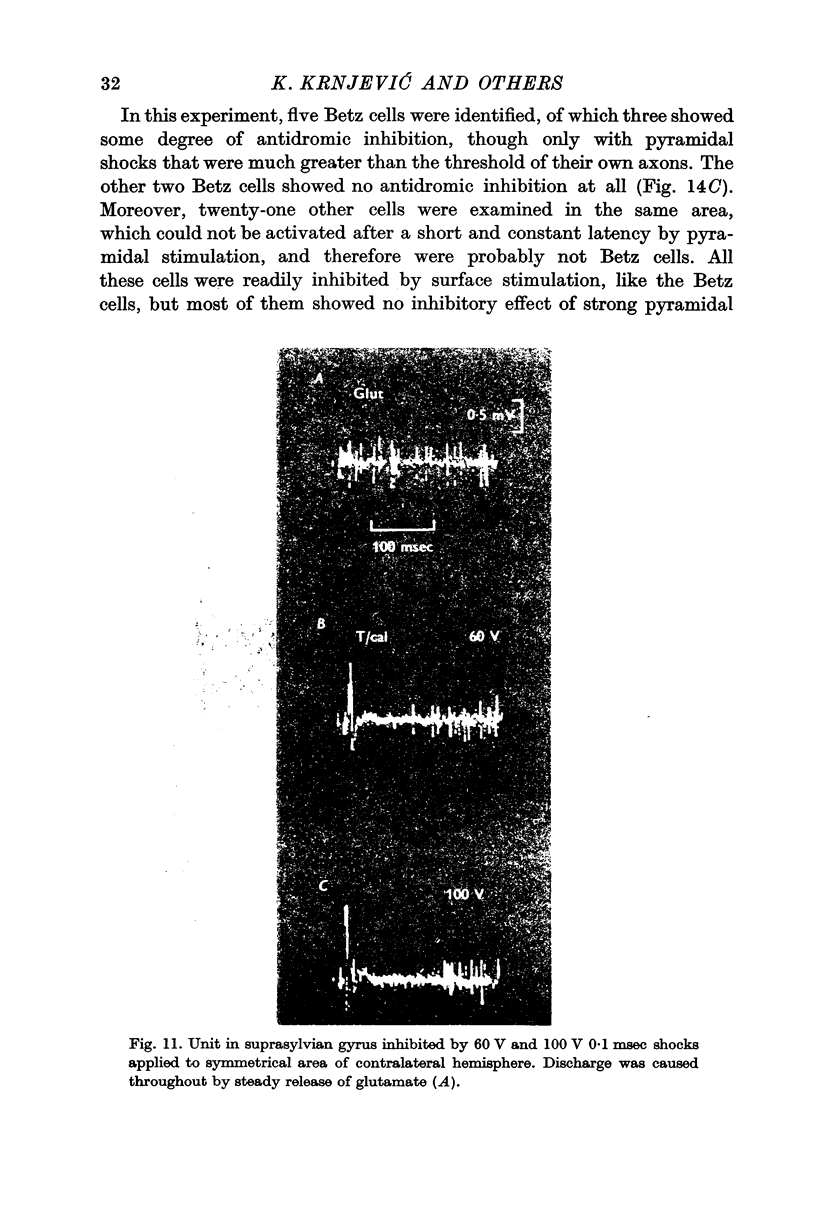
4. The most effective stimuli are direct cortical shocks, especially when applied within the cortex, below a depth of 0·6 mm; surface cathodal shocks are more effective than anodal shocks. These stimuli do not first excite the cells which are inhibited and they are not strong enough to cause appreciable local injury.
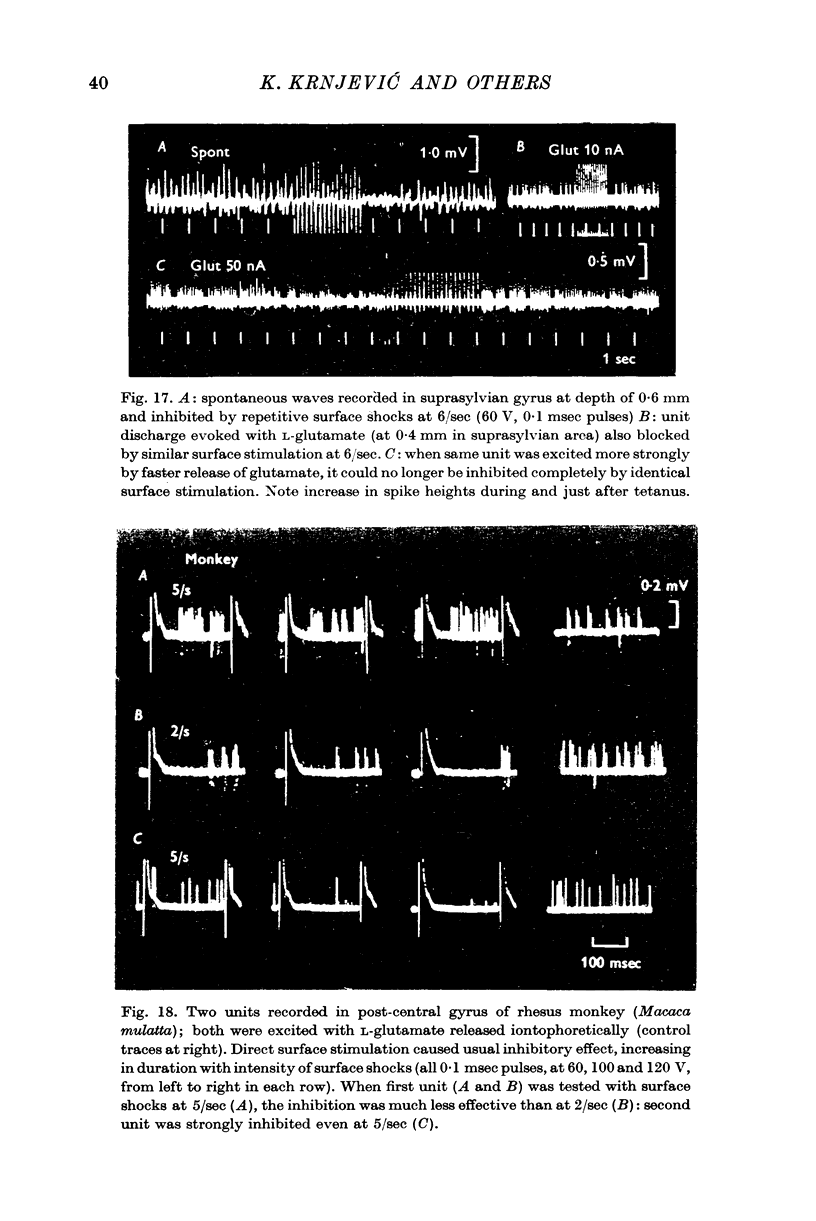
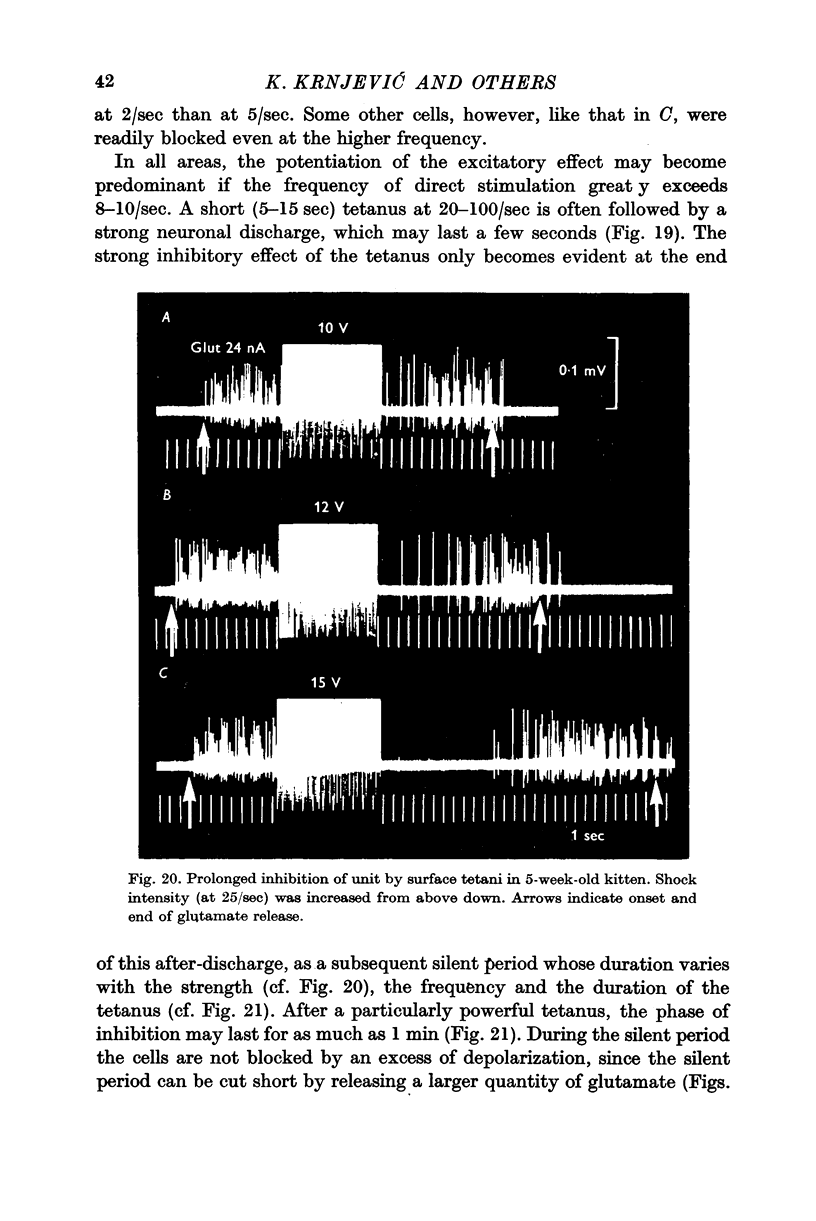
5. Because of its long duration, the inhibition is often readily maintained by repetitive stimulation at frequencies of 5-7/sec. A cumulative effect leads to a further silent period after the end of stimulation; this increases with the strength, frequency and duration of the tetanus, so that after stimulation at 50-100/sec, the silent period may last for over 1 min. During this time, a stronger depolarizing stimulus can initiate firing.
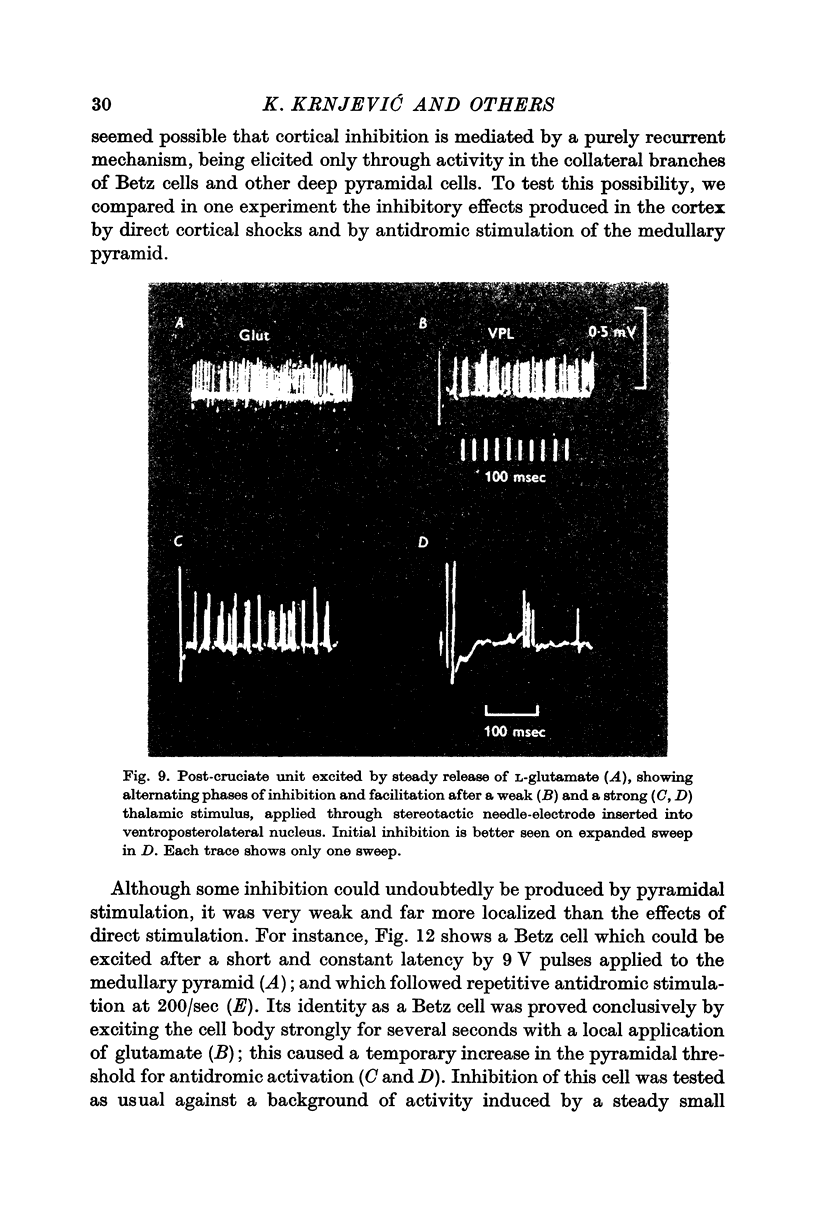
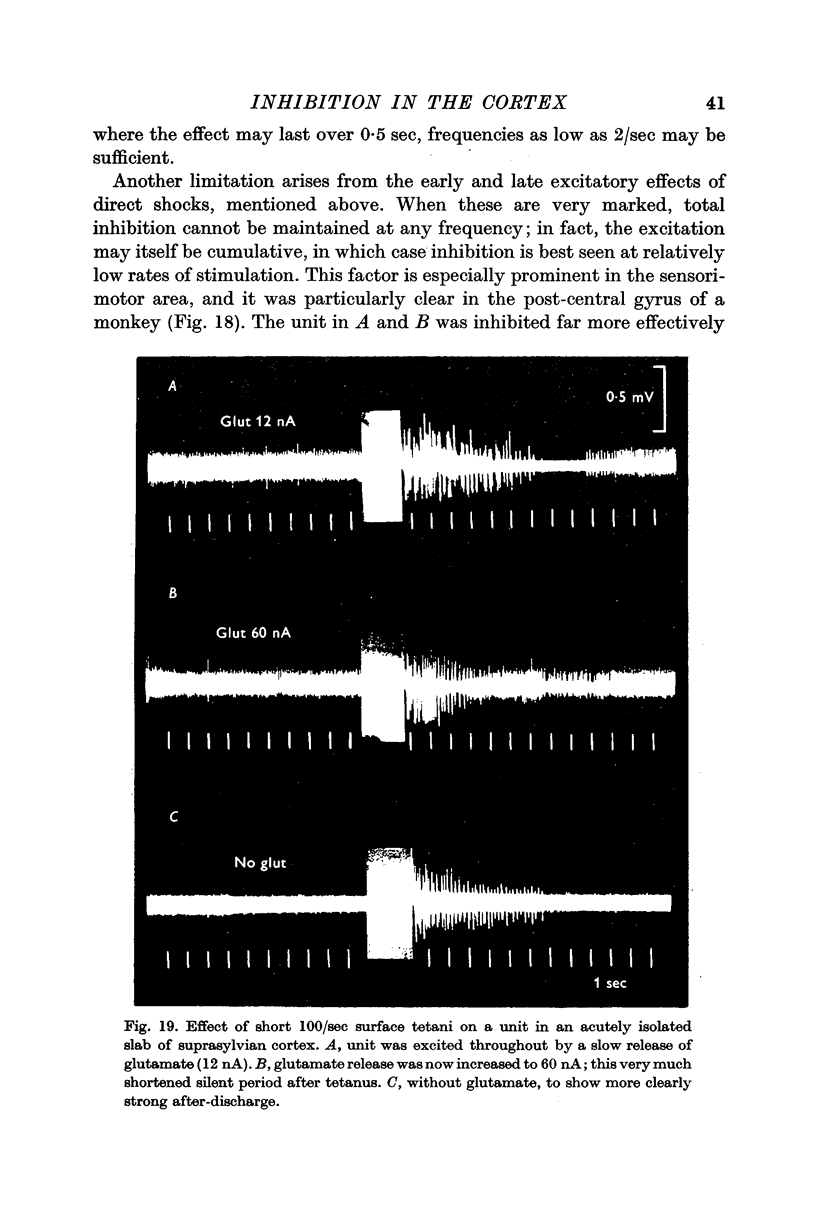
6. The inhibitory effect is often preceded and followed by phases of increased excitability; these may also show cumulative enhancement during repetitive stimulation, and a high frequency tetanus often leads to a short after-discharge, which is then followed by a long silent period, as above. Comparable changes take place in rabbits during spreading depression.
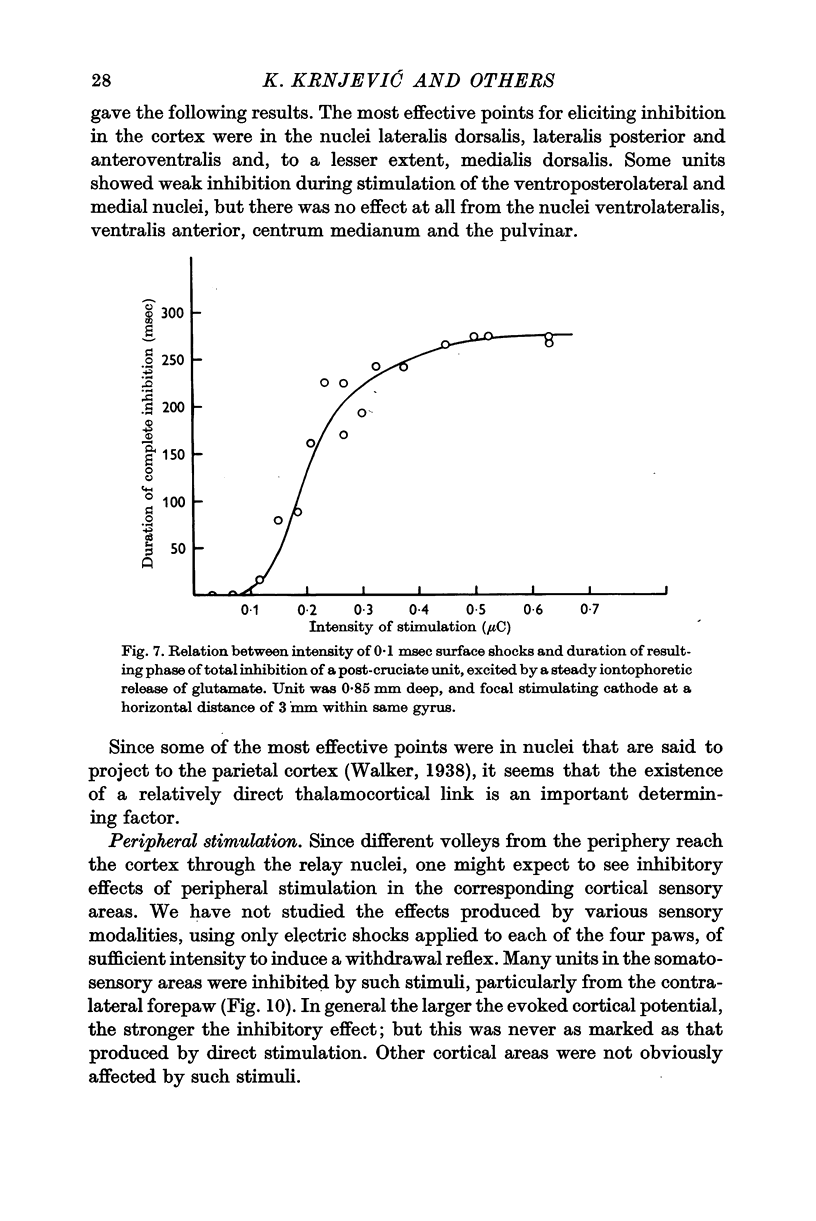
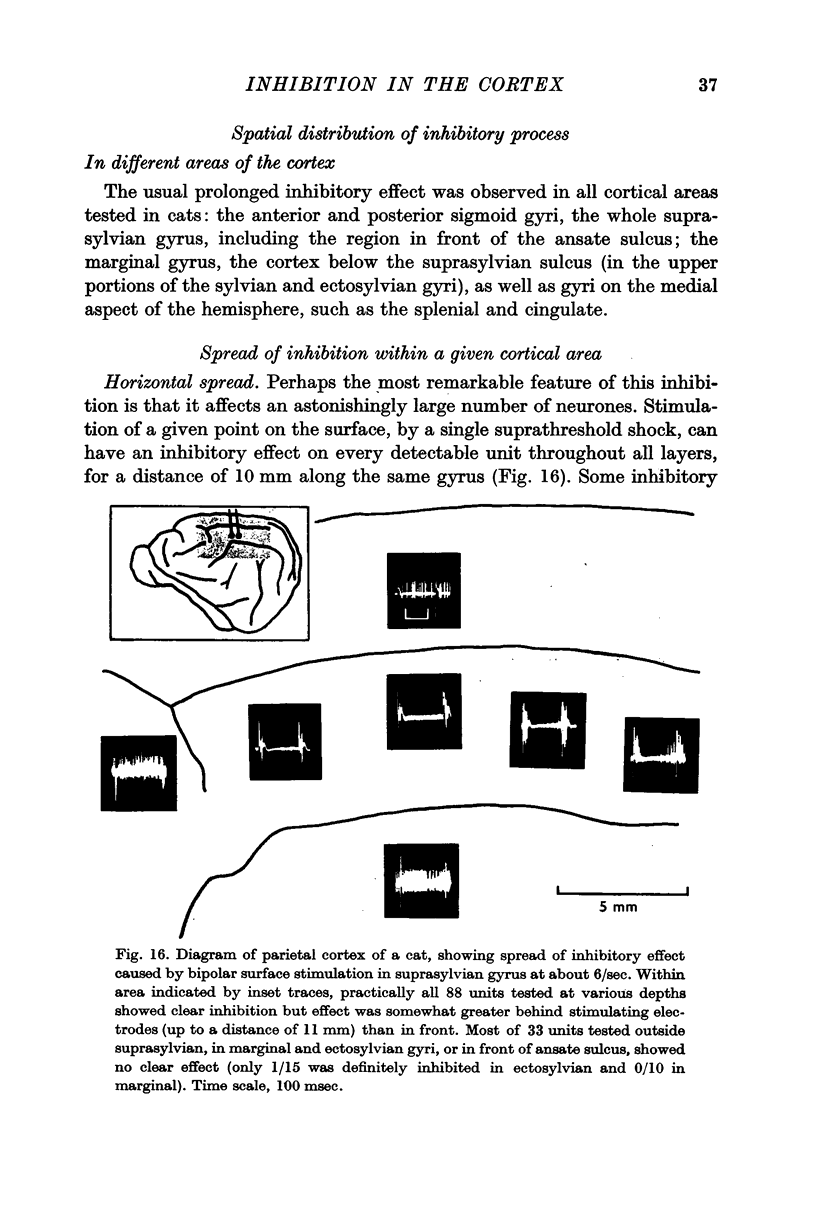
7. The inhibitory effect of a direct shock can spread over an area covering 1 cm of cortical surface, affecting the cells through all cortical layers; but the spread is uneven in different directions, being particularly poor under most sulci.
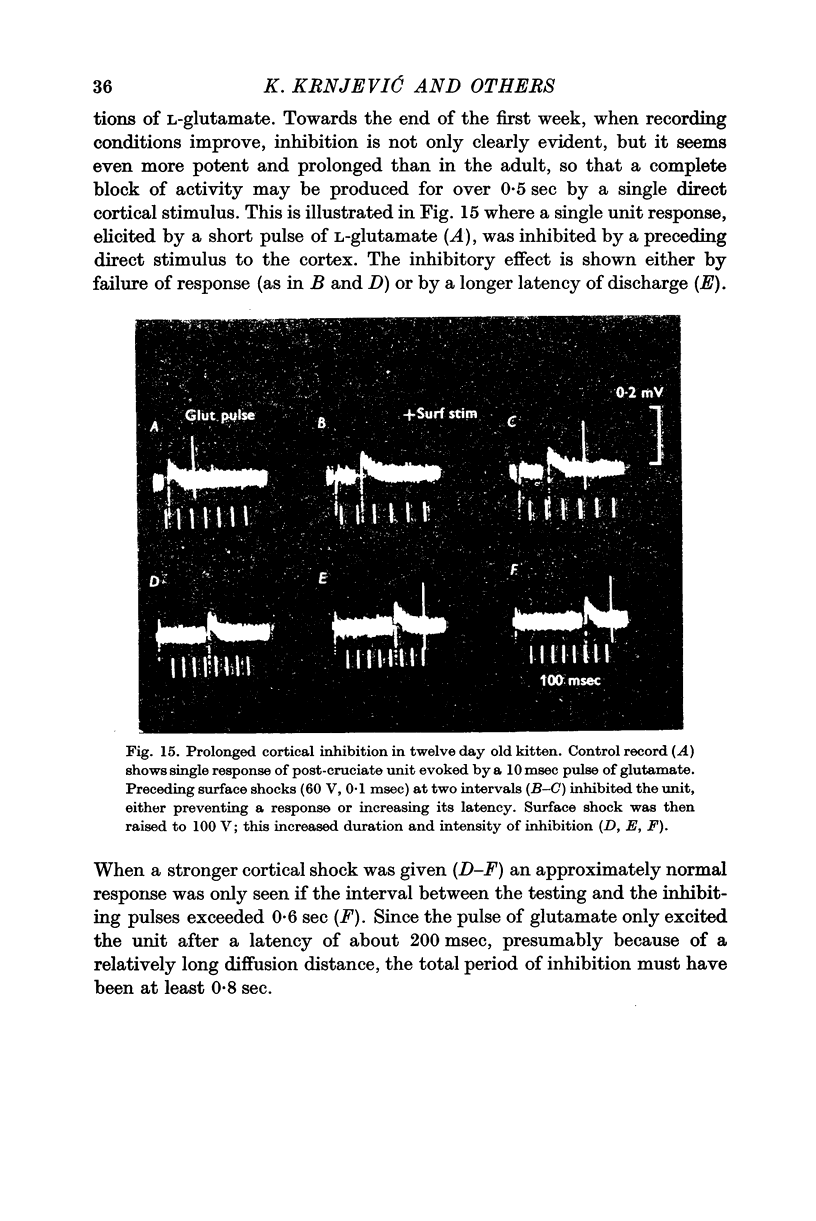
8. This type of inhibition can be elicited in all areas of the neocortex, and it is evident in kittens within a week of birth.
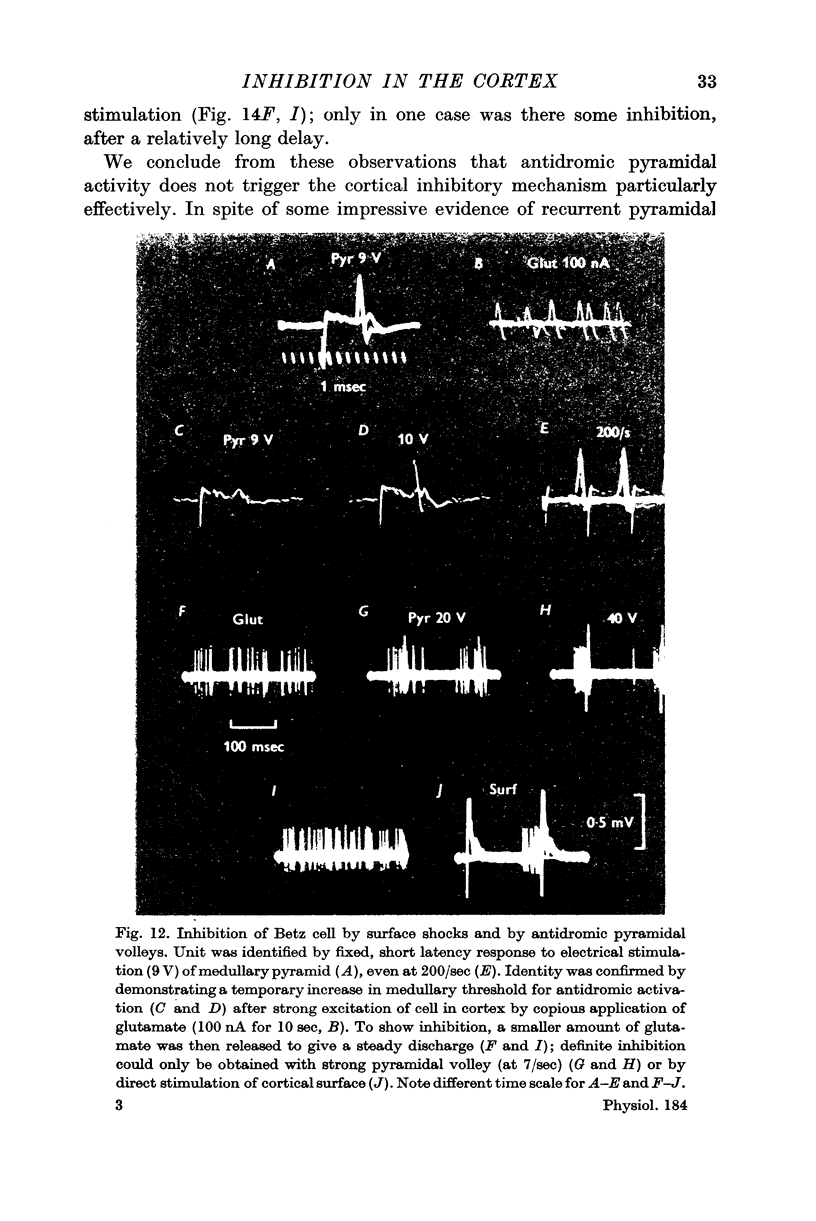
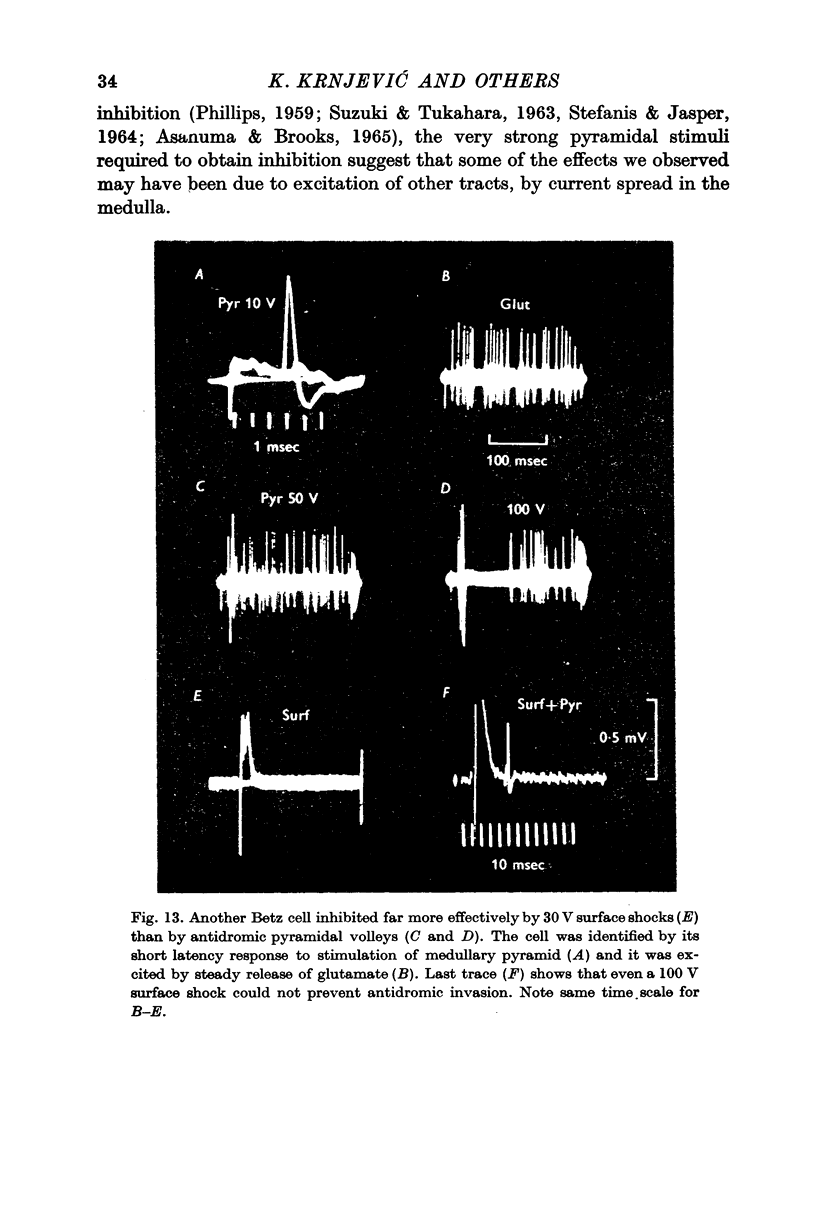
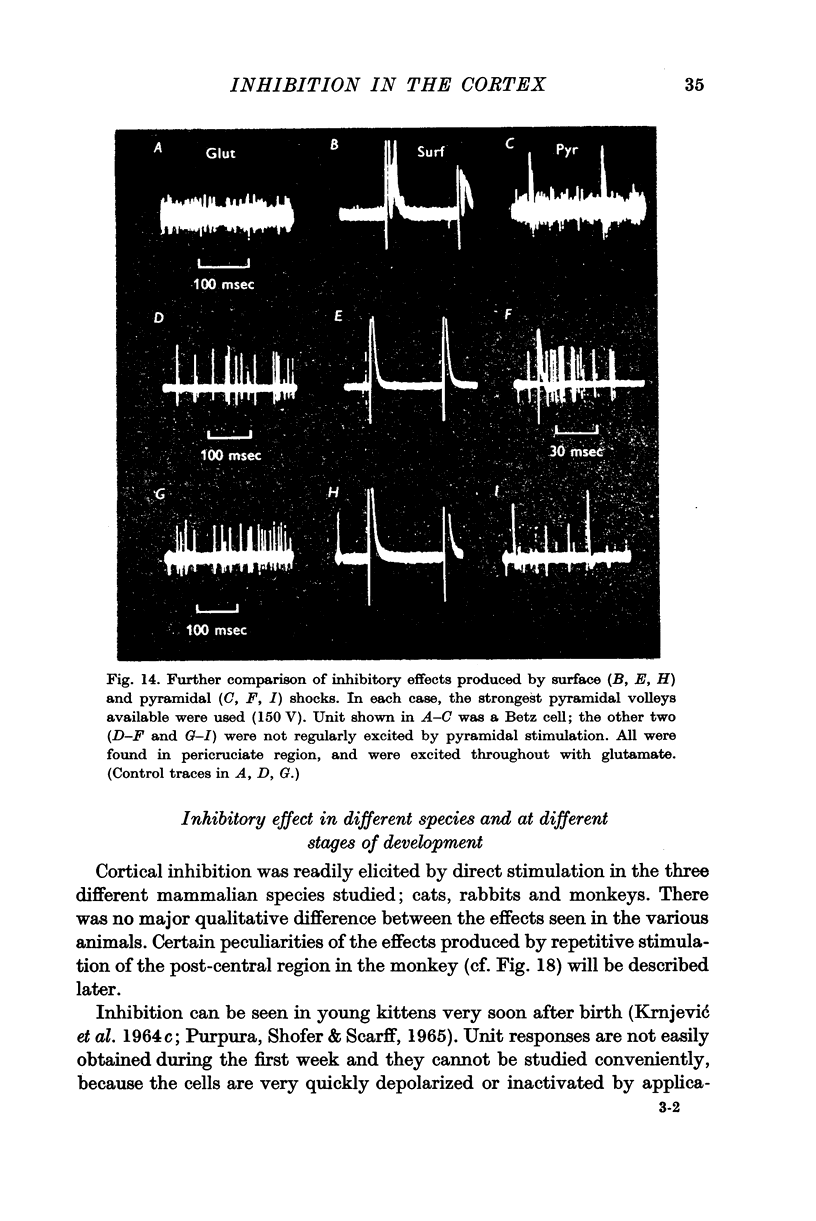
9. Antidromic pyramidal stimulation is very much less effective in evoking inhibition of Betz cells, and other cortical neurones, than direct cortical stimulation; the inhibition by direct shocks is therefore not likely to be mediated through pyramidal excitation.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMASSIAN V. E. Evoked single cortical unit activity in the somatic sensory areas. Electroencephalogr Clin Neurophysiol. 1953 Aug;5(3):415–438. doi: 10.1016/0013-4694(53)90084-4. [DOI] [PubMed] [Google Scholar]
- ASANUMA H., BROOKS V. B. RECURRENT CORTICAL EFFECTS FOLLOWING STIMULATION OF INTERNAL CAPSULE. Arch Ital Biol. 1965 Jun 10;103:220–246. [PubMed] [Google Scholar]
- ASANUMA H., OKAMOTO K. Unitary study on evoked activity of callosal neurons and its effect on pyramidal tract cell activity on cats. Jpn J Physiol. 1959 Dec 15;9:473–483. doi: 10.2170/jjphysiol.9.473. [DOI] [PubMed] [Google Scholar]
- Adrian E. D. The spread of activity in the cerebral cortex. J Physiol. 1936 Nov 6;88(2):127–161. doi: 10.1113/jphysiol.1936.sp003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMGARTNER G., CREUTZFELDT O., SCHOEN L. Reaktionen einzelner Neurone des senso-motorischen Cortex nach elektrischen Reizen. I. Hemmung und Erregung nach direkten und kontralateralen Einzelreizen. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1956;194(6):597–619. doi: 10.1007/BF00342874. [DOI] [PubMed] [Google Scholar]
- BRANCH C. L., MARTIN A. R. Inhibition of Betz cell activity by thalamic and cortical stimulation. J Neurophysiol. 1958 Jul;21(4):380–390. doi: 10.1152/jn.1958.21.4.380. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B., ASANUMA H. RECURRENT CORTICAL EFFECTS FOLLOWING STIMULATION OF MEDULLARY PYRAMID. Arch Ital Biol. 1965 Jun 10;103:247–278. [PubMed] [Google Scholar]
- BURNS B. D., SALMOIRAGHI G. C. Repetitive firing of respiratory neurones during their burst activity. J Neurophysiol. 1960 Jan;23:27–46. doi: 10.1152/jn.1960.23.1.27. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960 Mar;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, LANDAU W. M. Some relations between resistivity and electrical activity in the cerebral cortex of the cat. J Cell Physiol. 1955 Jun;45(3):377–392. doi: 10.1002/jcp.1030450305. [DOI] [PubMed] [Google Scholar]
- GRAFSTEIN B. Mechanism of spreading cortical depression. J Neurophysiol. 1956 Mar;19(2):154–171. doi: 10.1152/jn.1956.19.2.154. [DOI] [PubMed] [Google Scholar]
- HERN J. E., LANDGREN S., PHILLIPS C. G., PORTER R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon's motor cortex. J Physiol. 1962 Apr;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG R. Physiological basis of the electroencephalogram; neuronal discharge. Electroencephalogr Clin Neurophysiol Suppl. 1955;Suppl. 4:57–71. [PubMed] [Google Scholar]
- KREINER J. The myeloarchitectonics of the frontal cortex of the dog. J Comp Neurol. 1961 Apr;116:117–133. doi: 10.1002/cne.901160203. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., RANDIC M., STRAUGHAN D. W. CORTICAL INHIBITION. Nature. 1964 Mar 28;201:1294–1296. doi: 10.1038/2011294a0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Randić M., Straughan D. W. Pharmacology of cortical inhibition. J Physiol. 1966 May;184(1):78–105. doi: 10.1113/jphysiol.1966.sp007904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. L., CHOU S. N. Cortical intracellular synaptic potentials and direct cortical stimulation. J Cell Comp Physiol. 1962 Aug;60:1–16. doi: 10.1002/jcp.1030600102. [DOI] [PubMed] [Google Scholar]
- LI C. L., JASPER H. Microelectrode studies of the electrical activity of the cerebral cortex in the cat. J Physiol. 1953 Jul;121(1):117–140. doi: 10.1113/jphysiol.1953.sp004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. L. The inhibitory effect of stimulation of a thalamic nucleus on neuronal activity in the motor cortex. J Physiol. 1956 Jul 27;133(1):40–53. doi: 10.1113/jphysiol.1956.sp005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL W. H. Spreading cortical depression of Leao. Physiol Rev. 1959 Apr;39(2):239–279. doi: 10.1152/physrev.1959.39.2.239. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., DAVIES P. W., BERMAN A. L. Response properties of neurons of cat's somatic sensory cortex to peripheral stimuli. J Neurophysiol. 1957 Jul;20(4):374–407. doi: 10.1152/jn.1957.20.4.374. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POWELL T. P. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp. 1959 Oct;105:201–232. [PubMed] [Google Scholar]
- NACIMIENTO A. C., LUX H. D., CREUTZFELDT O. D. POSTSYNAPTISCHE POTENTIALE VON NERVENZELLEN DES MOTORISCHEN CORTEX NACH ELEKTRISCHER REIZUNG SPEZIFISCHER UND UNSPEZIFISCHER THALAMUSKERNE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Oct 5;281:152–169. [PubMed] [Google Scholar]
- PURPURA D. P., SHOFER R. J. CORTICAL INTRACELLULAR POTENTIALS DURING AUGMENTING AND RECRUITING RESPONSES. I. EFFECTS OF INJECTED HYPERPOLARIZING CURRENTS ON EVOKED MEMBRANE POTENTIAL CHANGES. J Neurophysiol. 1964 Mar;27:117–132. doi: 10.1152/jn.1964.27.2.117. [DOI] [PubMed] [Google Scholar]
- STEFANIS C., JASPER H. INTRACELLULAR MICROELECTRODE STUDIES OF ANTIDROMIC RESPONSES IN CORTICAL PYRAMIDAL TRACT NEURONS. J Neurophysiol. 1964 Sep;27:828–854. doi: 10.1152/jn.1964.27.5.828. [DOI] [PubMed] [Google Scholar]
- STOHR P. E., GOLDRING S., O'LEARY J. L. PATTERNS OF UNIT DISCHARGE ASSOCIATED WITH DIRECT CORTICAL RESPONSE IN MONKEY AND CAT. Electroencephalogr Clin Neurophysiol. 1963 Oct;15:882–888. doi: 10.1016/0013-4694(63)90177-9. [DOI] [PubMed] [Google Scholar]
- STRUMWASSER F., ROSENTHAL S. Prolonged and patterned direct extracellular stimulation of single neurons. Am J Physiol. 1960 Feb;198:405–413. doi: 10.1152/ajplegacy.1960.198.2.405. [DOI] [PubMed] [Google Scholar]
- von EULER, GREEN J. D. Activity in single hippocampal pyramids. Acta Physiol Scand. 1960 Mar 18;48:95–109. doi: 10.1111/j.1748-1716.1960.tb01850.x. [DOI] [PubMed] [Google Scholar]


