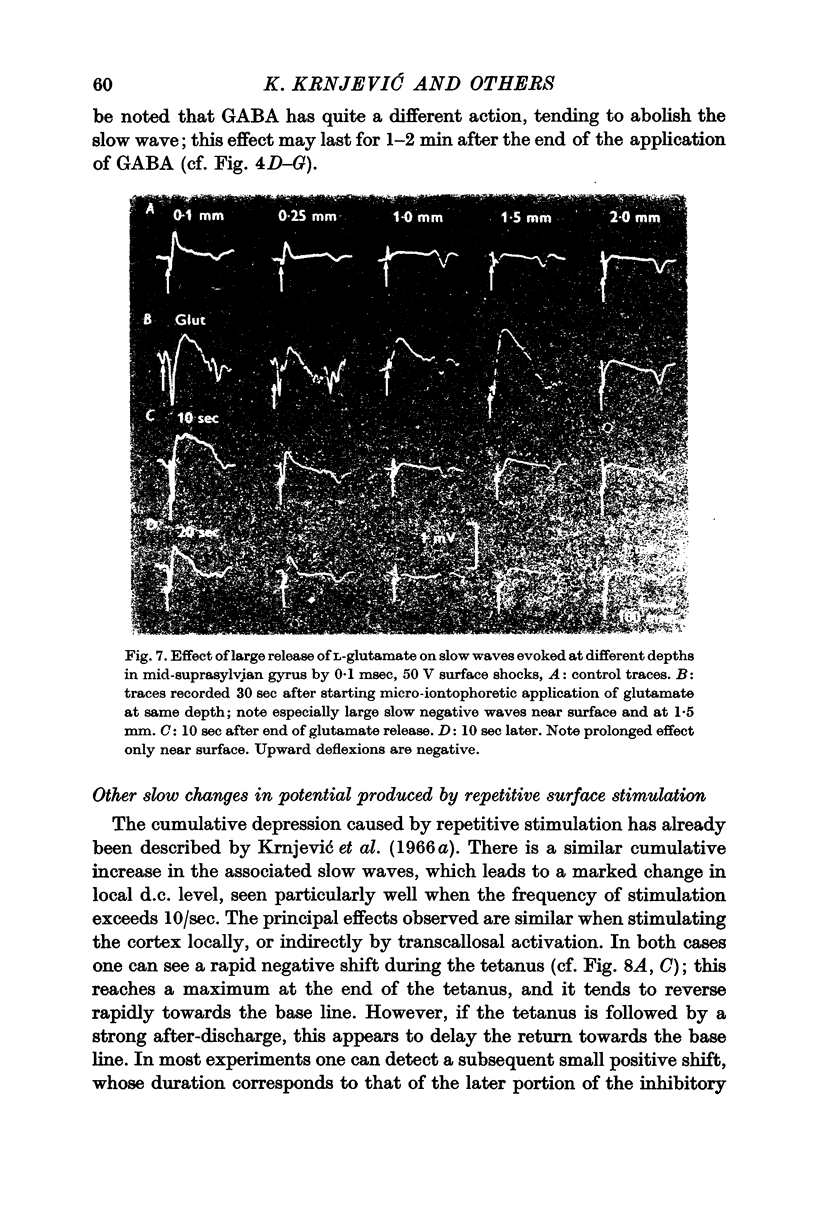
Abstract
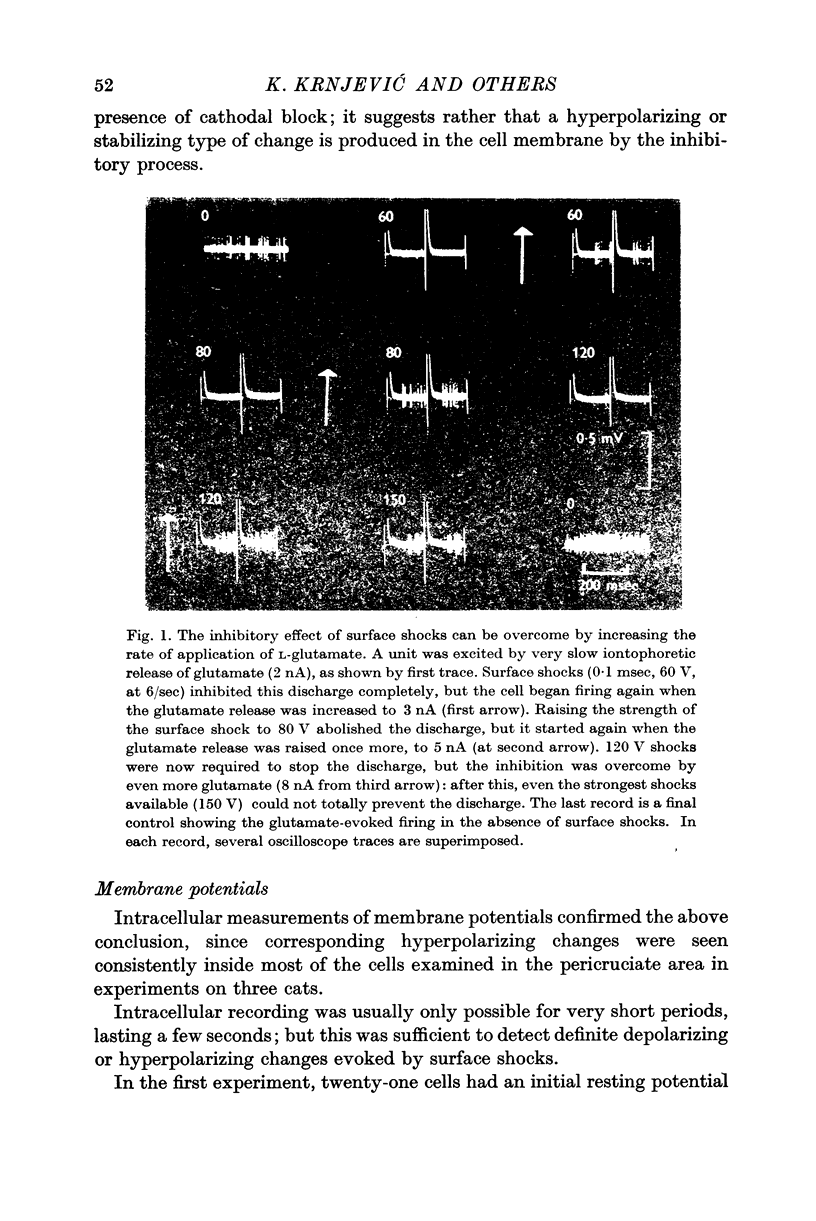
1. Since the inhibitory effect of direct or indirect cortical stimulation on cortical units can be overcome by excitation with even more L-glutamate, it is not likely to be due to an excessive depolarization.
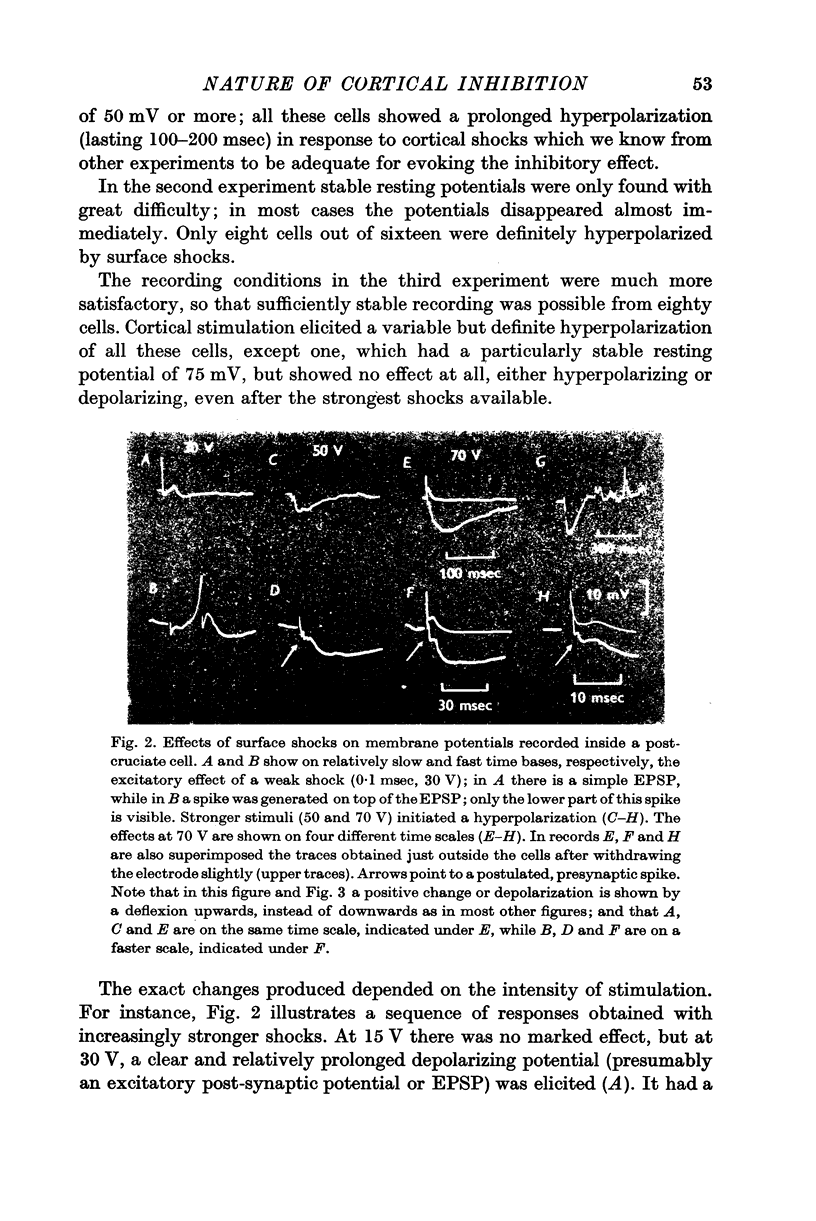
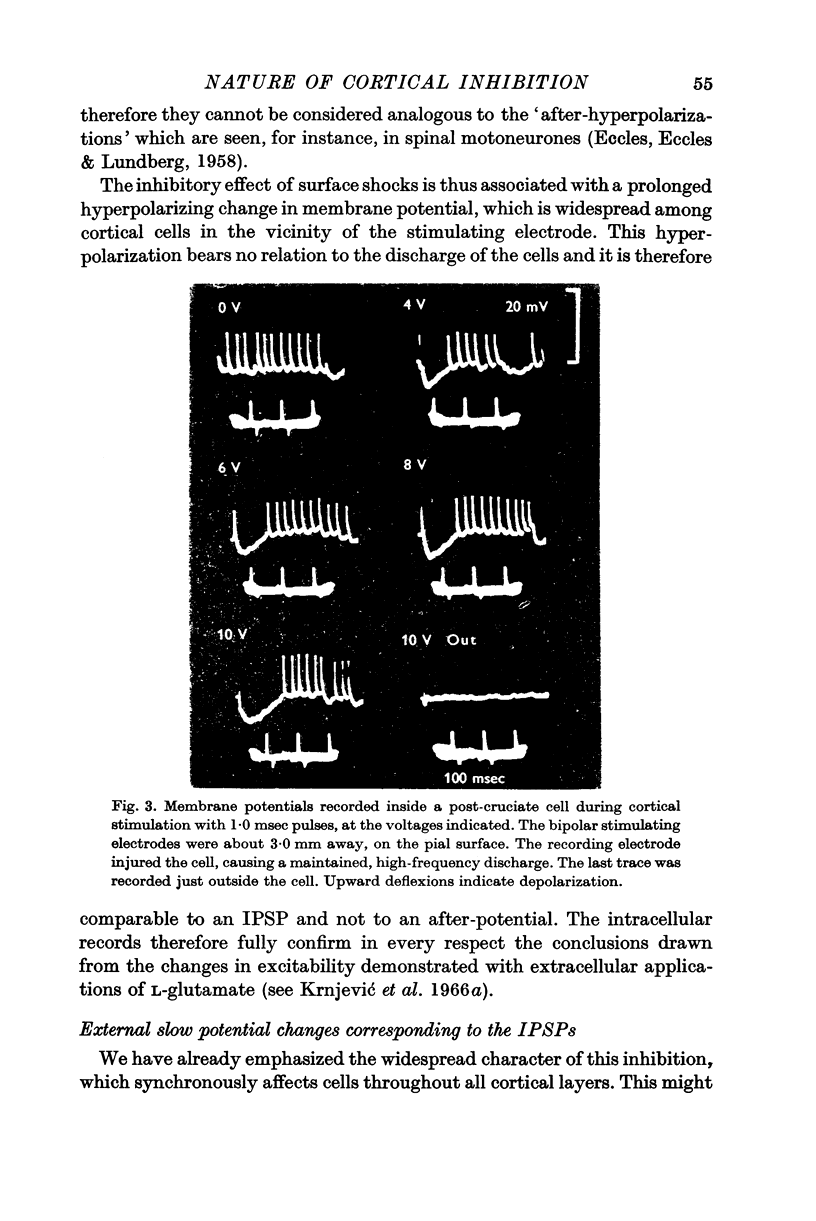
2. Further evidence that surface stimulation has a hyperpolarizing action on cortical cells was obtained by intracellular recording from over 120 pericruciate cells. Inhibitory post-synaptic potentials (IPSPs) are seen in most cells, which are comparable in threshold and duration with the inhibitory effect observed extracellularly. The IPSPs are usually not preceded by a discharge of the same cells.
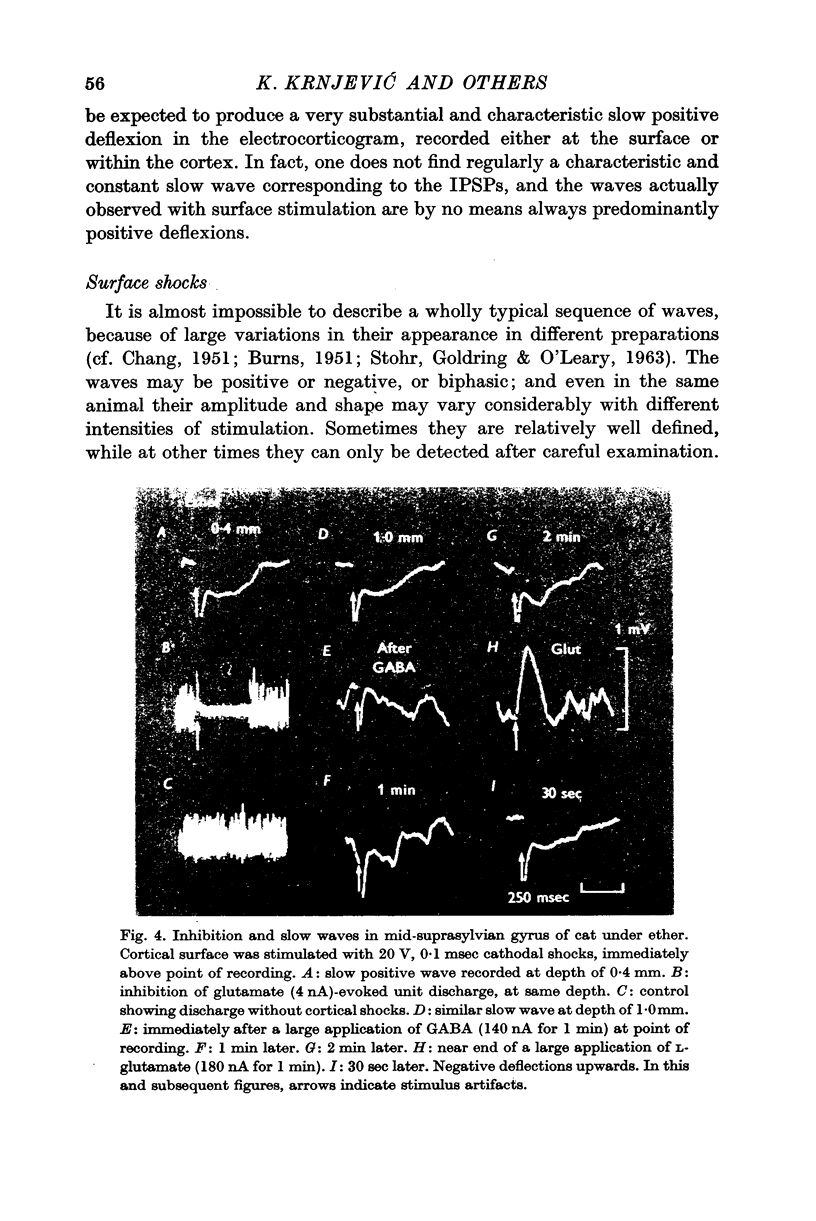
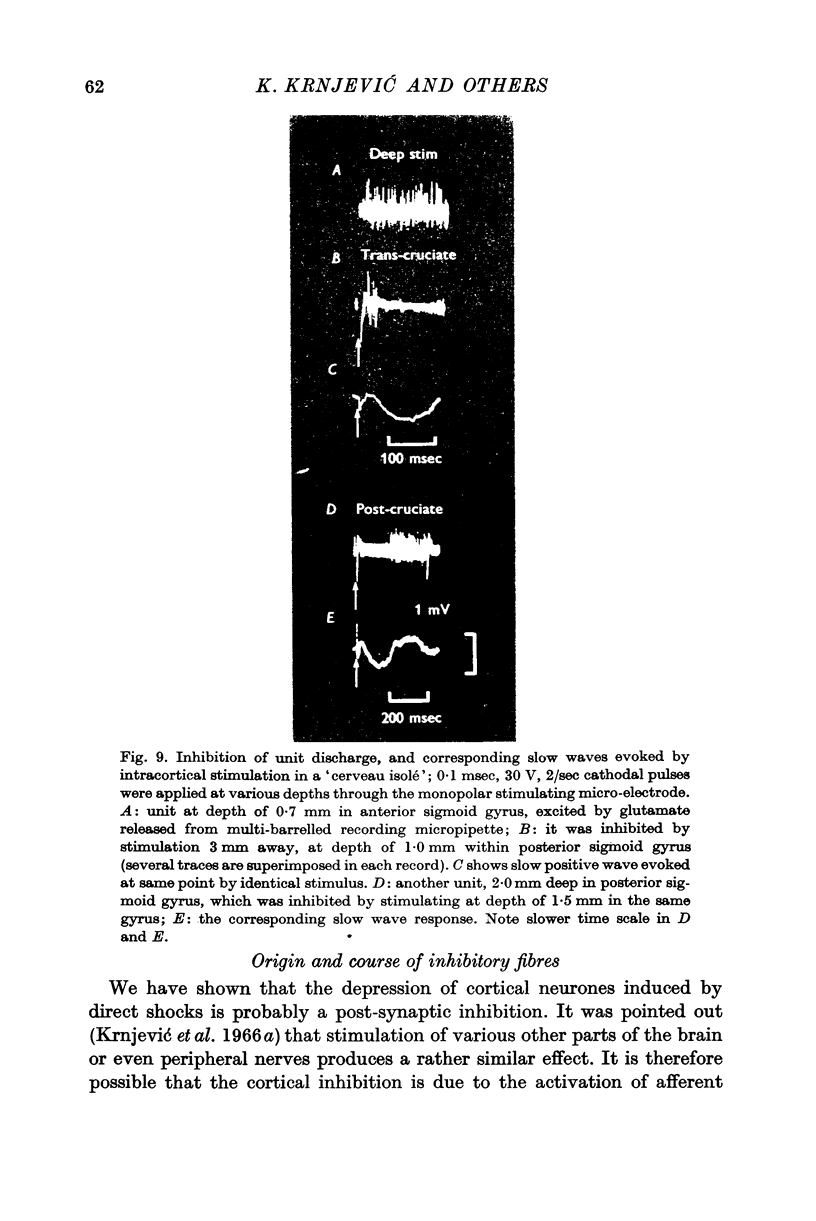
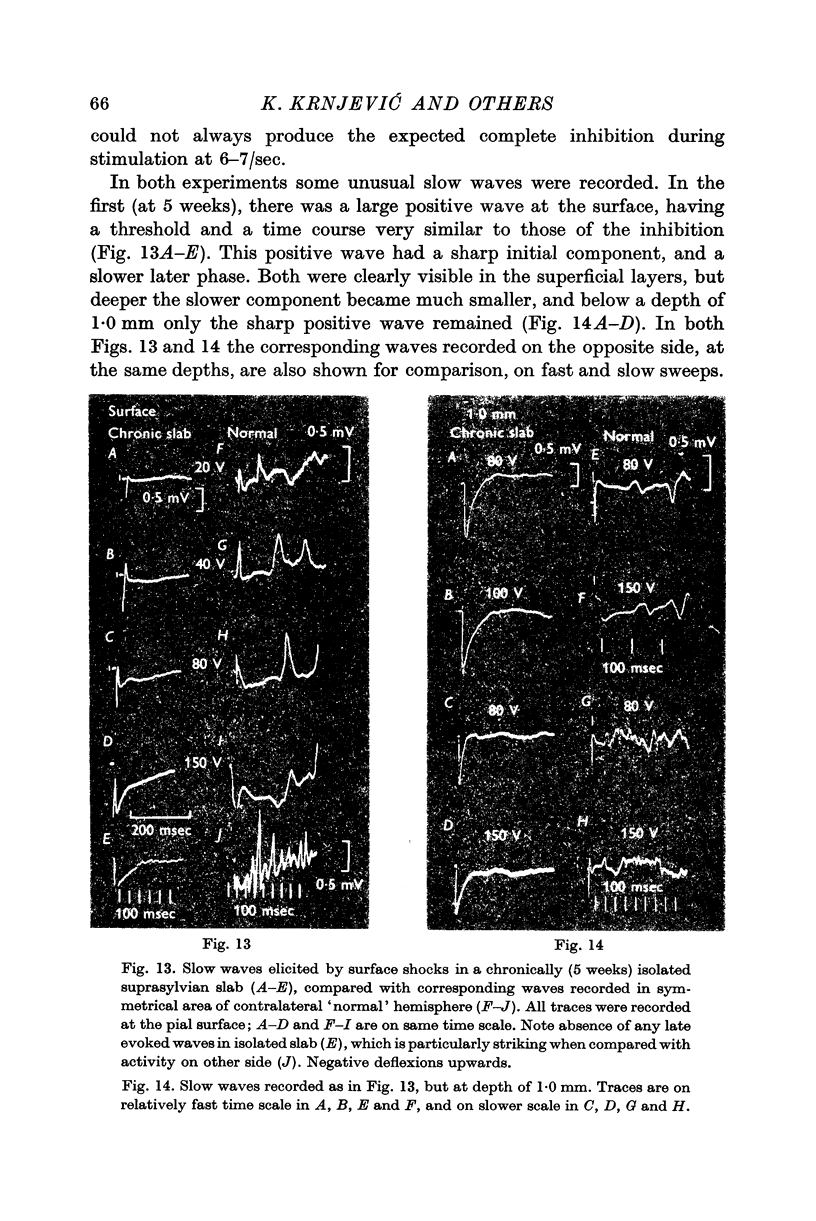
3. The extracellular slow wave corresponding to the inhibitory effect varies considerably with different preparations and different depths within the cortex. A predominantly positive wave is only seen occasionally. In general, the relevant wave recorded deep in the cortex tends to be mainly negative.
4. This negative slow wave can be much potentiated by tetanic stimulation, or, especially, by a large local release of L-glutamate; the last procedure is most effective either very near the surface, or below a depth of 1·0 mm. These observations suggest that inhibitory synapses occur more profusely in the superficial half of the grey matter.
5. Unlike L-glutamate, GABA tends to depress the `inhibitory' slow wave.
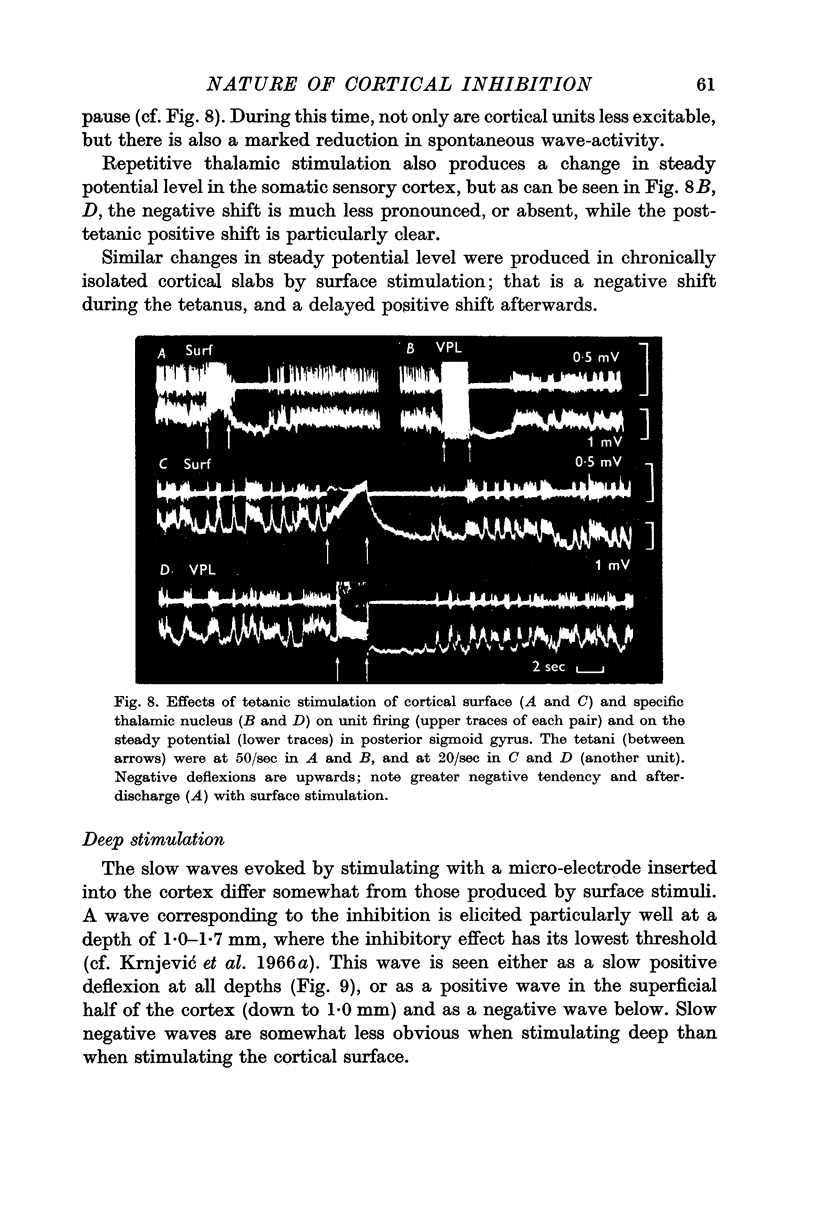
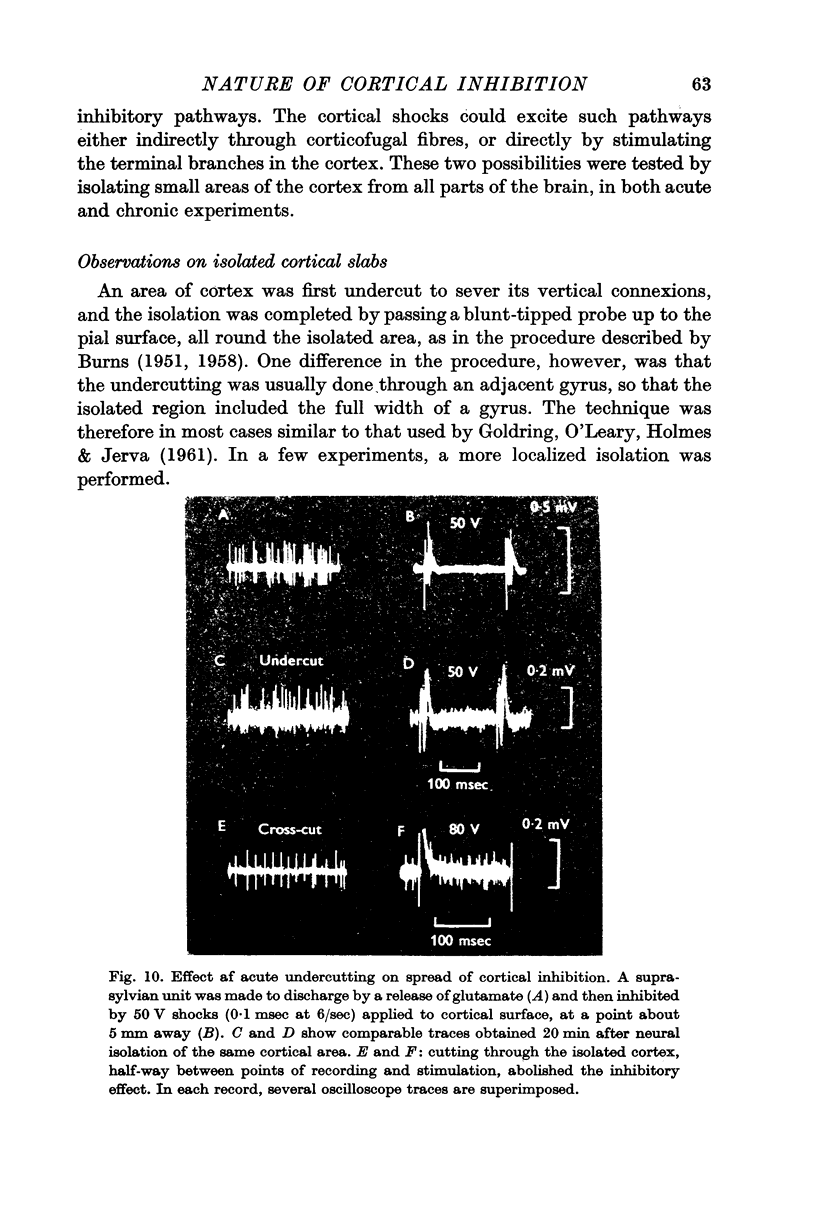
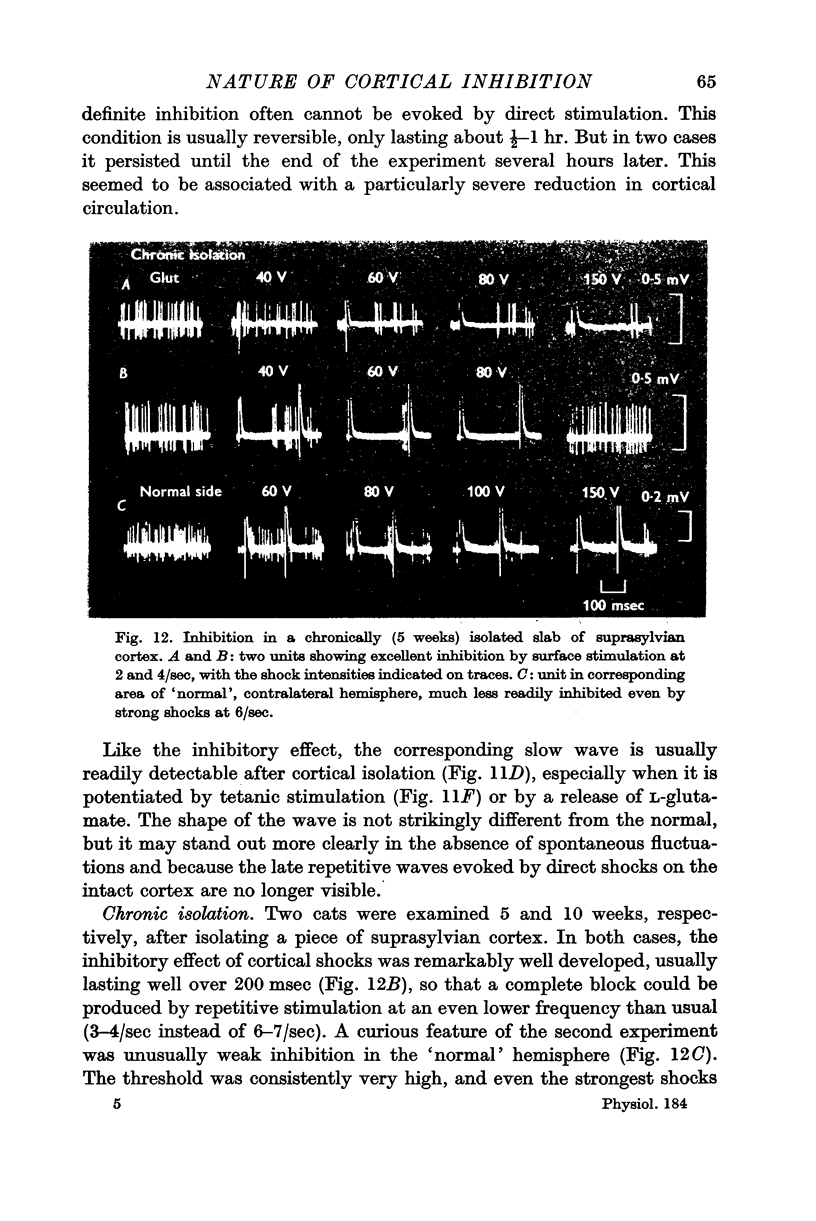
6. The inhibitory effect must be produced by intracortical neurones, since it is fully preserved in isolated cortical slabs. In both acute and chronic slabs, the inhibition is particularly well marked and long lasting, partly because spontaneous activity and the usual post-inhibitory rebound of excitability are absent.
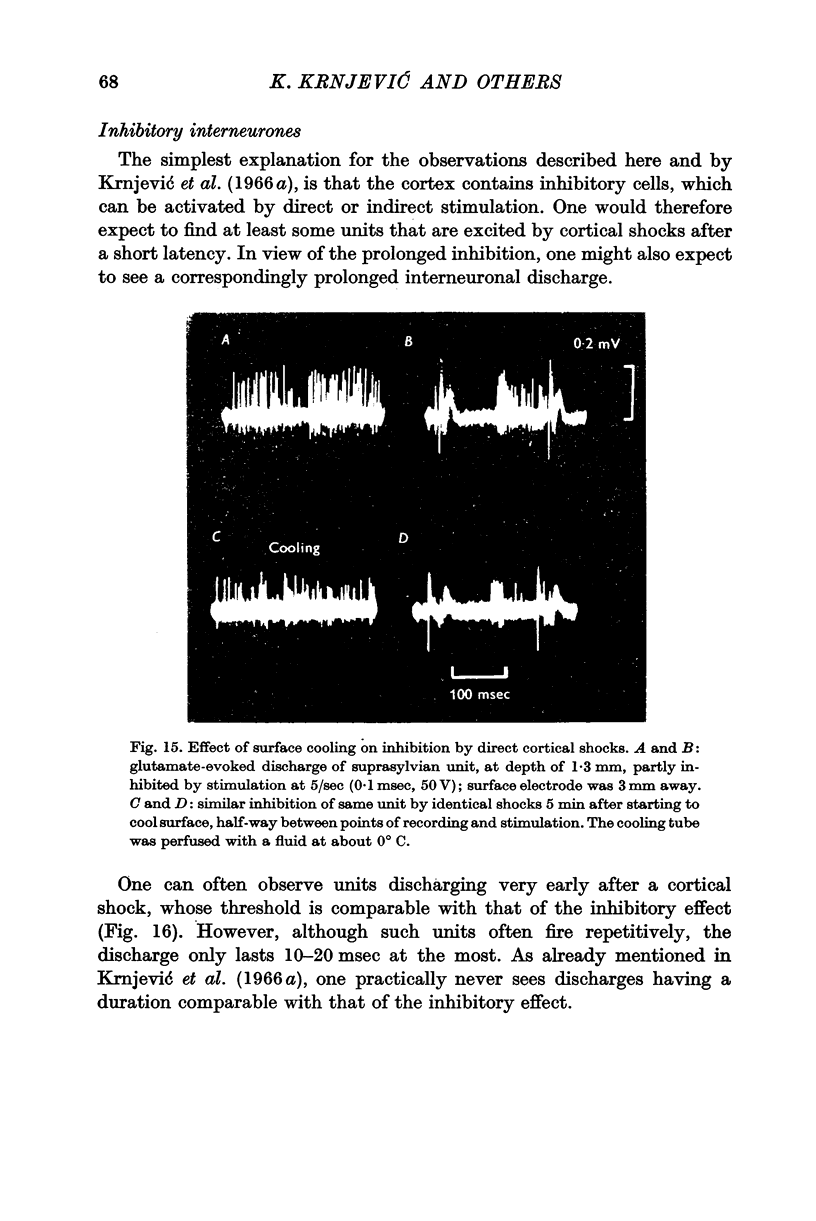
7. The intracortical pathways responsible for the spread of inhibition cannot be situated mainly in the superficial layers, as they are not readily blocked by surface cooling or the application of local anaesthetics.
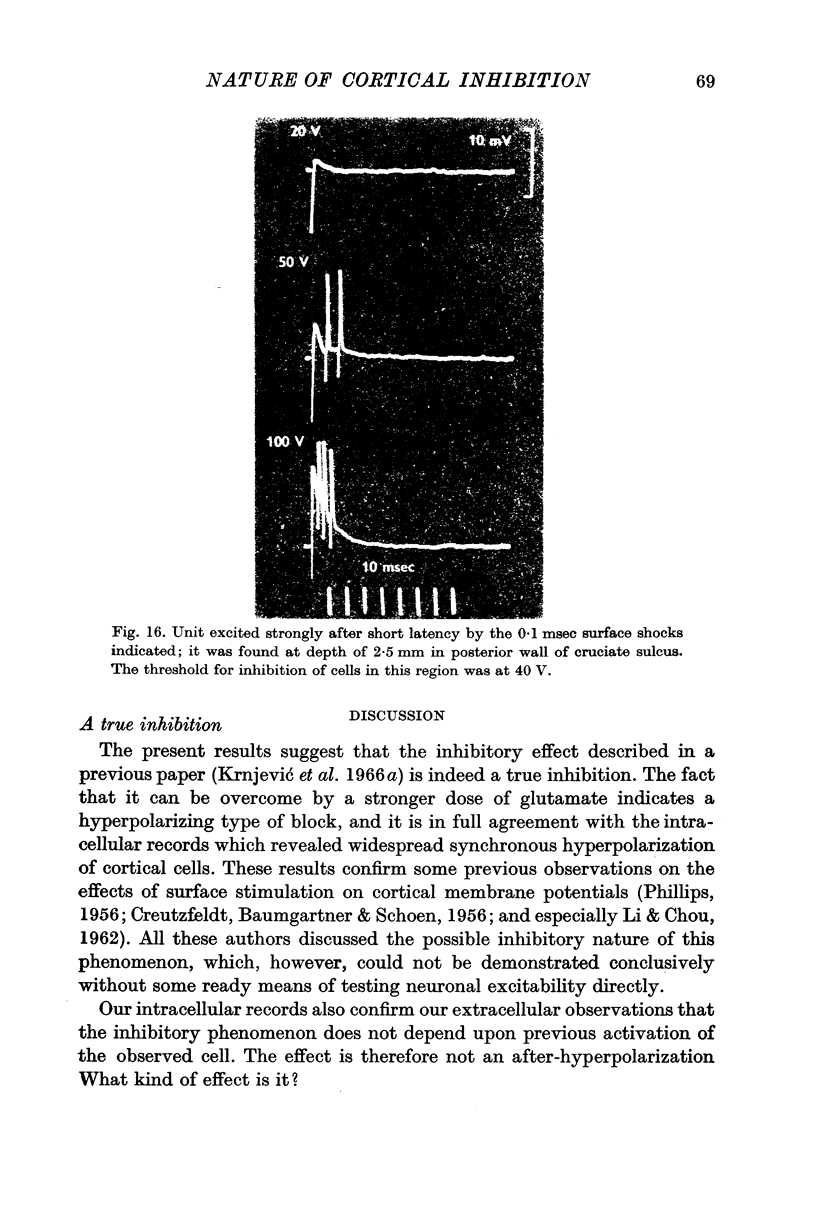
8. One can record unit discharges immediately after a surface shock. Some of these discharges could be from inhibitory interneurones, but they do not last more than 10-20 msec.
9. We conclude from the observations described in this and a previous paper (Krnjević, Randić & Straughan, 1966a) that a widespread system of intracortical interneurones can be activated by direct or indirect stimulation of the cortex; these interneurones have a powerful and prolonged inhibitory action on most cortical cells.
10. The identity and distribution of the postulated inhibitory interneurones is discussed in the light of some relevant morphological evidence.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963 May 11;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J., VOORHOEVE P. E. INHIBITORY SYNAPSES ON SOMAS OF PURKINJE CELLS IN THE CEREBELLUM. Nature. 1963 Aug 17;199:655–656. doi: 10.1038/199655a0. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., SEARS T. A. THE ROLE OF INHIBITION IN THE PHASING OF SPONTANEOUS THALAMO-CORTICAL DISCHARGE. J Physiol. 1964 Oct;173:459–480. doi: 10.1113/jphysiol.1964.sp007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian E. D. Afferent discharges to the cerebral cortex from peripheral sense organs. J Physiol. 1941 Sep 8;100(2):159–191. doi: 10.1113/jphysiol.1941.sp003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMGARTNER G., CREUTZFELDT O., SCHOEN L. Reaktionen einzelner Neurone des senso-motorischen Cortex nach elektrischen Reizen. I. Hemmung und Erregung nach direkten und kontralateralen Einzelreizen. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1956;194(6):597–619. doi: 10.1007/BF00342874. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B., ASANUMA H. PHARMACOLOGICAL STUDIES OF RECURRENT CORTICAL INHIBITION AND FACILITATION. Am J Physiol. 1965 Apr;208:674–681. doi: 10.1152/ajplegacy.1965.208.4.674. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B., ASANUMA H. RECURRENT CORTICAL EFFECTS FOLLOWING STIMULATION OF MEDULLARY PYRAMID. Arch Ital Biol. 1965 Jun 10;103:247–278. [PubMed] [Google Scholar]
- BURNS B. D. Some properties of isolated cerebral cortex in the unanesthetized cat. J Physiol. 1951 Jan;112(1-2):156–175. doi: 10.1113/jphysiol.1951.sp004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG H. T. Dendritic potential of cortical neurons produced by direct electrical stimulation of the cerebral cortex. J Neurophysiol. 1951 Jan;14(1):1–21. doi: 10.1152/jn.1951.14.1.1. [DOI] [PubMed] [Google Scholar]
- CREUTZFELDT O., STRUCK G. [Neurophysiology and morphology of the chronically isolated cortical islet in the cat: brain potentials and neuron activity of an isolated nerve cell population without afferent fibers]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:708–731. doi: 10.1007/BF00352735. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDRING S., O 'L EARY J. L., HOLMES T. G., JERVA M. J. Direct response of isolated cerebral cortex of cat. J Neurophysiol. 1961 Nov;24:633–650. doi: 10.1152/jn.1961.24.6.633. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959 Oct;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- HERN J. E., LANDGREN S., PHILLIPS C. G., PORTER R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon's motor cortex. J Physiol. 1962 Apr;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., RANDIC M., STRAUGHAN D. W. CORTICAL INHIBITION. Nature. 1964 Mar 28;201:1294–1296. doi: 10.1038/2011294a0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Randić M., Straughan D. W. An inhibitory process in the cerebral cortex. J Physiol. 1966 May;184(1):16–48. doi: 10.1113/jphysiol.1966.sp007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Randić M., Straughan D. W. Pharmacology of cortical inhibition. J Physiol. 1966 May;184(1):78–105. doi: 10.1113/jphysiol.1966.sp007904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. L., CHOU S. N. Cortical intracellular synaptic potentials and direct cortical stimulation. J Cell Comp Physiol. 1962 Aug;60:1–16. doi: 10.1002/jcp.1030600102. [DOI] [PubMed] [Google Scholar]
- MARSHALL W. H. Spreading cortical depression of Leao. Physiol Rev. 1959 Apr;39(2):239–279. doi: 10.1152/physrev.1959.39.2.239. [DOI] [PubMed] [Google Scholar]
- NACIMIENTO A. C., LUX H. D., CREUTZFELDT O. D. POSTSYNAPTISCHE POTENTIALE VON NERVENZELLEN DES MOTORISCHEN CORTEX NACH ELEKTRISCHER REIZUNG SPEZIFISCHER UND UNSPEZIFISCHER THALAMUSKERNE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Oct 5;281:152–169. [PubMed] [Google Scholar]
- O'LEARY J. L., GOLDRING S. D-C POTENTIALS OF THE BRAIN. Physiol Rev. 1964 Jan;44:91–125. doi: 10.1152/physrev.1964.44.1.91. [DOI] [PubMed] [Google Scholar]
- PURPURA D. P. Analysis of axodendritic synaptic organizations in immature cerebral cortex. Ann N Y Acad Sci. 1961 Sep 6;94:604–654. doi: 10.1111/j.1749-6632.1961.tb35561.x. [DOI] [PubMed] [Google Scholar]
- STEFANIS C., JASPER H. INTRACELLULAR MICROELECTRODE STUDIES OF ANTIDROMIC RESPONSES IN CORTICAL PYRAMIDAL TRACT NEURONS. J Neurophysiol. 1964 Sep;27:828–854. doi: 10.1152/jn.1964.27.5.828. [DOI] [PubMed] [Google Scholar]
- STOHR P. E., GOLDRING S., O'LEARY J. L. PATTERNS OF UNIT DISCHARGE ASSOCIATED WITH DIRECT CORTICAL RESPONSE IN MONKEY AND CAT. Electroencephalogr Clin Neurophysiol. 1963 Oct;15:882–888. doi: 10.1016/0013-4694(63)90177-9. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., TUKAHARA Y. RECURRENT INHIBITION OF THE BETZ CELL. Jpn J Physiol. 1963 Aug 15;13:386–398. doi: 10.2170/jjphysiol.13.386. [DOI] [PubMed] [Google Scholar]


