Abstract
1. End-plate potentials (e.p.p.s) were recorded intracellularly from neuromuscular junctions in curarized or Mg-paralysed rat diaphragm-phrenic nerve preparations in vitro. In Mg-paralysed preparations after 1000 impulses at 100/sec the amplitude of e.p.p.s elicited at 1/sec before and after the tetanus was on average greater than the control amplitude for 120 ± 30 sec.
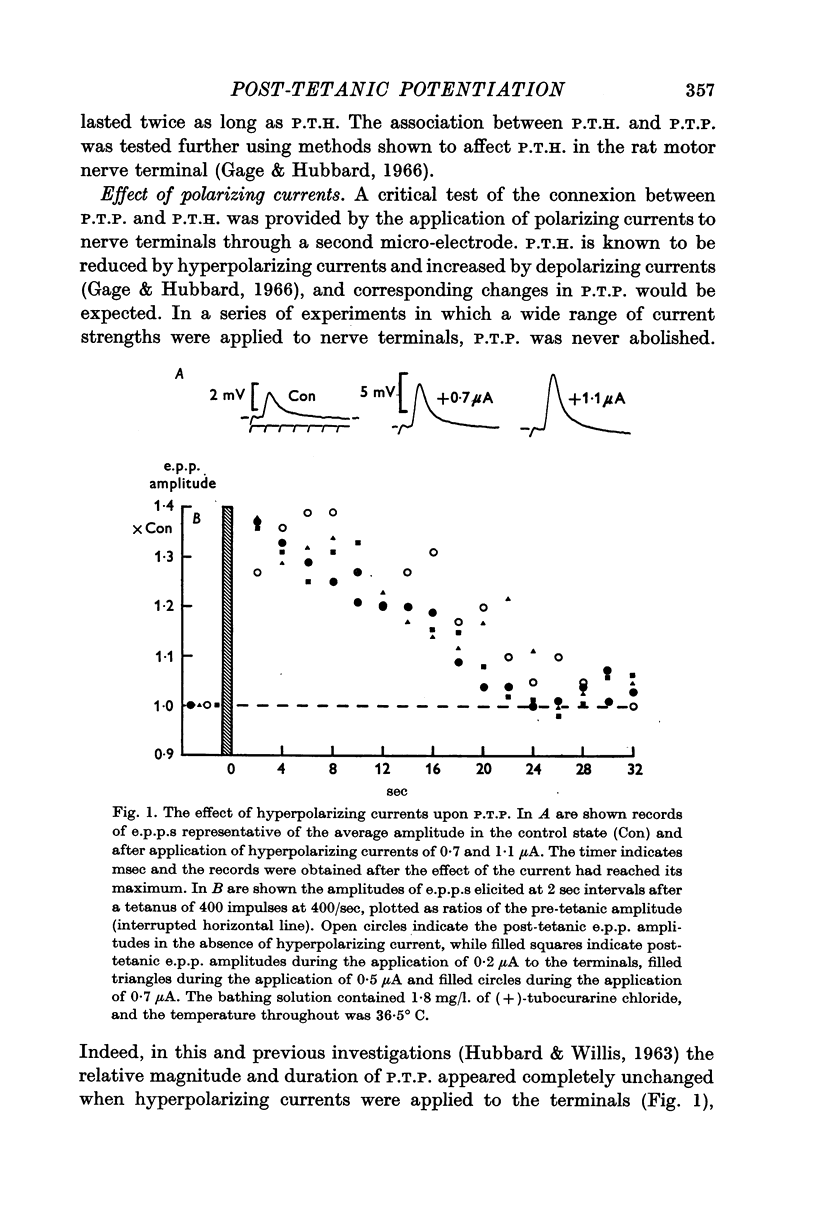
2. The post-tetanic potentiation (P.T.P.) of e.p.p. amplitudes was not thought to be dependent upon post-tetanic hyperpolarization (P.T.H.) of nerve terminals as it lasted longer than the hyperpolarization generated by an identical tetanus; was unaffected by hyperpolarizing currents which reduced P.T.H. or depolarizing currents which prolonged P.T.H.; and was diminished in solutions containing 30% of the normal NaCl concentration or 1% ethyl alcohol, both of which procedures prolong P.T.H. The magnitude and duration of P.T.P. were influenced by the pH of the bathing solution in the range 7-7·5 although there was no change in P.T.H. under these conditions. The inability of polarizing currents to influence P.T.P. was also thought inconsistent with the hypothesis that P.T.P. is due to an increase in available transmitter.
3. P.T.P. was not thought to be due to sodium accumulation in nerve terminals, for P.T.P. was reduced or abolished by procedures which would be expected to increase the intraterminal sodium ion concentration. These procedures were: exhibition of metabolic inhibitors (1·8 × 10-6 M antimycin A, 3-5 mM sodium azide or 1 mM sodium iodoacetate), exhibition of cardiac glycosides (7·7 × 10-6 M digoxin or 0·42 mM ouabain), and omission of glucose or potassium ions from the bathing solution. Abolition of P.T.P. by potassium-free solutions was also thought to be inconsistent with the hypothesis that P.T.P. is due to a reduction in the potassium concentration in nerve terminals.
4. P.T.P. was not thought to be due to terminal volume changes, for no consistent effect upon the quantal content of e.p.p.s could be detected in hypo- or hyperosmotic solutions.
5. It was concluded that the only hypothesis for P.T.P. not excluded by our experiments was that P.T.P. is due to some change in ionized calcium at a membrane site important in transmitter release.
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R. I. THE ROLE OF SODIUM IONS IN THE METABOLISM OF ACETYLCHOLINE. Can J Biochem Physiol. 1963 Dec;41:2573–2597. [PubMed] [Google Scholar]
- BIRKS R. I. The effects of a cardiac glycoside on subcellular structures within nerve cells and their processes in sympathetic ganglia and skeletal muscle. Can J Biochem Physiol. 1962 Feb;40:303–315. [PubMed] [Google Scholar]
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., RALL W. Effects induced in a monosynaptic reflex path by its activation. J Neurophysiol. 1951 Sep;14(5):353–376. doi: 10.1152/jn.1951.14.5.353. [DOI] [PubMed] [Google Scholar]
- ENNOR A. H., MORRISON J. F. Biochemistry of the phosphagens and related guanidines. Physiol Rev. 1958 Oct;38(4):631–674. doi: 10.1152/physrev.1958.38.4.631. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAGE P. W. EFFECT OF CARDIAC GLYCOSIDES ON NEUROMUSCULAR TRANSMISSION. Nature. 1965 Jan 2;205:84–85. doi: 10.1038/205084a0. [DOI] [PubMed] [Google Scholar]
- GERSMEYER G., HOLLAND W. C. Influence of ouabain on contractile force, resting tension, Ca45 entry and tissue Ca content in rat atria. Circ Res. 1963 Jun;12:620–622. doi: 10.1161/01.res.12.6.620. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- GREEFF K., WESTERMANN E. Untersuchungen über die muskellähmende Wirkung des Strophanthins. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1955;226(2):103–113. [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Metabolic studies on the hyperpolarization following activity in mammalian non-myelinated nerve fibres. J Physiol. 1962 May;161:414–423. doi: 10.1113/jphysiol.1962.sp006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. The origin of the post-tetanic hyperpolarization of mammalian motor nerve terminals. J Physiol. 1966 May;184(2):335–352. doi: 10.1113/jphysiol.1966.sp007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Influence of sodium ions on transmitter release. Nature. 1965 Jun 5;206(988):1047–1048. doi: 10.1038/2061047a0. [DOI] [PubMed] [Google Scholar]
- HILL D. K. The volume change resulting from stimulation of a giant nerve fibre. J Physiol. 1950 Oct 16;111(3-4):304–327. doi: 10.1113/jphysiol.1950.sp004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN L. M., SHERROD T. R. ACTION OF OUABAIN ON ISOMETRIC TWITCH TENSION IN STRIATED MUSCLE. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:940–943. doi: 10.3181/00379727-113-28537. [DOI] [PubMed] [Google Scholar]
- HOLMES O. Effects of pH, changes in potassium concentration and metabolic inhibitors on the after-potentials of mammalian non-medullated nerve fibres. Arch Int Physiol Biochim. 1962 Mar;70:211–245. doi: 10.3109/13813456209092855. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I., GAGE P. W. ABOLITION OF POST-TETANIC POTENTIATION. Nature. 1964 Apr 18;202:299–300. doi: 10.1038/202299a0. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Reduction of transmitter output by depolarization. Nature. 1962 Mar 31;193:1294–1295. doi: 10.1038/1931294a0. [DOI] [PubMed] [Google Scholar]
- HUGHES J. R. Post-tetanic potentiation. Physiol Rev. 1958 Jan;38(1):91–113. doi: 10.1152/physrev.1958.38.1.91. [DOI] [PubMed] [Google Scholar]
- HURWITZ L., BATTLE F., WEISS G. B. Action of the calcium antagonists cocaine and ethanol on contraction and potassium efflux of smooth muscle. J Gen Physiol. 1962 Nov;46:315–332. doi: 10.1085/jgp.46.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F. Effect of choline on neuromuscular transmission in the cat. J Physiol. 1952 Jun;117(2):241–250. doi: 10.1113/jphysiol.1952.sp004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F. Post-tetanic restoration of neuromuscular transmission blocked by D-tubocurarine. J Physiol. 1952 Oct;118(2):216–227. doi: 10.1113/jphysiol.1952.sp004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- KELLY J. S. ANTAGONISM BETWEEN NA+ AND CA2+ AT THE NEUROMUSCULAR JUNCTION. Nature. 1965 Jan 16;205:296–297. doi: 10.1038/205296a0. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K., MIYAMOTO S. Release of calcium-45 from frog nerves during electrical activity. Nature. 1961 Feb 4;189:402–403. doi: 10.1038/189402a0. [DOI] [PubMed] [Google Scholar]
- KOKETSU K., MIYAMOTO S. Significance of membrane calcium in calcium-free and potassium-rich media. Nature. 1961 Feb 4;189:403–404. doi: 10.1038/189403a0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Failure of neuromuscular propagation in rats. J Physiol. 1958 Mar 11;140(3):440–461. [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- LLOYD D. P. C. Post-tetanic potentiation of response in monosynaptic reflex pathways of the spinal cord. J Gen Physiol. 1949 Nov;33(2):147–170. doi: 10.1085/jgp.33.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- SHANES A. M., BIANCHI C. P. Radiocalcium release by stimulated and potassium-treated sartorius muscles of the frog. J Gen Physiol. 1960 Jan;43:481–493. doi: 10.1085/jgp.43.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLATER E. C. The constitution of the respiratory chain in animal tissues. Adv Enzymol Relat Subj Biochem. 1958;20:147–199. doi: 10.1002/9780470122655.ch6. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Changes in potassium concentration around motor nerve terminals, produced by current flow, and their effects on neuromuscular transmission. J Physiol. 1961 Jan;155:46–58. doi: 10.1113/jphysiol.1961.sp006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOBIAS J. M., AGIN D. P., PAWLOWSKI R. Phospholipidcholesterol membrane model. Control of resistance by ions or current flow. J Gen Physiol. 1962 May;45:989–1001. doi: 10.1085/jgp.45.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]


