Abstract
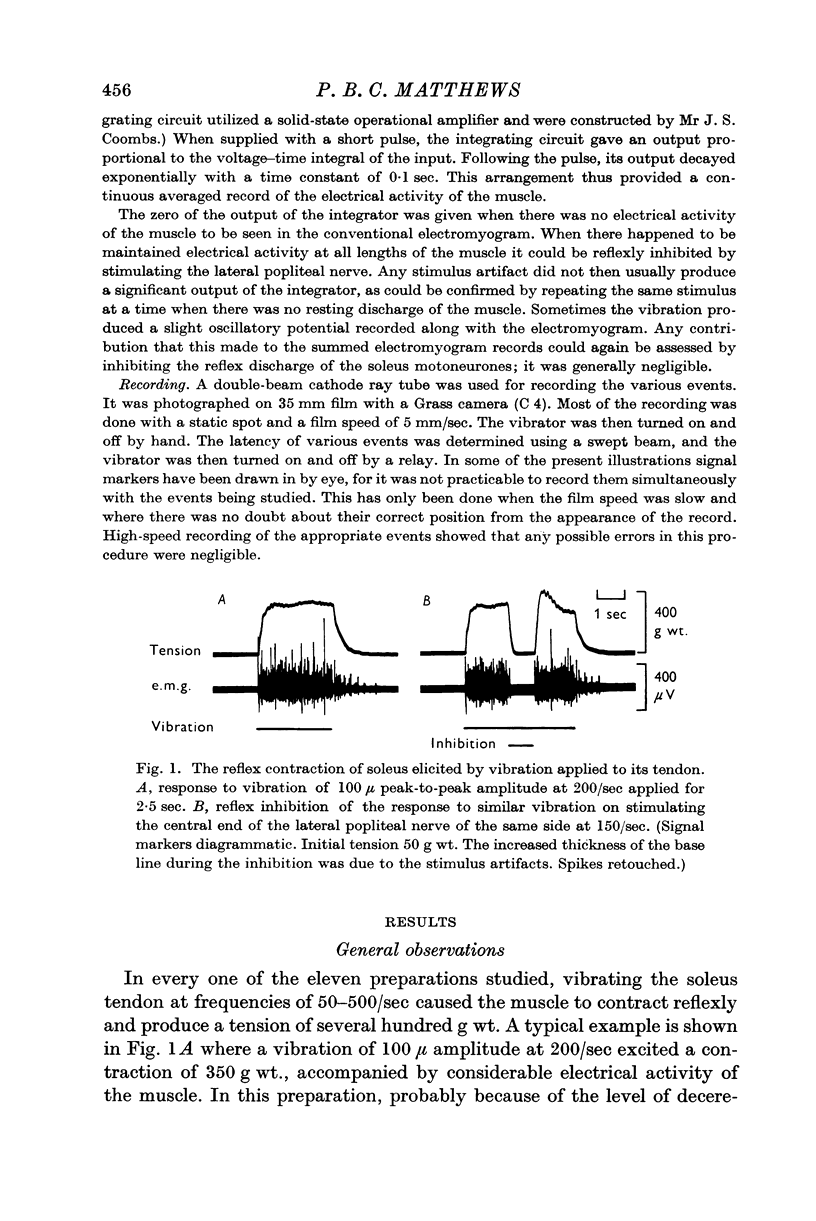
1. Vibration was applied longitudinally to the fully innervated soleus muscle of the decerebrate cat by attaching its tendon to a vibrator. Vibration at frequencies of 50-500/sec with amplitudes of 10 μ upwards caused the muscle to contract reflexly for as long as the vibration was maintained. The response was recorded myographically by a myograph mounted upon the vibrator, and electromyographically by gross `belly-tendon' leads. The reflex contraction produced several hundred g wt. of tension and involved too many motor units for their discharges to be separable. The maintained reflex was abolished by making the preparation spinal or by anaesthetizing it with pentobarbitone, but it persisted after removing the cerebellum.
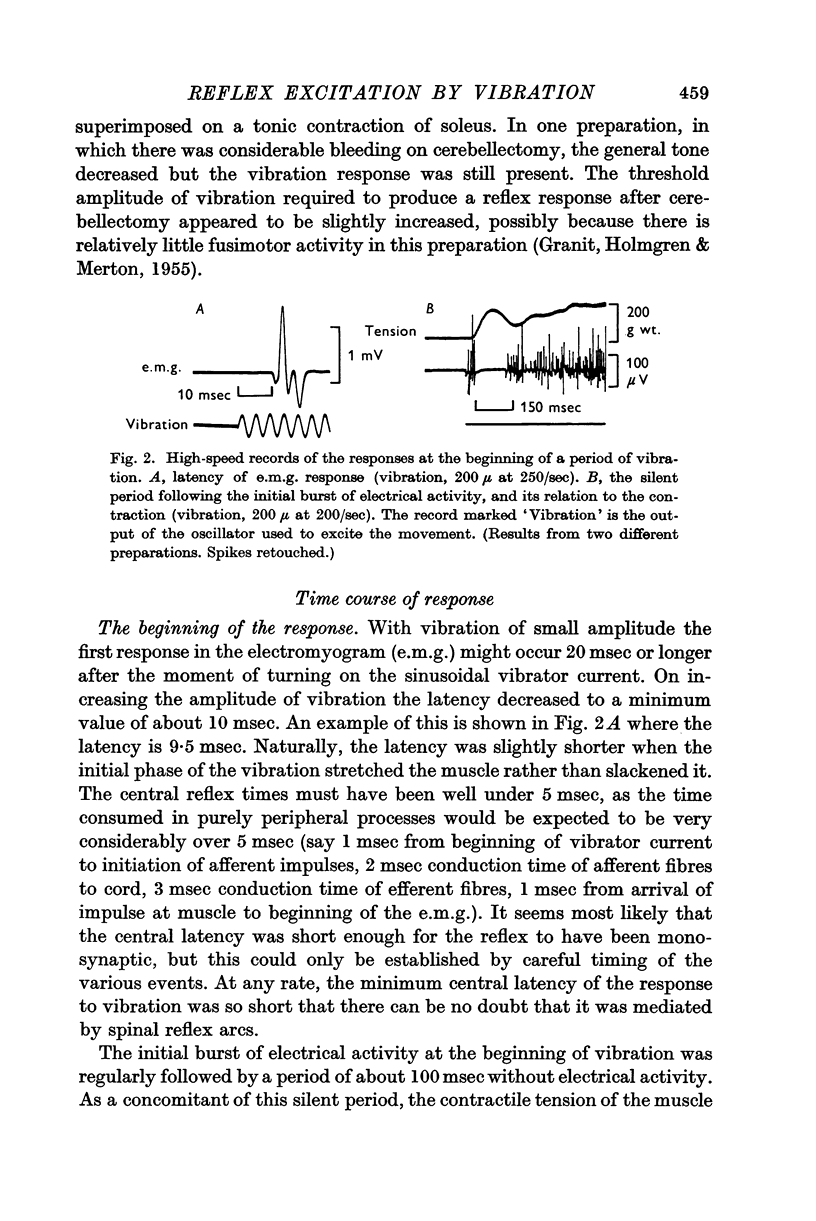
2. The minimum latency for the appearance of the reflex response at the beginning of a period of vibration was about 10 msec. The latency of cessation of the response at the end of vibration was similarly short.
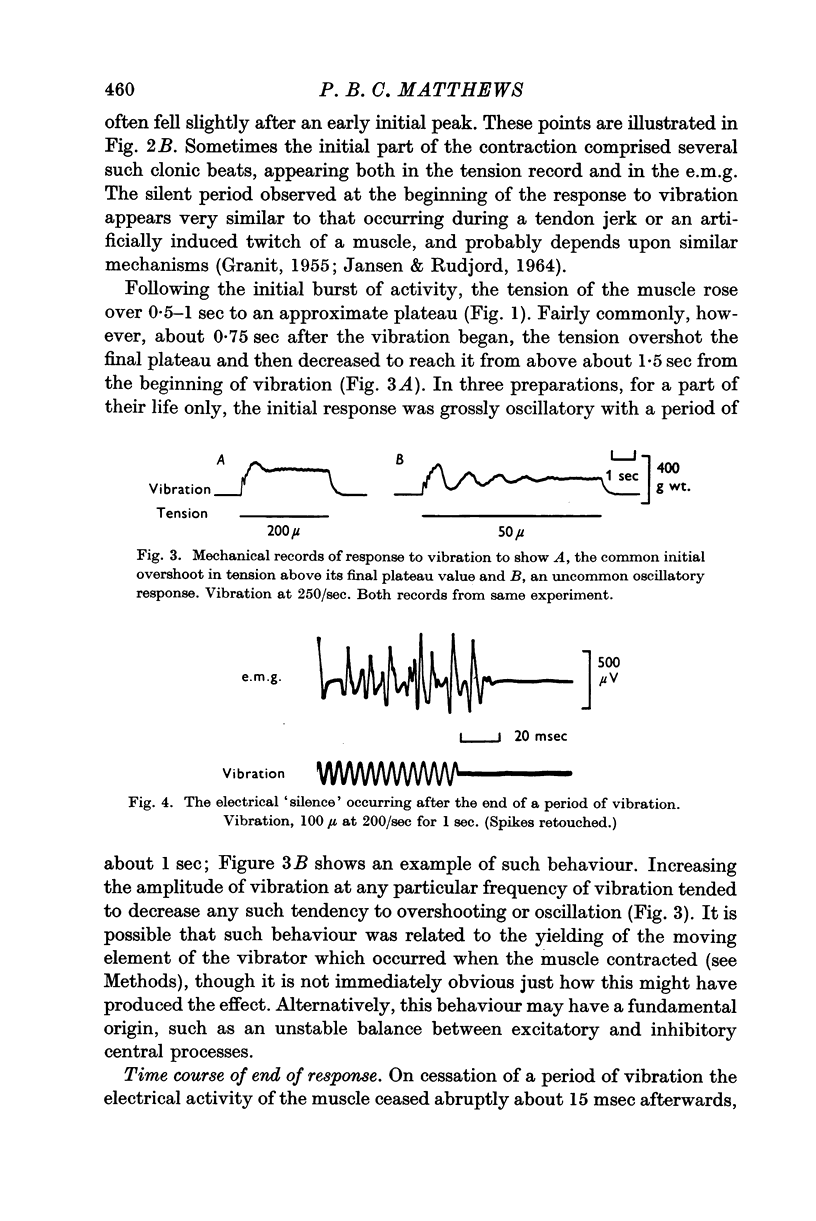
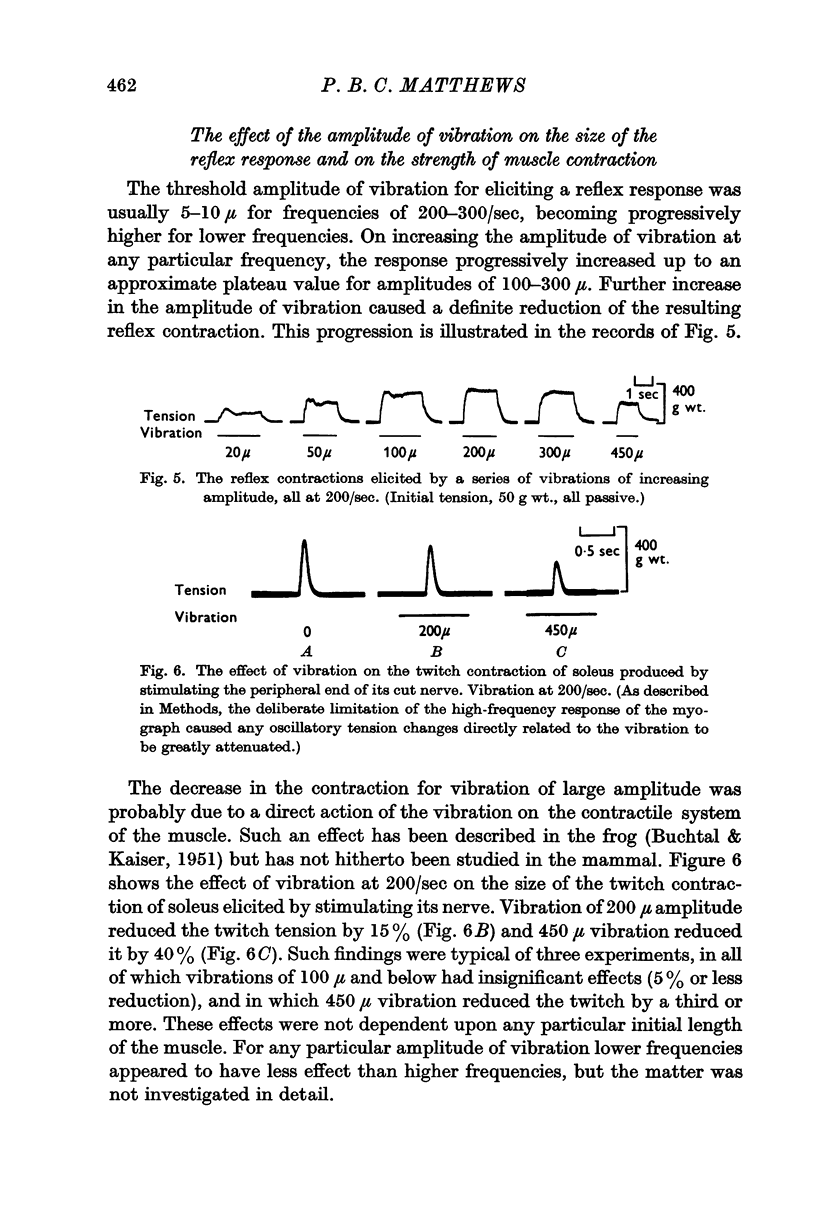
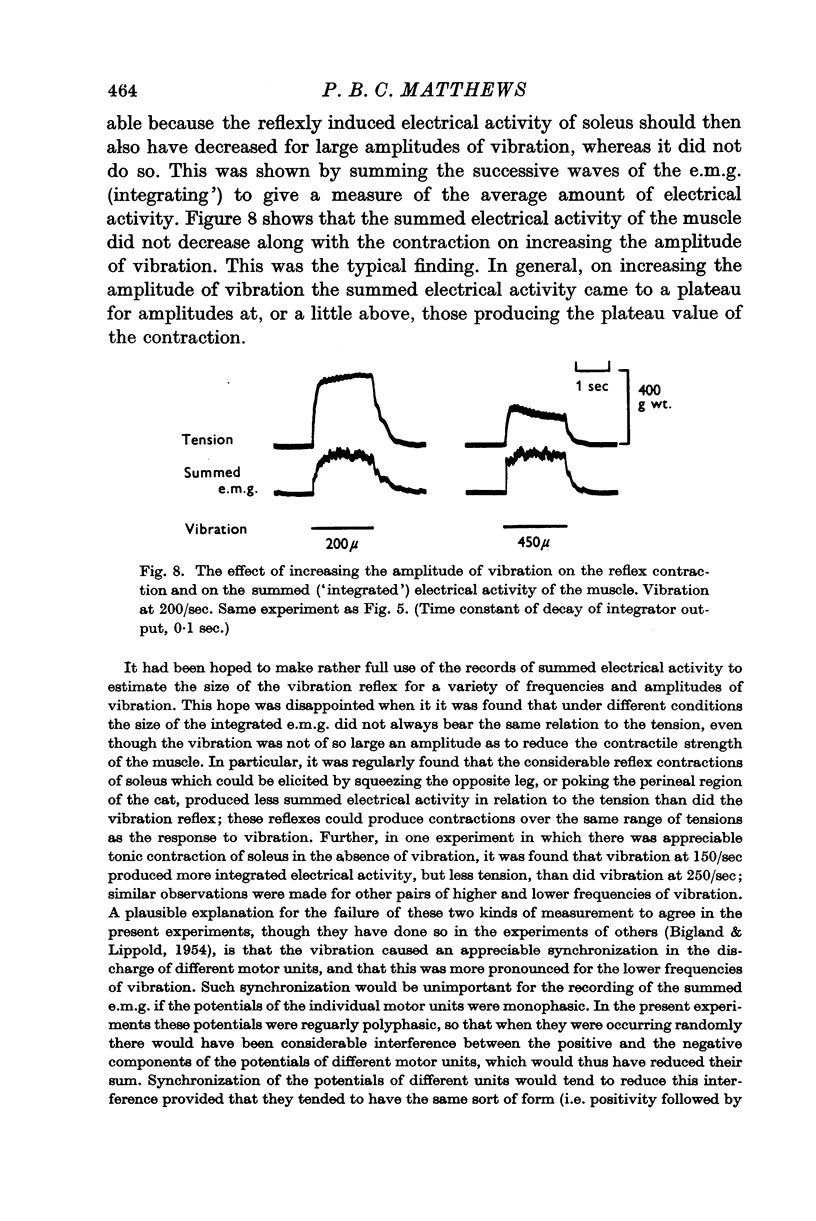
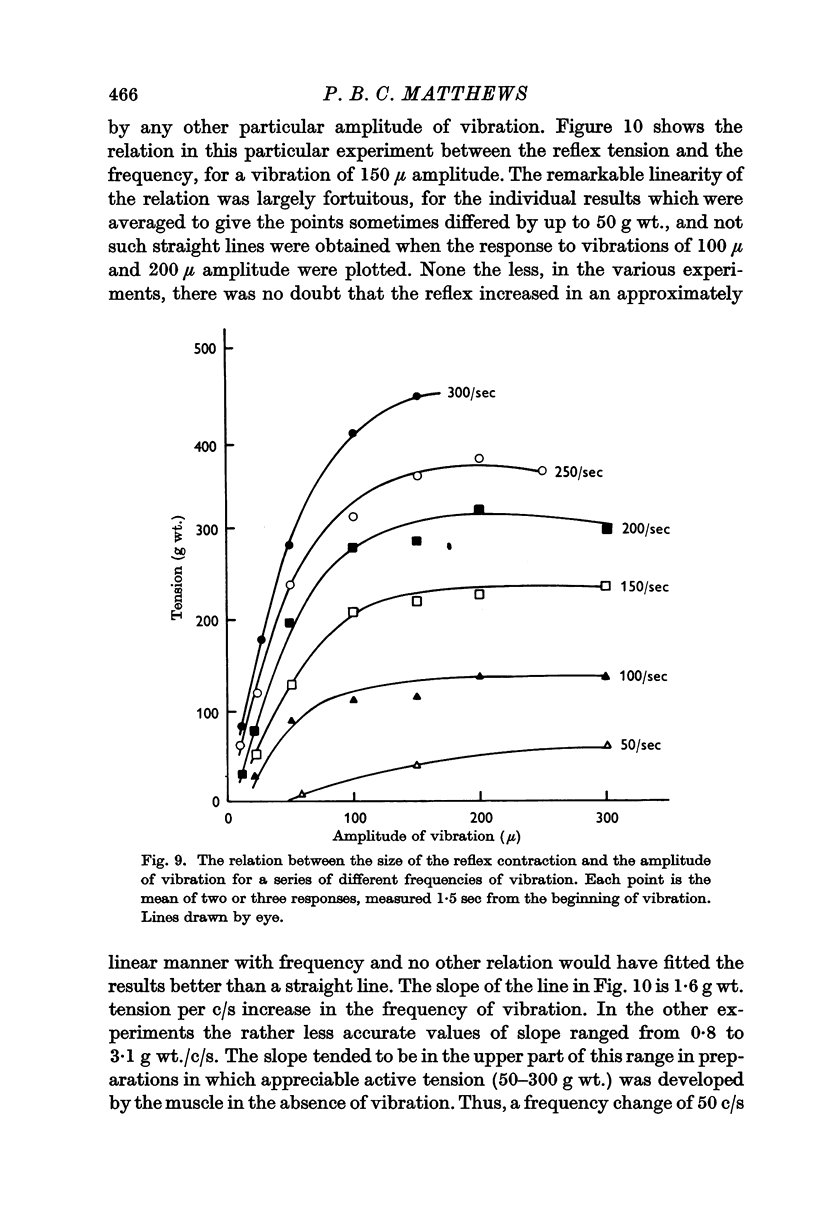
3. On increasing the amplitude of vibration at any particular frequency in the range 100-300/sec the resulting reflex tension increased to an approximate plateau for amplitudes of vibration of 100-200 μ. Further increase in the amplitude decreased the size of the contraction, though there was no such reduction in records of the `integrated' electromyogram.
4. Such large amplitudes of vibration also reduced the tension, and shortened the duration, of a twitch contraction of the muscle elicited by stimulating its nerve. The strength of a tetanic contraction was much less affected by vibration than was that of the twitch contraction, and the muscle action potential elicited by stimulation of the nerve was unaffected. Thus, large-amplitude vibration influenced the contractile mechanism of the muscle (cf. Buchtal & Kaiser, 1951).
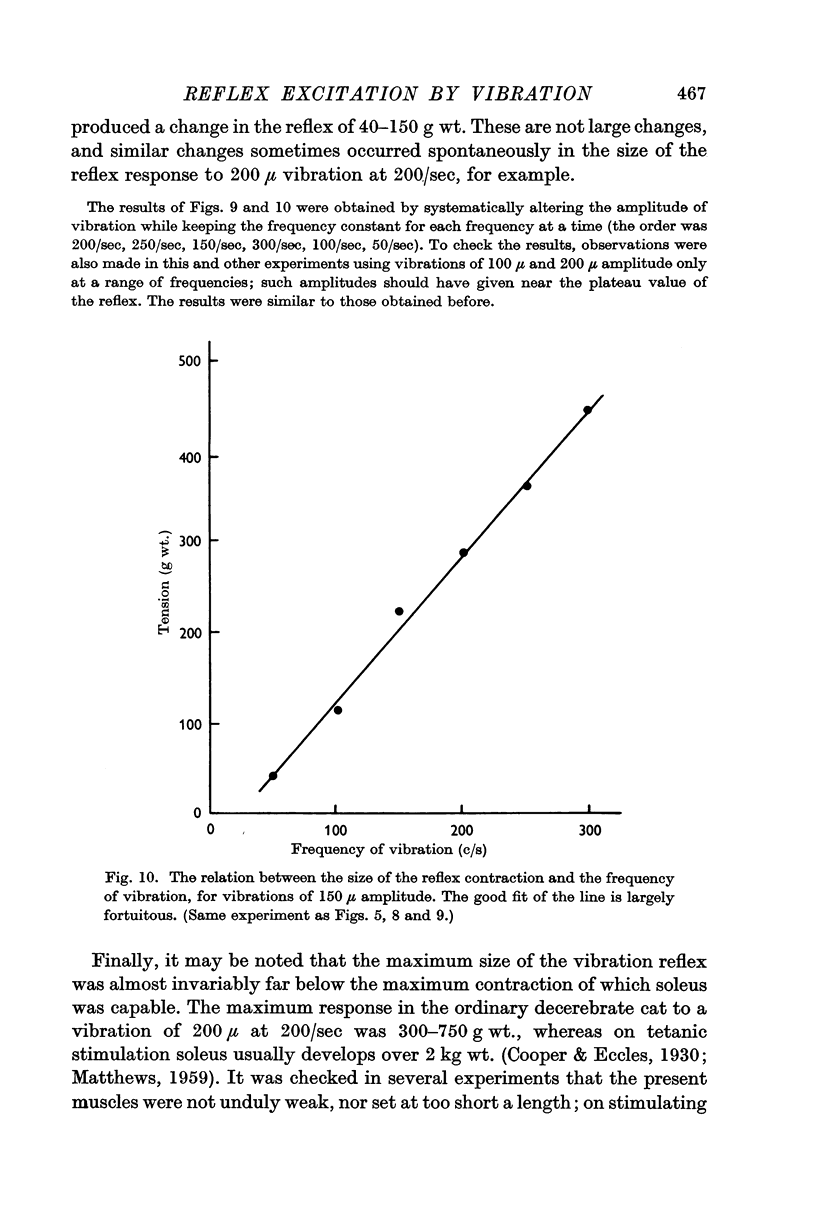
5. Increasing the frequency of vibration increased the value of the plateau tension reached on increasing the amplitude. The effect was, however, relatively small and the largest increase seen was 3 g wt. of contractile tension per c/s increase in vibration frequency.
6. The primary afferent ending of the muscle spindle is considered to be the receptor whose excitation leads to the reflex response to vibration. The vibration reflex thus appears to be the well-known stretch reflex, elicited by a rather unusual form of stretching. The size of the vibration reflex and its variation with frequency are discussed in relation to the servo theory of muscular contraction.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIANCONI R., van der MEULEN J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963 Jan;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- BIGLAND B., LIPPOLD O. C. The relation between force, velocity and integrated electrical activity in human muscles. J Physiol. 1954 Jan;123(1):214–224. doi: 10.1113/jphysiol.1954.sp005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. FURTHER STUDIES OF STATIC AND DYNAMIC FUSIMOTOR FIBRES. J Physiol. 1964 Oct;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. The isometric responses of mammalian muscles. J Physiol. 1930 Jun 27;69(4):377–385. doi: 10.1113/jphysiol.1930.sp002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDRED E., GRANIT R., MERTON P. A. Supraspinal control of the muscle spindles and its significance. J Physiol. 1953 Dec 29;122(3):498–523. doi: 10.1113/jphysiol.1953.sp005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echlin F., Fessard A. Synchronized impulse discharges from receptors in the deep tissues in response to a vibrating stimulus. J Physiol. 1938 Sep 16;93(4):312–334. doi: 10.1113/jphysiol.1938.sp003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D. Gamma control of dynamic properties of muscle spindles. J Neurophysiol. 1956 Jul;19(4):356–366. doi: 10.1152/jn.1956.19.4.356. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HOLMGREN B., MERTON P. A. The two routes for excitation of muscle and their subservience to the cerebellum. J Physiol. 1955 Oct 28;130(1):213–224. doi: 10.1113/jphysiol.1955.sp005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R. Neuromuscular interaction in postural tone of the cat's isometric soleus muscle. J Physiol. 1958 Oct 31;143(3):387–402. doi: 10.1113/jphysiol.1958.sp006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The central control of the dynamic response of muscle spindle receptors. J Physiol. 1962 May;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The effects of fusimotor activity on the static responsiveness of primary and secondary endings of muscle spindles in the decerebrate cat. Acta Physiol Scand. 1962 Aug;55:376–386. doi: 10.1111/j.1748-1716.1962.tb02451.x. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., RUDJORD T. ON THE SILENT PERIOD AND GOLGI TENDON ORGANS OF THE SOLEUS MUSCLE OF THE CAT. Acta Physiol Scand. 1964 Dec;62:364–379. doi: 10.1111/j.1748-1716.1964.tb10435.x. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. MUSCLE SPINDLES AND THEIR MOTOR CONTROL. Physiol Rev. 1964 Apr;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The dependence of tension upon extension in the stretch reflex of the soleus muscle of the decerebrate cat. J Physiol. 1959 Oct;147(3):521–546. doi: 10.1113/jphysiol.1959.sp006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTRIDGE L. D., GLASER G. H. Adaptation in regulation of movement and posture. A study of stretch responses in spastic animals. J Neurophysiol. 1960 May;23:257–268. doi: 10.1152/jn.1960.23.3.257. [DOI] [PubMed] [Google Scholar]
- PRINGLE J. W. Models of muscle. Symp Soc Exp Biol. 1960;14:41–68. [PubMed] [Google Scholar]
- Sherrington C. S. Decerebrate Rigidity, and Reflex Coordination of Movements. J Physiol. 1898 Feb 17;22(4):319–332. doi: 10.1113/jphysiol.1898.sp000697. [DOI] [PMC free article] [PubMed] [Google Scholar]


