Abstract
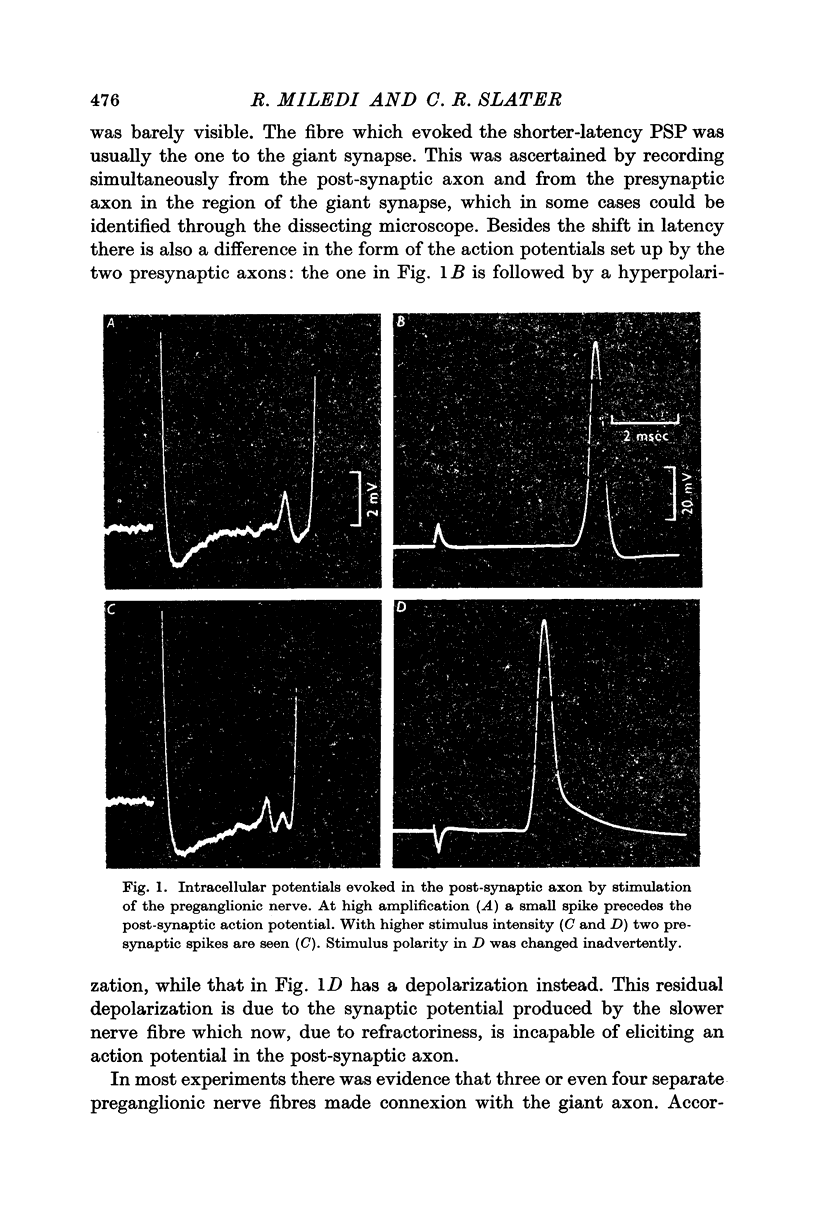
1. The isolated stellate ganglion of the squid (L. pealii) was studied with intracellular and extracellular micro-electrodes. Three or four nerve fibres in the preganglionic nerve establish synaptic relations with the giant axon in the last stellar nerve. Accordingly, 1-3 small presynaptic spikes (< 1 mV) could be recorded from within the post-synaptic axon.
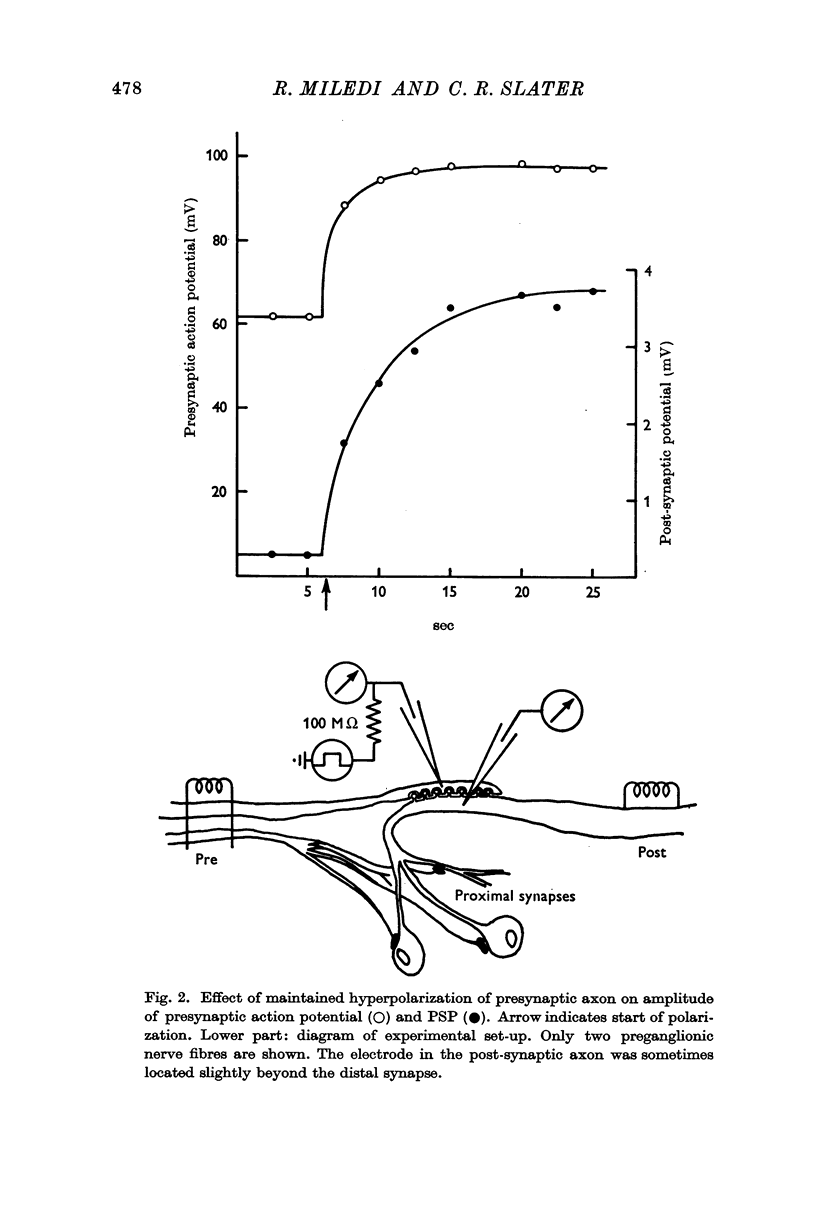
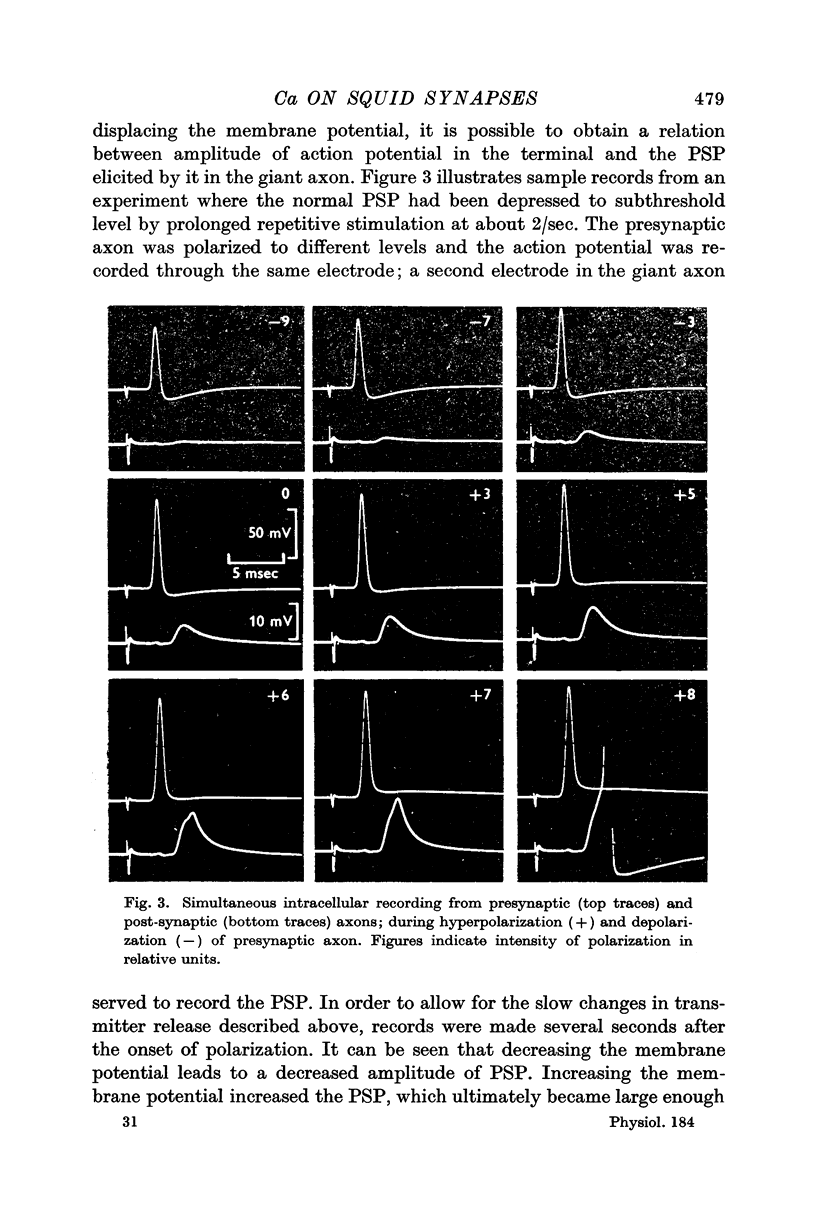
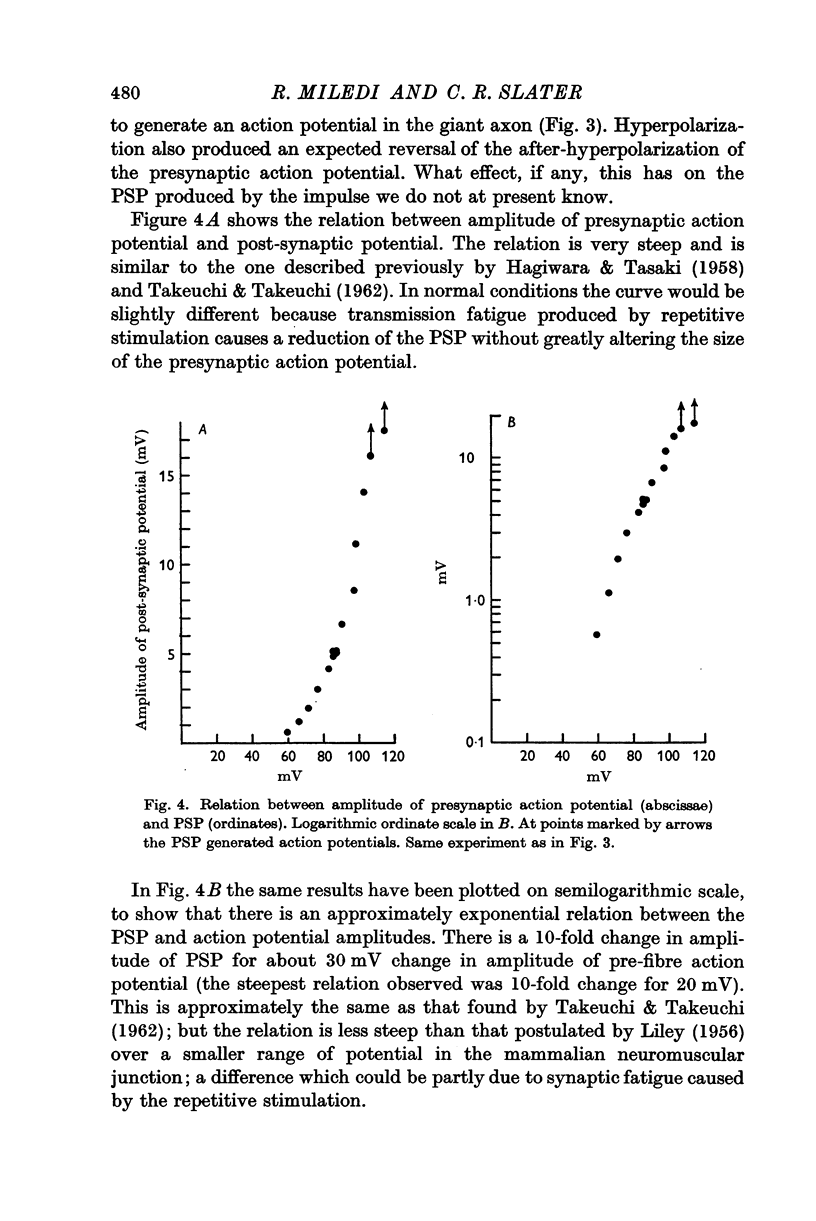
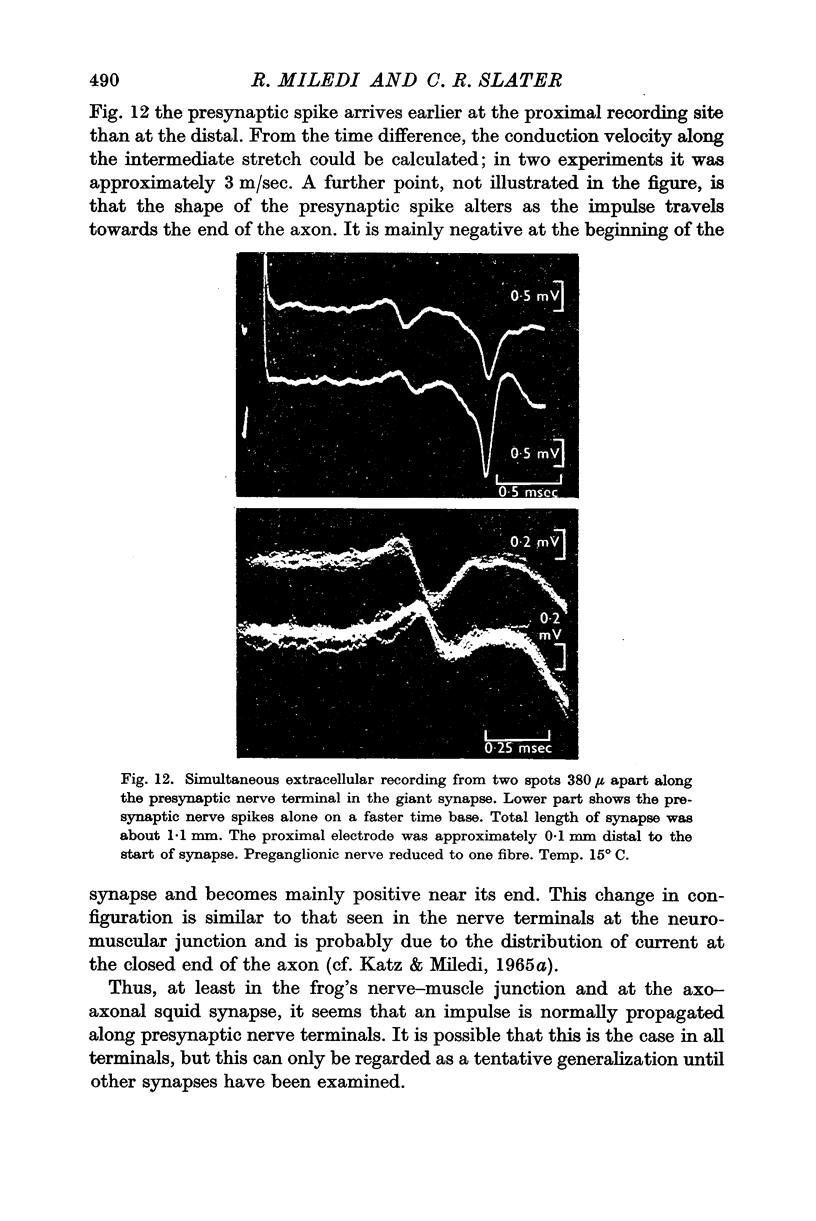
2. A micro-electrode was inserted in the presynaptic fibre and used to polarize and record simultaneously. In the distal (giant) synapse, hyperpolarization of the ending produced an increase in the size of the presynaptic action potential and post-synaptic potential (PSP). Depolarization had the opposite effect. These effects of polarization took more than 10 sec to develop fully, and declined with a similar time course at the end of polarization. Analogous results were obtained with two other preganglionic fibres, which make contacts in the proximal synaptic region.
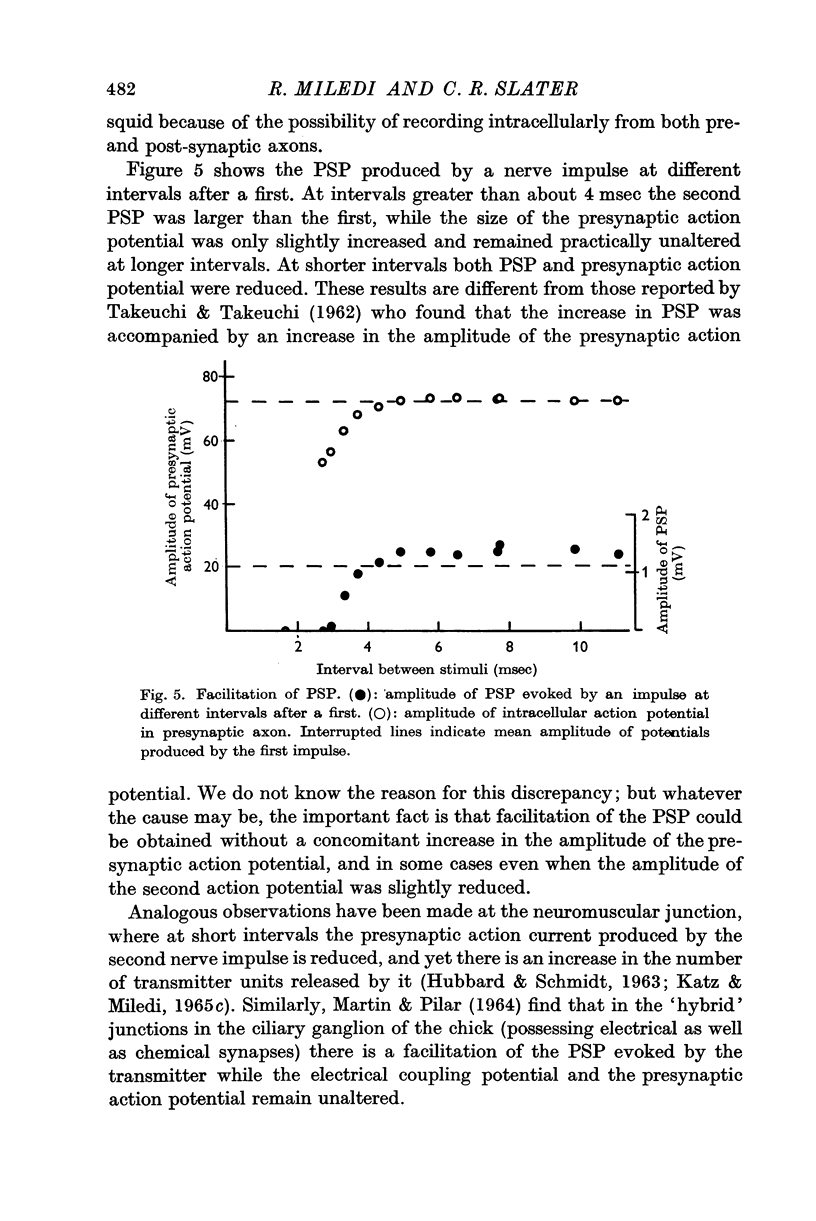
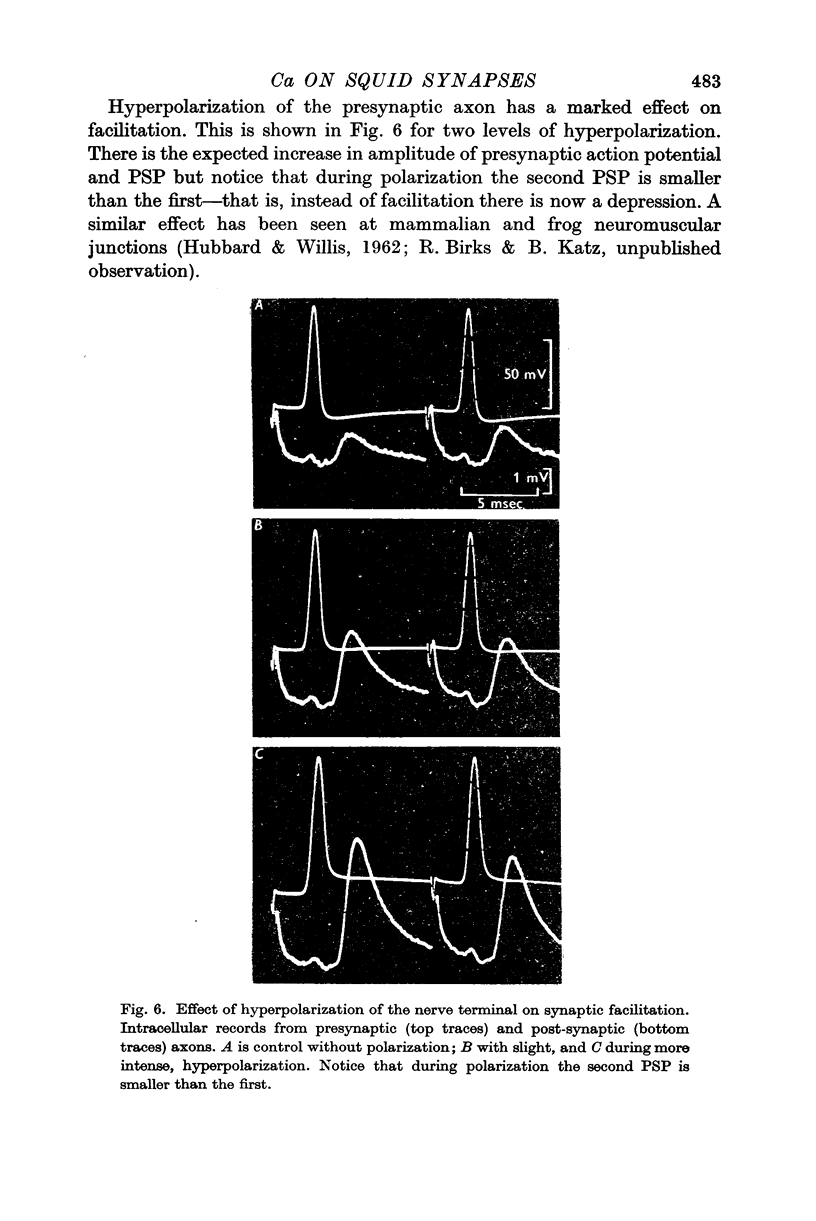
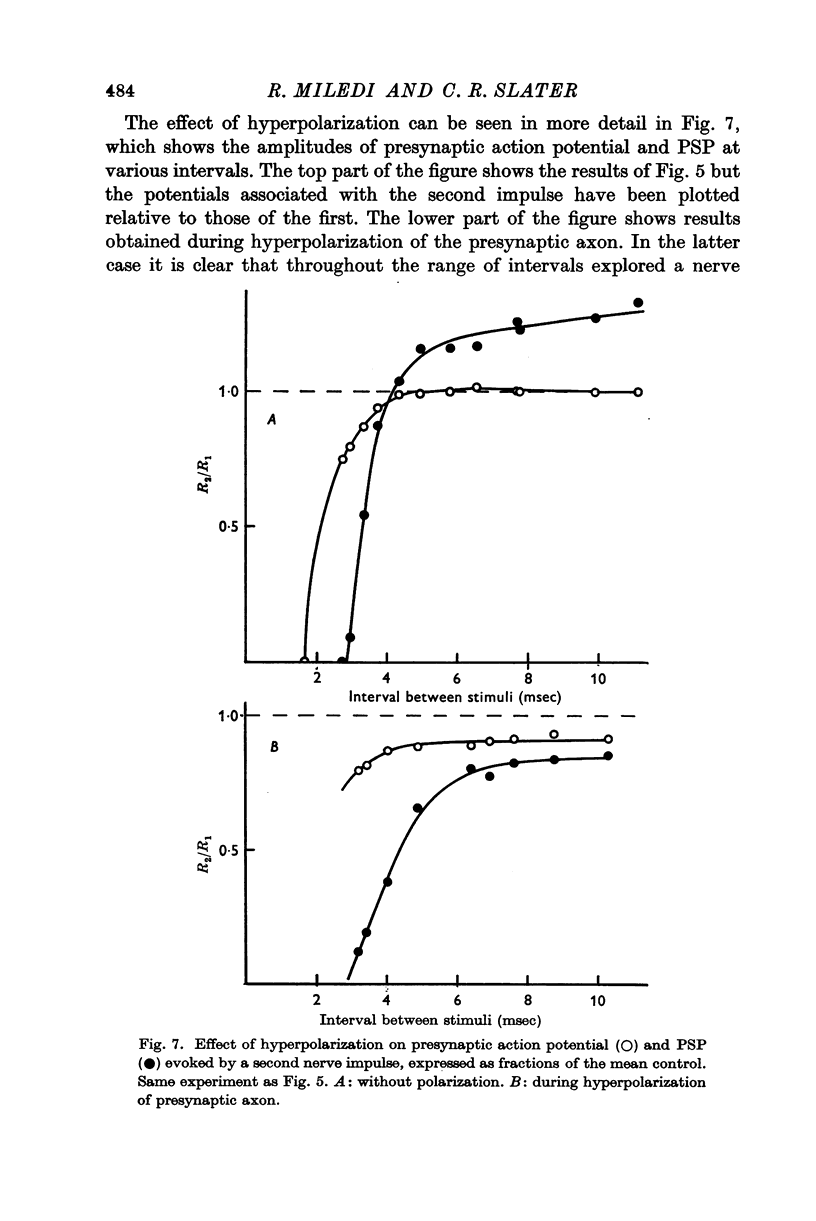
3. The second of a pair of preganglionic impulses evoked a PSP larger than the first. This facilitation of PSP was sometimes accompanied by a small increase in the size of the second action potential in the presynaptic axon. At some shorter intervals, the second presynaptic action potential was reduced in amplitude, but the PSP was still increased. Hyperpolarization of the presynaptic terminal increased the size of both PSPs in a pair and abolished the facilitation. With stronger hyperpolarization the second PSP was even smaller than the first.
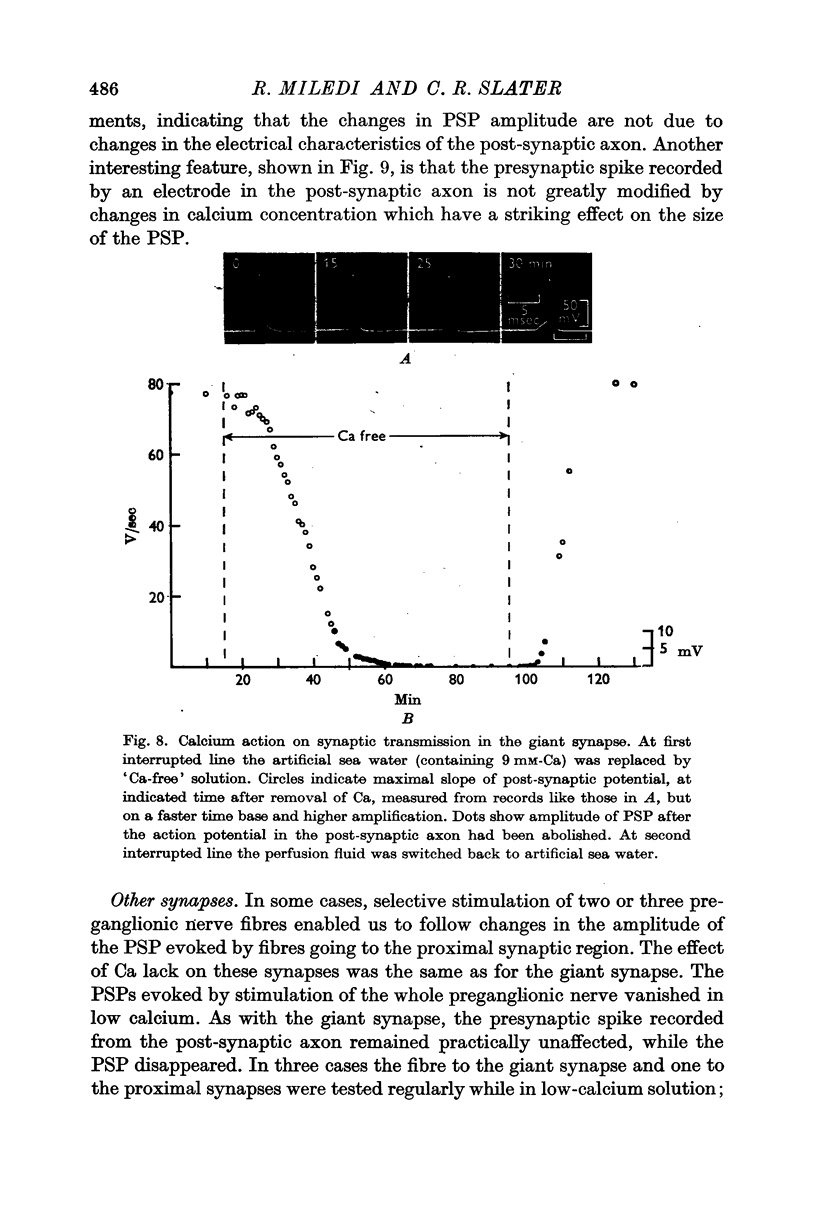
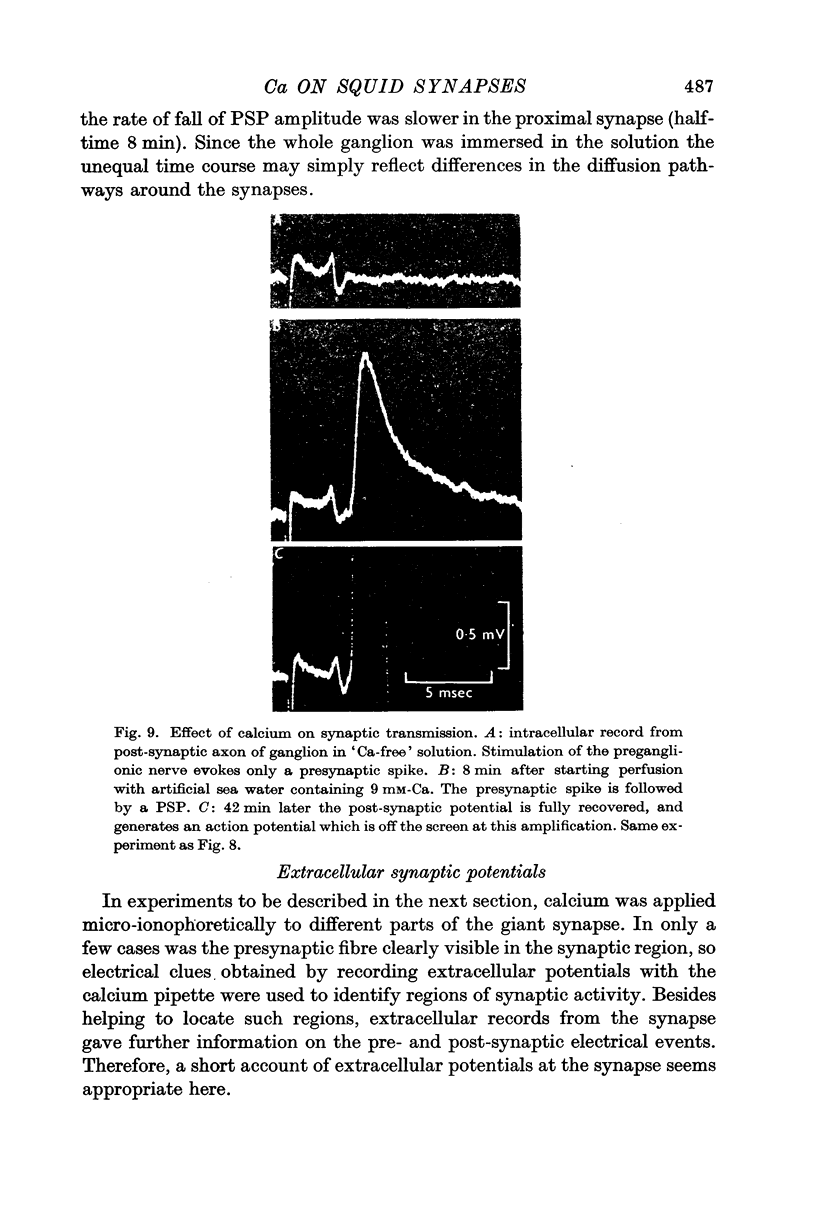
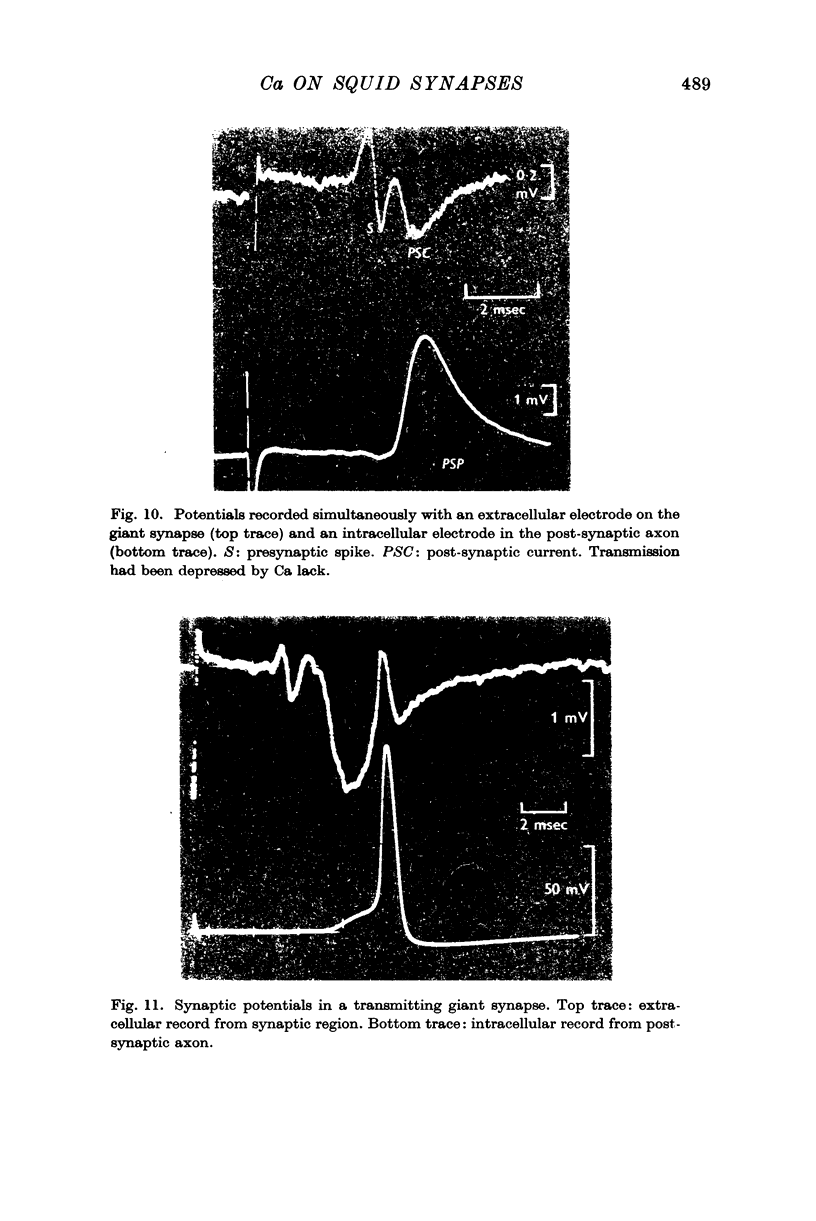
4. Removing or reducing the Ca in the bathing fluid reversibly abolished the post-synaptic response. The small presynaptic spikes remained practically unaffected. In these conditions a nerve impulse still invaded the ending and normal action potentials could be recorded from the pre-synaptic terminal. This shows that electrical coupling between pre- and post-synaptic axons is insufficient to account for synaptic transmission.
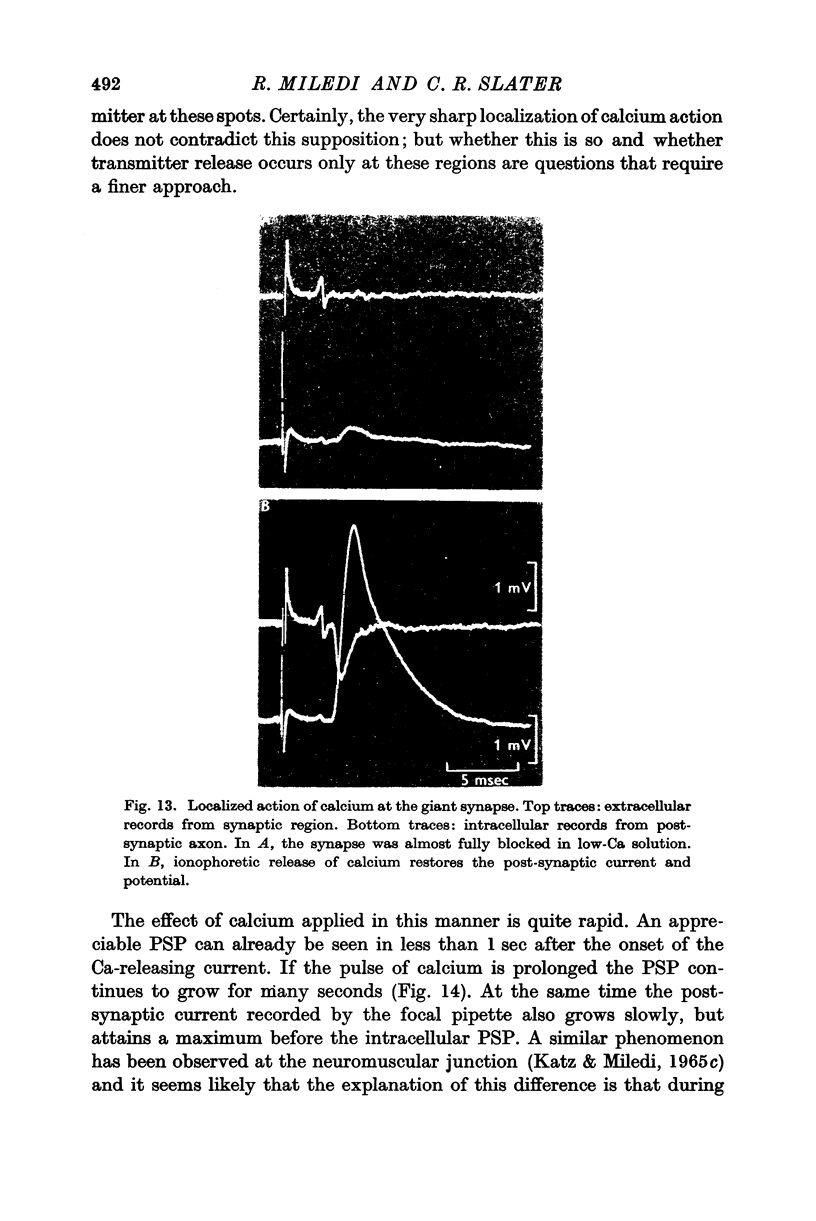
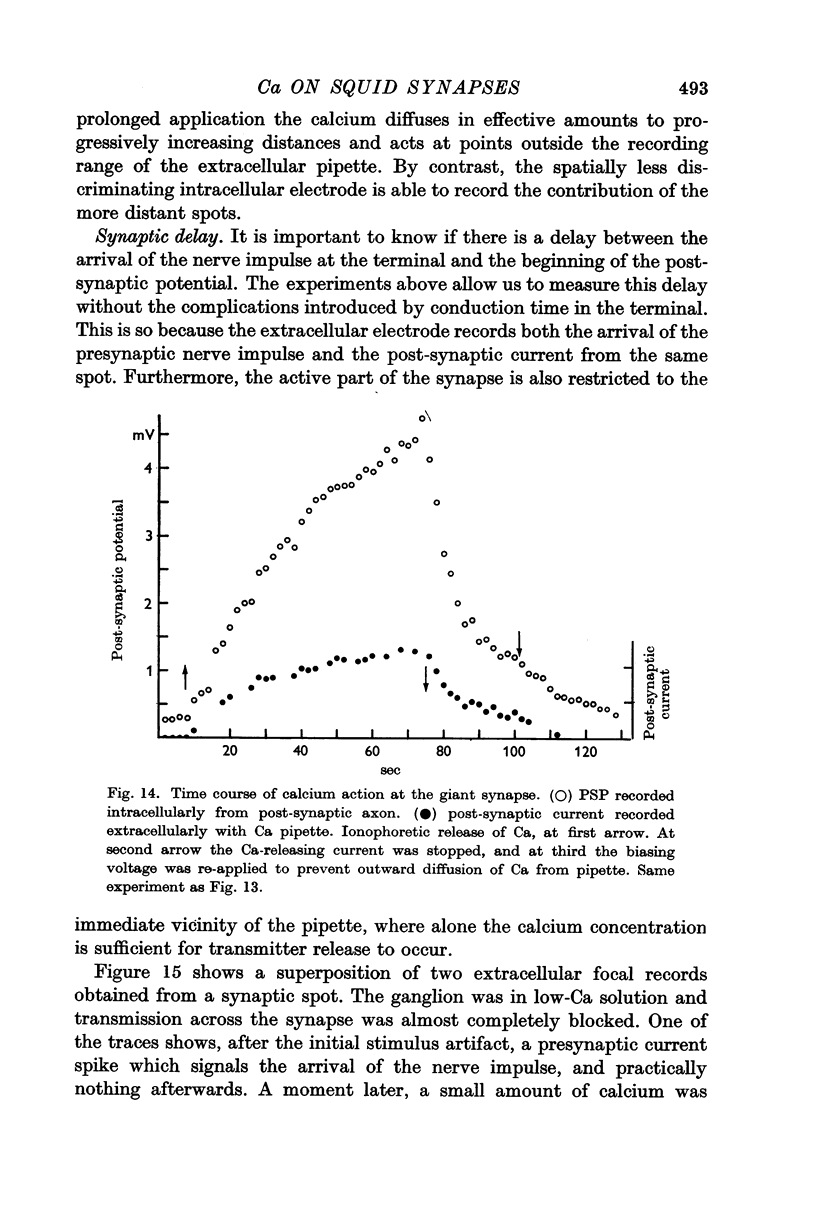
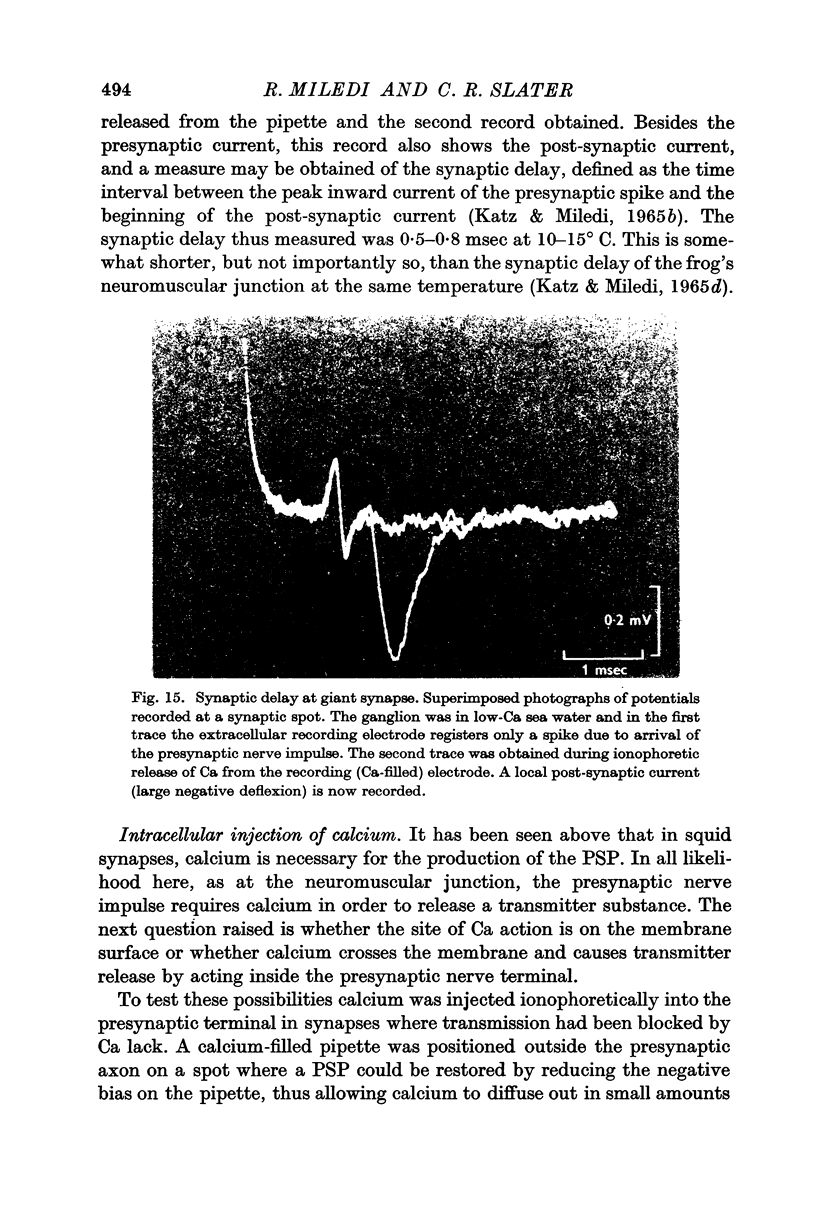
5. In low-Ca solution synaptic transmission could be restored locally by extracellular ionophoretic application of Ca to a small portion of the synapse. At sensitive spots a post-synaptic current (recorded with the Ca pipette) and PSP could be detected earlier than 1 sec after commencing the application of Ca.
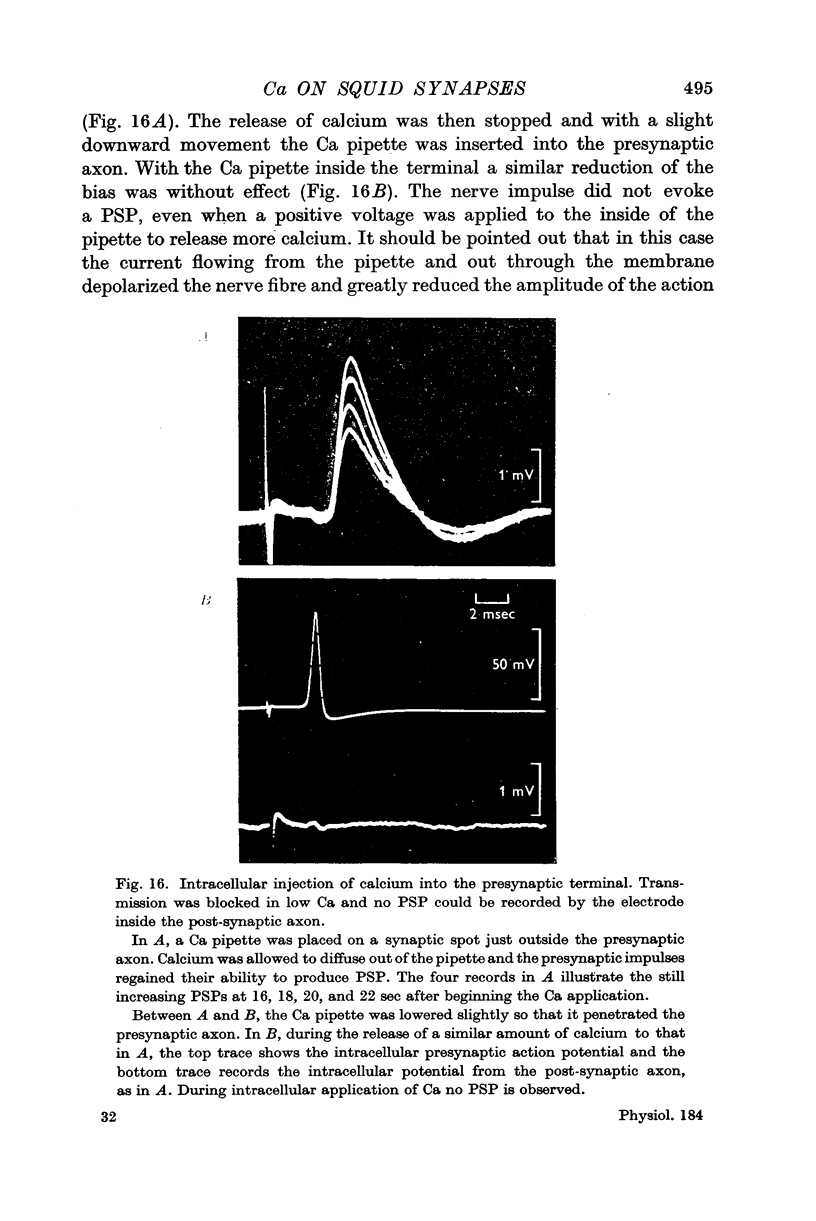
6. Ca was ineffective when injected intracellularly into the presynaptic fibre at a spot where extracellular ionophoresis of Ca restored the PSP.
7. The results indicate that synaptic transmission in the squid stellate ganglion is not electrical but due to the release of an unidentified transmitter. Release of this transmitter by the presynaptic nerve impulse requires the presence of Ca in the external medium. During the impulse Ca would combine with a `Ca-receptor' in the membrane and initiate the reactions which lead to transmitter release. It appears that the `Ca-receptor' is only accessible from the outside of the membrane.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT S. H. The function of the proximal synapses of the squid stellate ganglion. J Gen Physiol. 1959 Jan 20;42(3):609–616. doi: 10.1085/jgp.42.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT S. H. Transmission in squid giant synapses: the importance of oxygen supply and the effects of drugs. J Gen Physiol. 1958 Jan 20;41(3):473–484. doi: 10.1085/jgp.41.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLOCK T. H., HAGIWARA S. Intracellular recording from the giant synapse of the squid. J Gen Physiol. 1957 Mar 20;40(4):565–577. doi: 10.1085/jgp.40.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Mechanism of facilitation at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:530–542. doi: 10.1113/jphysiol.1961.sp006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., TASAKI I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958 Aug 29;143(1):114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMA K. Some observations on the fine structure of the giant synapse in the stellate ganglion of the squid, Doryteuphis bleekeri. Z Zellforsch Mikrosk Anat. 1962;56:437–444. doi: 10.1007/BF00335624. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- KUNO M. MECHANSIM OF FACILITATION AND DEPRESSION OF THE EXCITATORY SYNAPTIC POTENTIAL IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:100–112. doi: 10.1113/jphysiol.1964.sp007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. Ultrastructure of two invertebrate synapses. Proc Soc Exp Biol Med. 1953 Feb;82(2):219–223. doi: 10.3181/00379727-82-20071. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]


