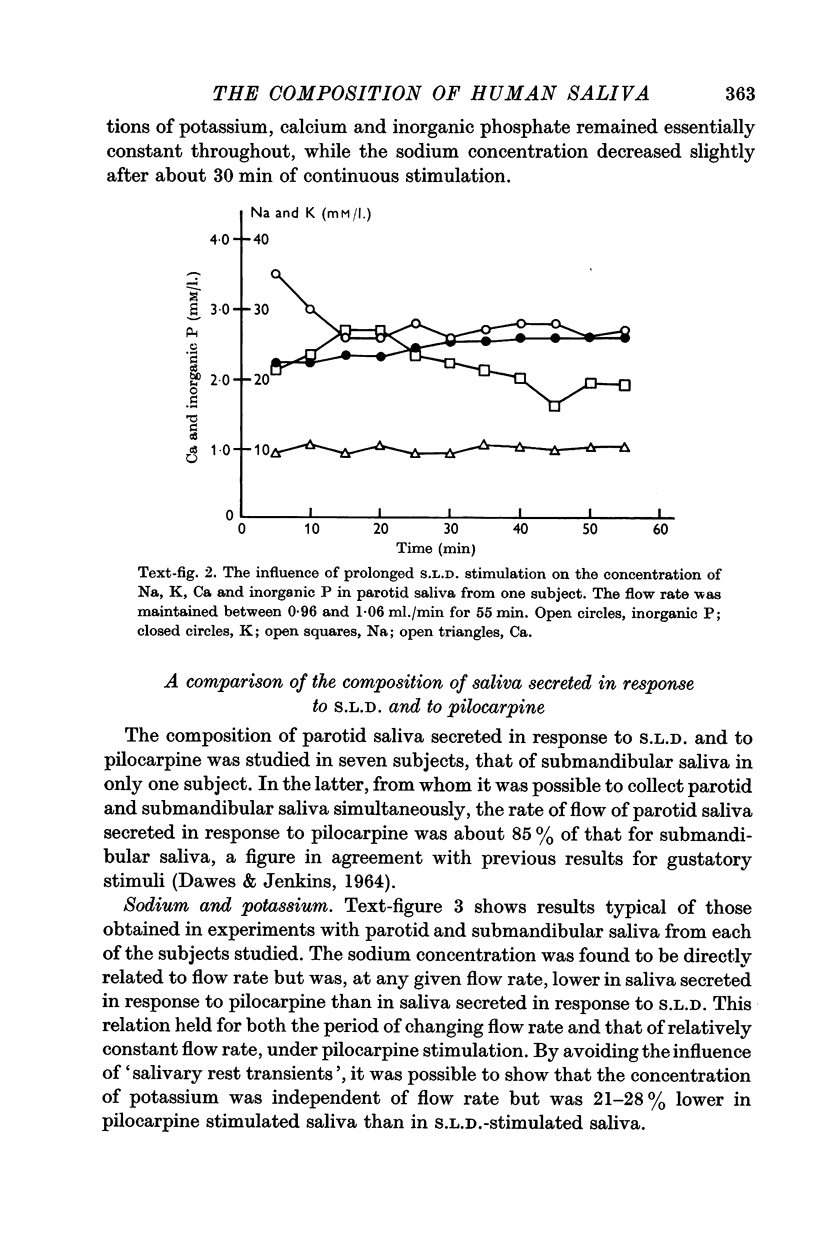
Abstract
1. The composition of human saliva secreted in response to sour lemon drops (S.L.D.), and pilocarpine, was studied.
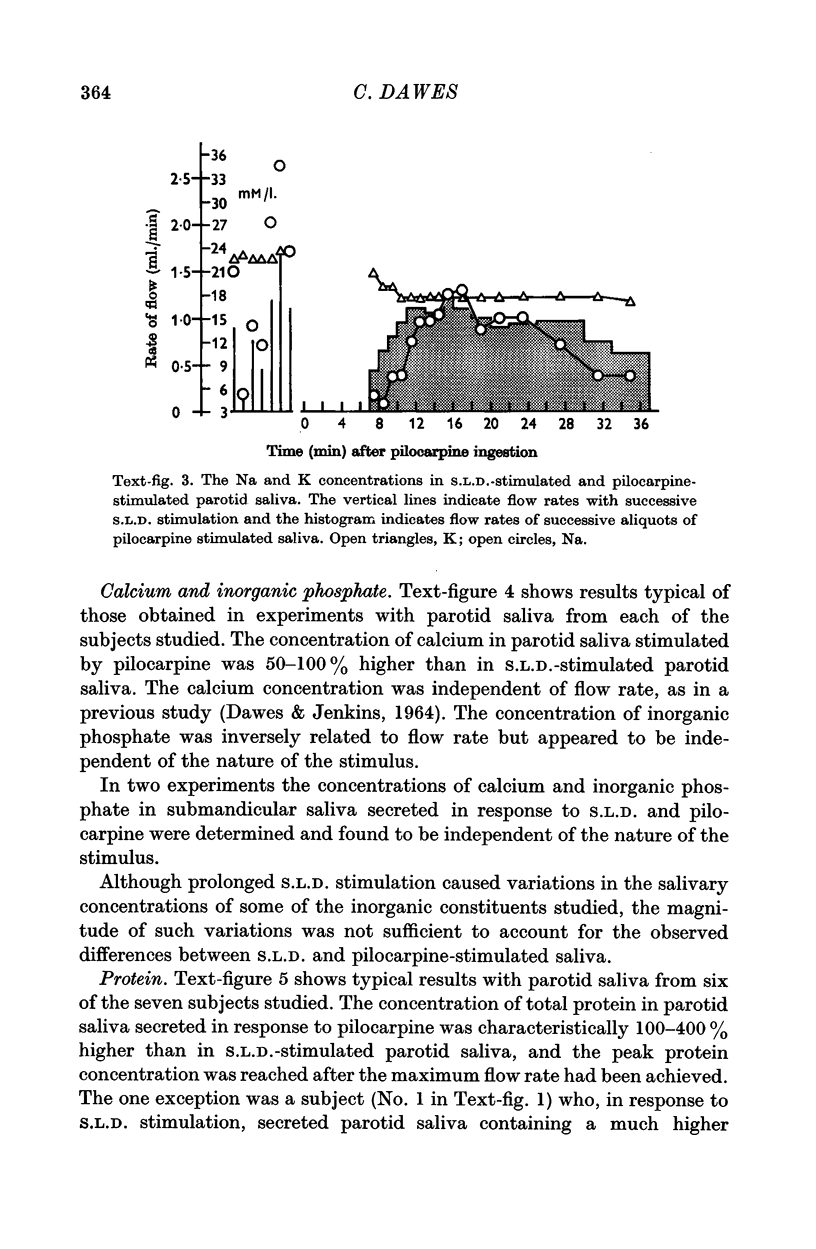
2. At a given flow rate, pilocarpine-stimulated submandibular and parotid saliva contained less sodium and potassium and an equivalent amount of inorganic phosphate, and parotid saliva also contained more calcium and protein than did the corresponding types of S.L.D.-stimulated saliva.
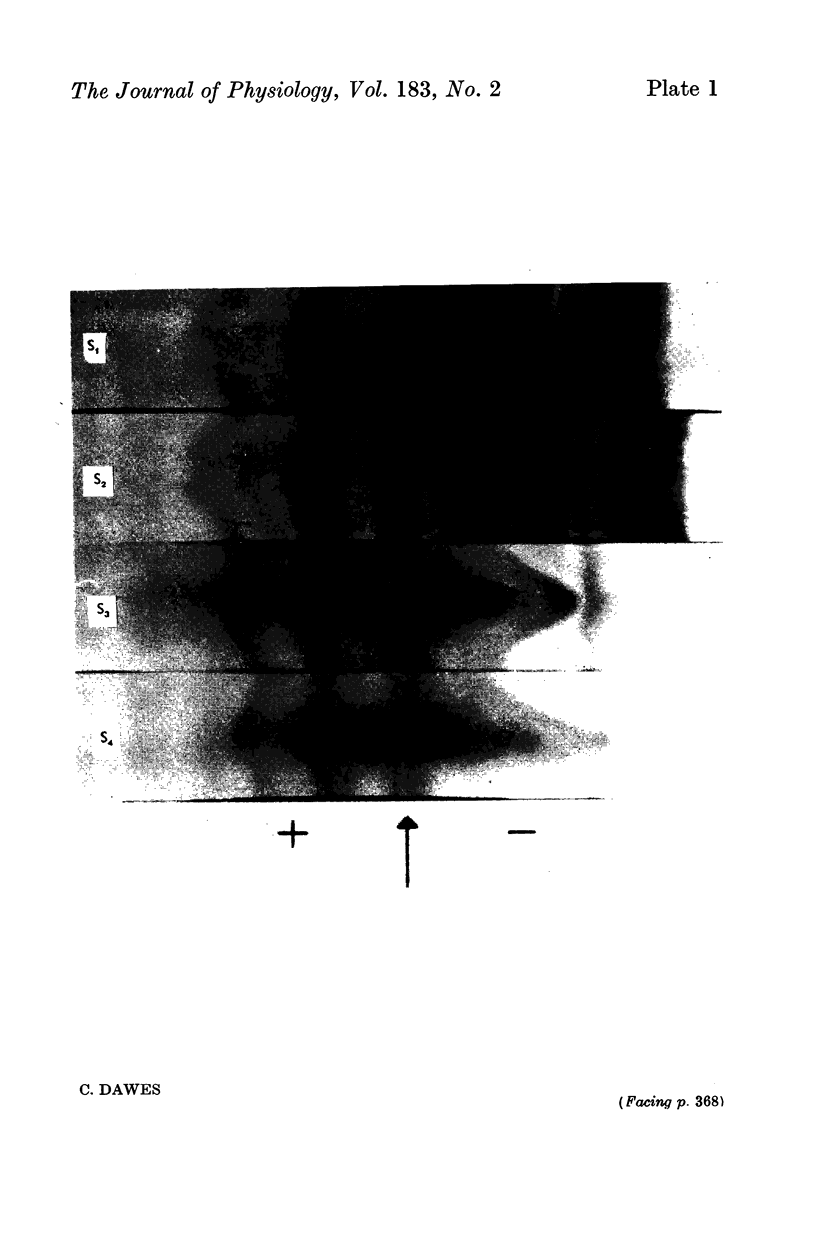
3. Prolonged S.L.D. stimulation did not cause a depletion in the protein concentration of either parotid or submandibular saliva and neither this procedure nor pilocarpine stimulation altered the proportions of the different proteins secreted.
4. Pilocarpine was judged to be an inadequate substitute for more physiological, gustatory stimuli.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anrep G. V. The metabolism of the salivary glands: I. The relation of the chorda tympani to the nitrogen metabolism of the submaxillary gland. J Physiol. 1921 Mar 15;54(5-6):319–331. doi: 10.1113/jphysiol.1921.sp001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY B. A., MATTHEWS J., SMYTH D. H. Transfer of glucose and fluid by different parts of the small intestine of the rat. J Physiol. 1961 Jul;157:279–288. doi: 10.1113/jphysiol.1961.sp006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY R. J., DIKSTEIN S., MATTHEWS J., SMYTH D. H., WRIGHT E. M. ELECTRICAL POTENTIALS ASSOCIATED WITH INTESTINAL SUGAR TRANSFER. J Physiol. 1964 Jun;171:316–338. doi: 10.1113/jphysiol.1964.sp007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. Determination of maltase and isomaltase activities with a glucose-oxidase reagent. Biochem J. 1961 Sep;80:547–551. doi: 10.1042/bj0800547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES C. DISODIUM ETHYLENEDIAMINETETRAACETATE AS AN AID FOR THE RECONSTITUTION OF LYOPHILIZED HUMAN SALIVARY PROTEINS BEFORE PAPER ELECTROPHORESIS. Arch Oral Biol. 1963 Sep-Oct;8:653–656. doi: 10.1016/0003-9969(63)90079-7. [DOI] [PubMed] [Google Scholar]
- DAWES C., JENKINS G. N. THE EFFECTS OF DIFFERENT STIMULI ON THE COMPOSITION OF SALIVA IN MAN. J Physiol. 1964 Jan;170:86–100. doi: 10.1113/jphysiol.1964.sp007315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES C., SHAW J. H. THE EFFECTS OF CHANGES IN THE PROPORTION AND TYPE OF DIETARY CARBOHYDRATE ON THE AMYLASE AND PROTEIN CONCENTRATIONS IN RAT SALIVA. Arch Oral Biol. 1965 Mar-Apr;10:261–267. doi: 10.1016/0003-9969(65)90028-2. [DOI] [PubMed] [Google Scholar]
- DAWES C. SOME CHARACTERISTICS OF PAROTID AND SUBMANDIBULAR SALIVARY PROTEINS. Arch Oral Biol. 1965 Mar-Apr;10:269–278. doi: 10.1016/0003-9969(65)90029-4. [DOI] [PubMed] [Google Scholar]
- Dawes C., Shaw J. H. Dietary phosphate supplementation and its effects on dental caries and salivary and serum concentrations of calcium and inorganic phosphate in the rat. Arch Oral Biol. 1965 Jul-Aug;10(4):567–577. doi: 10.1016/0003-9969(65)90002-6. [DOI] [PubMed] [Google Scholar]
- Dickens F., Weil-Malherbe H. Metabolism of normal and tumour tissue: A note on the metabolism of medulla of kidney. Biochem J. 1936 Apr;30(4):659–660. doi: 10.1042/bj0300659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERICSSON Y. THE ROLE OF SOME SALIVARY CONSTITUENTS IN ORAL PATHOLOGY, WITH SPECIAL REGARD TO CARIES EXPERIMENTS WITH RODENTS. Int Ser Monogr Oral Biol. 1964;3:281–297. doi: 10.1016/b978-1-4832-2871-6.50024-2. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY P. J., WILLIAMS R. A. A STUDY OF THE ORAL SECRETIONS OF RATS STIMULATED BY PILOCARPINE. Arch Oral Biol. 1965 Mar-Apr;10:237–244. doi: 10.1016/0003-9969(65)90025-7. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- LEVIN R. J., SMYTH D. H. THE EFFECT OF THE THYROID GLAND ON INTESTINAL ABSORPTION OF HEXOSES. J Physiol. 1963 Dec;169:755–769. doi: 10.1113/jphysiol.1963.sp007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NEWEY H., PARSONS B. J., SMYTH D. H. The site of action of phlorrhizin in inhibiting intestinal absorption of glucose. J Physiol. 1959 Oct;148:83–92. doi: 10.1113/jphysiol.1959.sp006274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMYTH D. H., TAYLOR C. B. Transfer of water and solutes by an in vitro intestinal preparation. J Physiol. 1957 May 23;136(3):632–648. doi: 10.1113/jphysiol.1957.sp005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEENEY E. A., SHAW J. H., CHILDS, EL, WEISBERGER D. Studies on the protein composition of rodent saliva. I. Application of methods of paper electrophoresis to two strains of laboratory rat. Arch Oral Biol. 1962 Sep-Oct;7:621–632. doi: 10.1016/0003-9969(62)90069-9. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954 Jan;123(1):116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]



