Abstract
1. An in vitro method, using fluorescent γ-globulin and everted neonatal pig's intestinal slices, for the study of the active transport of large molecules is described.
2. Uptake of γ-globulin occurred within 15 min and required no exogenous substrates.
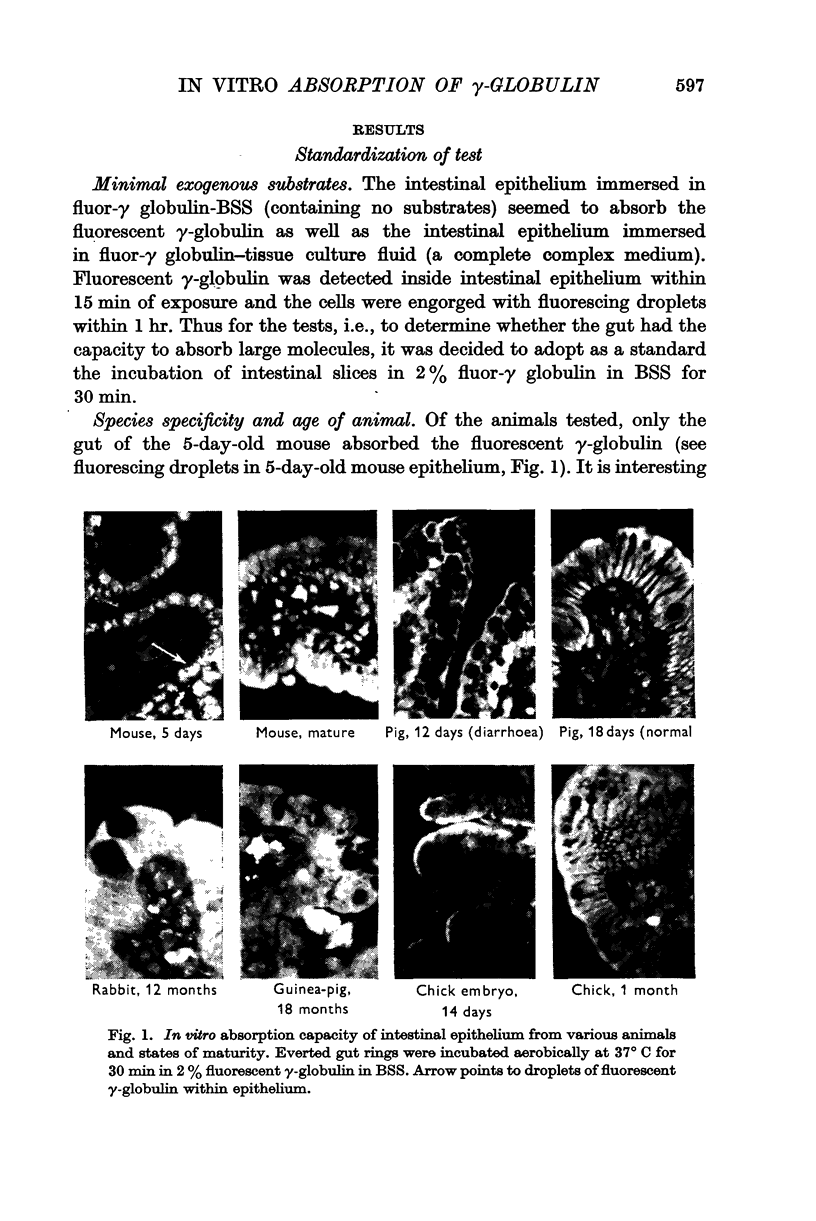
3. In vitro absorption of γ-globulin by intestinal epithelium was limited to the neonatal pig and 5-day-old mouse. No uptake was seen in intestines from a mature mouse, a pig with diarrhoea, a normal pig, a mature rabbit, a guinea-pig, a chick, and a chick embryo. Chick embryo yolk sac readily took up γ-globulin.
4. Rings of everted intestinal epithelium remained active (still absorbed γ-globulin) after incubating for 4-6 hr in balanced salt solution (BSS).
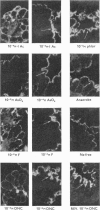
5. Uptake of γ-globulin required oxygen and sodium and was reversibly inhibited by metabolic antagonists such as iodoacetate, arsenate, fluoride, 4,6-dinitro-ϕ-cresol, phlorrhizin, anaerobiosis and cold. Under the conditions of the test, large colloidal molecules did not inhibit uptake of γ-globulin.
6. Similar results (although not as clear-cut) with metabolic inhibitors were obtained with preparations of chick embryo yolk sacs.
7. Injuring mature pig's intestinal epithelium with surface-active agents did not produce non-specific absorption artifacts that resembled the specific absorption found in immature pig's intestinal epithelium.
Full text
PDF



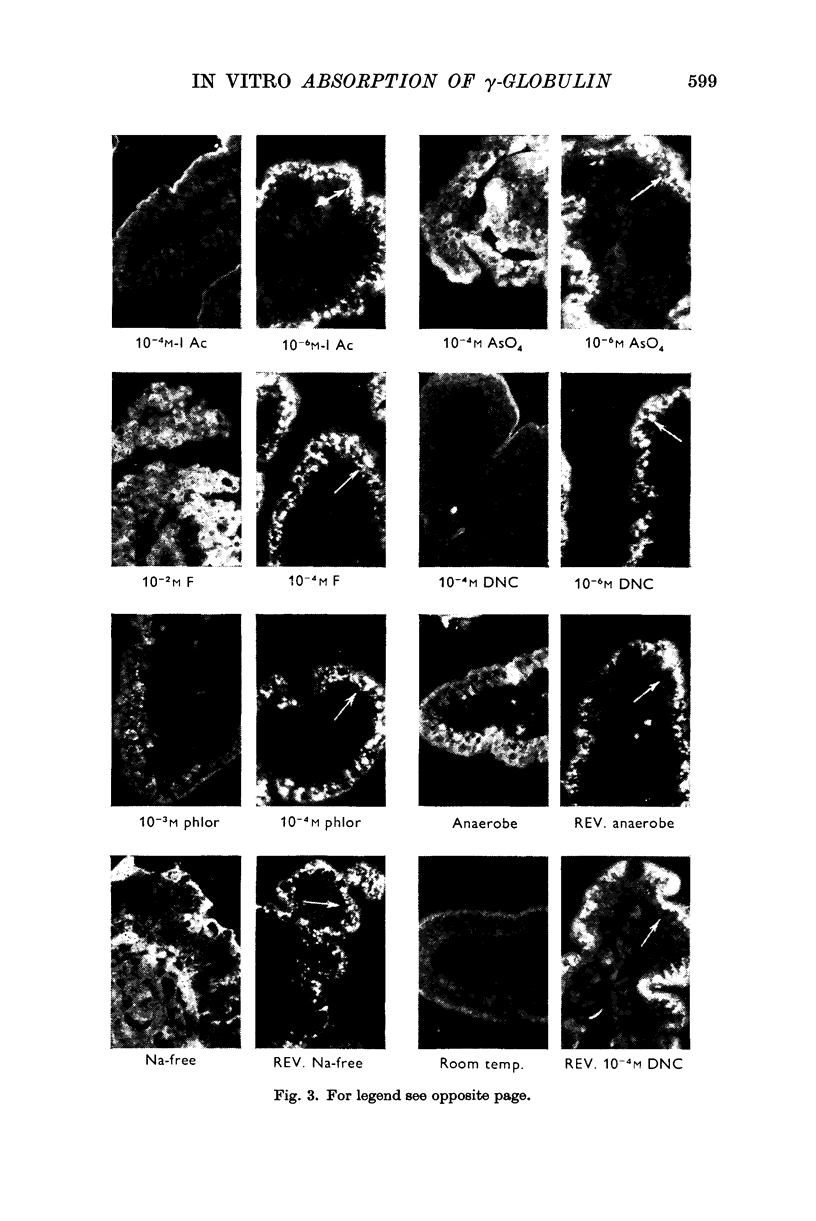

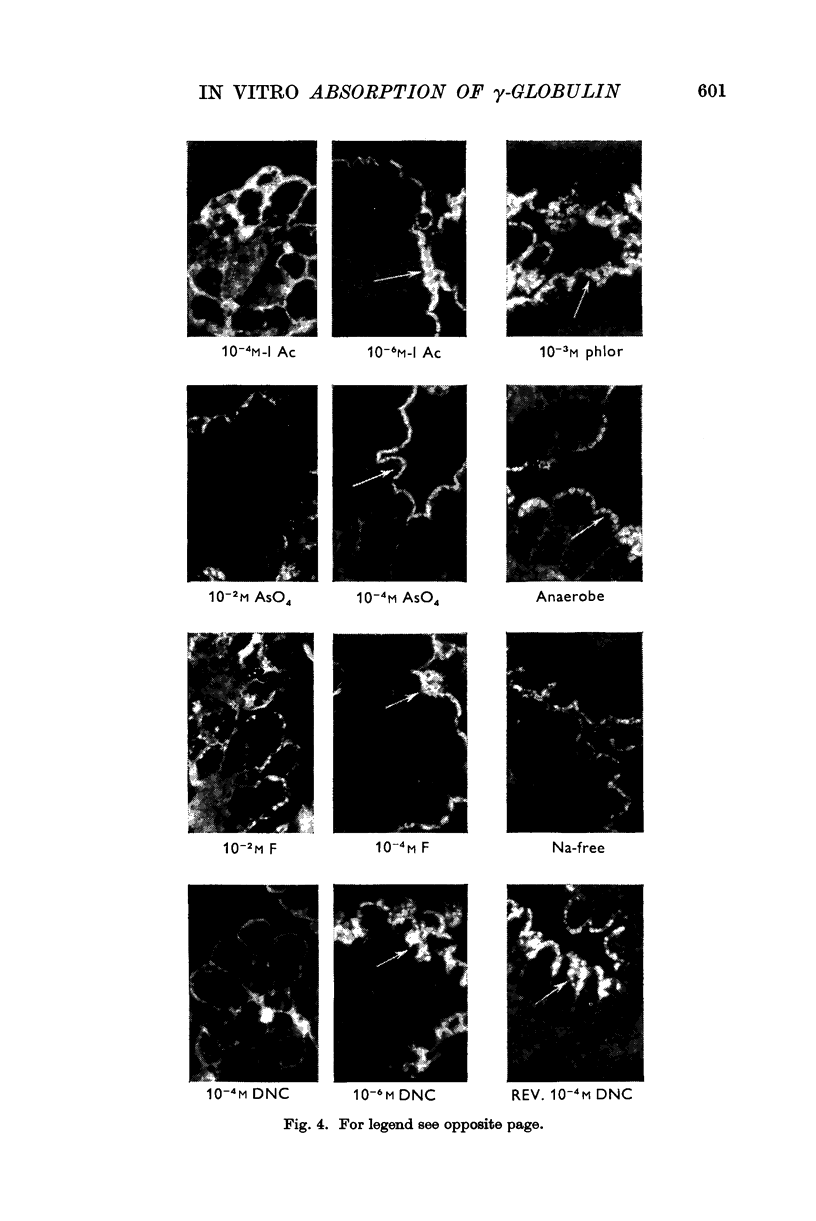



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIHLER I., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. V. The influence of several cations and anions on the active transport of sugars, in vitro, by various preparations of hamster small intestine. Biochim Biophys Acta. 1962 May 7;59:78–93. doi: 10.1016/0006-3002(62)90699-6. [DOI] [PubMed] [Google Scholar]
- CLARK S. L., Jr The ingestion of proteins and colloidal materials by columnar absorptive cells of the small intestine in suckling rats and mice. J Biophys Biochem Cytol. 1959 Jan 25;5(1):41–50. doi: 10.1083/jcb.5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K. Hypothesis for mechanism of intestinal active transport of sugars. Fed Proc. 1962 Nov-Dec;21:891–895. [PubMed] [Google Scholar]
- CRANE R. K., MANDELSTAM P. The active transport of sugars by various preparations of hamster intestine. Biochim Biophys Acta. 1960 Dec 18;45:460–476. doi: 10.1016/0006-3002(60)91482-7. [DOI] [PubMed] [Google Scholar]
- CSAKY T. Z., THALE M. Effect of ionic environment on intestinal sugar transport. J Physiol. 1960 Apr;151:59–65. [PMC free article] [PubMed] [Google Scholar]
- HOLTER H. Pinocytosis. Int Rev Cytol. 1959;8:481–504. doi: 10.1016/s0074-7696(08)62738-2. [DOI] [PubMed] [Google Scholar]
- HOLTZER H., HOLTZER S. The in vitro uptake of fluorescein labelled plasma proteins. I. Mature cells. C R Trav Lab Carlsberg. 1960;31:373–408. [PubMed] [Google Scholar]
- RYSER H. J. THE MEASUREMENT OF I131-SERUM ALBUMIN UPTAKE BY TUMOR CELLS IN TISSUE CULTURE. Lab Invest. 1963 Oct;12:1009–1017. [PubMed] [Google Scholar]
- RYSER H., AUB J. C., CAULFIELD J. B. Studies on protein uptake by isolated tumor cells. II. Quantitative data on the adsorption and uptake of I-131-serum albumin by Ehrlich ascites tumor cells. J Cell Biol. 1962 Dec;15:437–449. doi: 10.1083/jcb.15.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]