Abstract
1. Tritiated water, [14C]urea, [14C]thiourea, [14C]sucrose and [59Fe]-haemoglobin were used to study the permeability of a semi-isolated piece of the great curvature of the canine stomach.
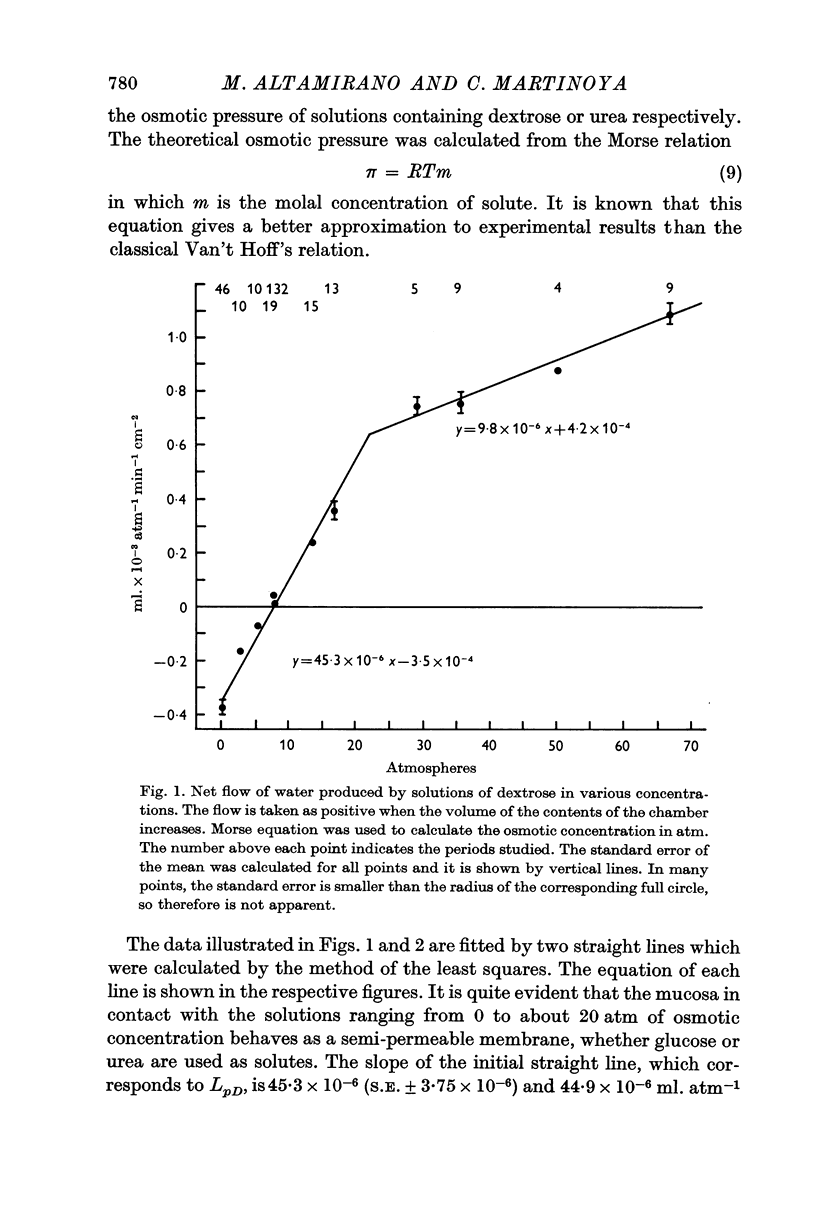
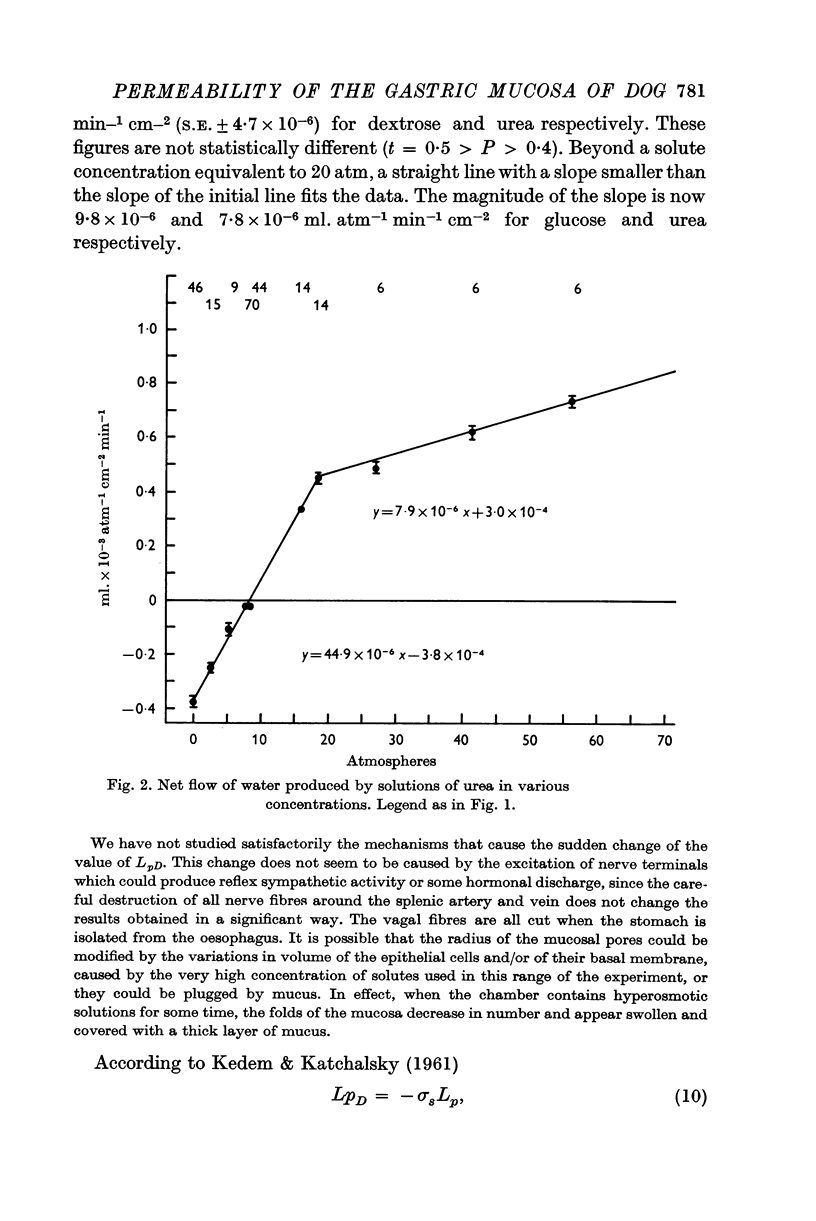
2. The osmotic pressure of the solutions placed in contact with the secretory surface of the epithelium was changed by means of dextrose or urea. The mucosa behaved as a semi-permeable membrane, meaning that water flowed under gradients of osmotic pressure. Regardless of the solute used, about 45 × 10-6 ml. of water flowed/cm2/min under a gradient of one atmosphere.
3. The permeability constants of the probing molecules were determined under zero net volume flow obtained by placing isosmotic dextrose or isosmotic urea in the chamber. The constants decreased as the molecular volume of the probing molecules increased.
4. The transport of all the non-electrolytes across the epithelium decreased significantly when the chamber contained isosmotic dextrose. Basically, this effect seems to be a result of the reduction of the area available for diffusion caused by the high molecular volume of dextrose.
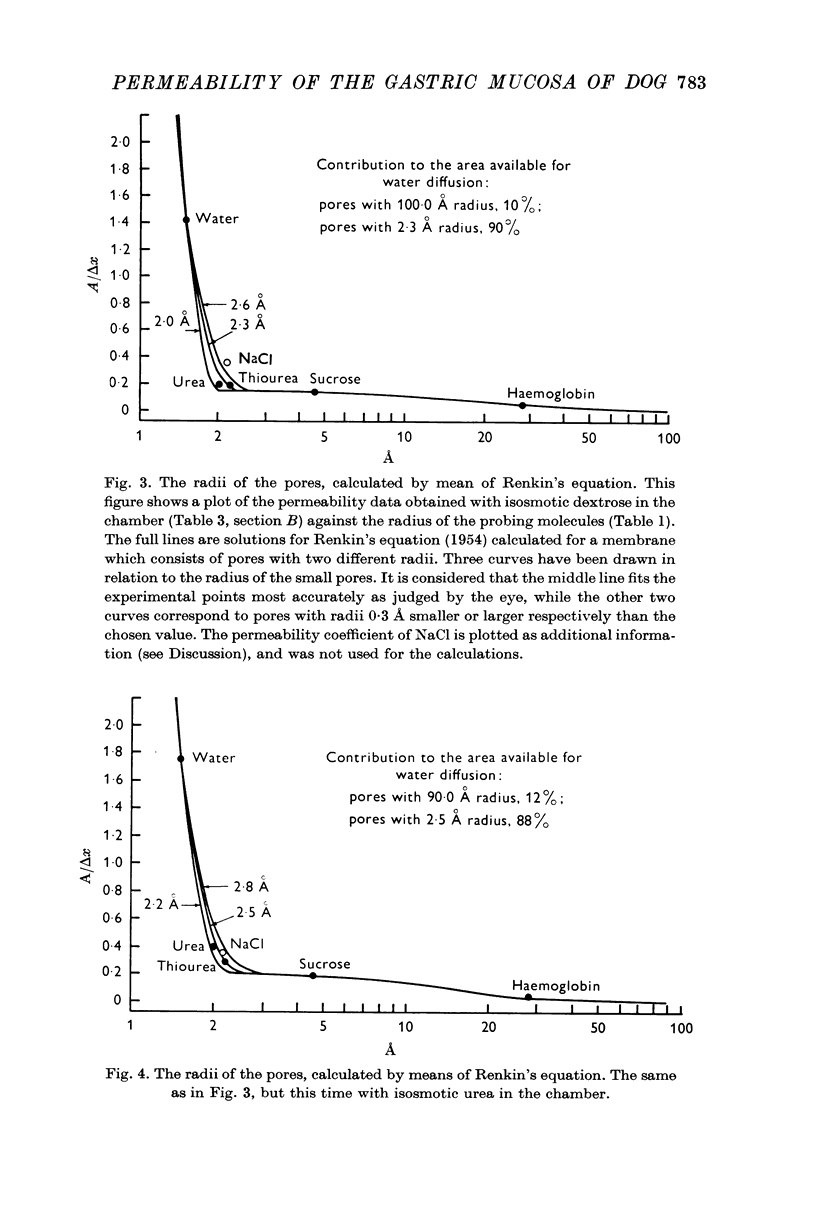
5. The increased hindrance to diffusion of the probing molecules caused by the added solutes is considered as good evidence that the probing molecules diffuse by way of pores filled with water.
6. The equation derived by Renkin (1954) fits the results obtained if we assume that the equivalent membrane has pores of at least two different radii. The calculated radii vary somewhat with the solute placed in the chamber, though about 88% of the area available for diffusion consists of pores with radii smaller than 2·5 Å.
7. The equivalent pore radius, calculated from Kedem & Katchalsky's (1961) formula for pores of one single radius, contradicts some experimental findings. Once again, the results obtained would be reproduced more accurately by an equivalent membrane pierced by parallel pores of at least two different diameters.
8. A procedure is suggested for calculating the proportion of pores of different radii. It seems likely that the pore radii vary in a continuous distribution from the large pores which allow the diffusion of haemoglobin, to pores hardly permitting the passage of water. The wide pores would form a small fraction of the total area available for the diffusion of water.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTAMIRANO M. ALKALINE SECRETION PRODUCED BY INTRA-ARTERIAL ACETYLCHOLINE. J Physiol. 1963 Oct;168:787–803. doi: 10.1113/jphysiol.1963.sp007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTAMIRANO M., CHIANG L., BRAVO I. Effect of sympathetic stimulation on gastric secretion of pepsinogen. Am J Physiol. 1960 Jul;199:131–135. doi: 10.1152/ajplegacy.1960.199.1.131. [DOI] [PubMed] [Google Scholar]
- CODE C. F., SCHOLER J. F., HIGHTOWER N. C., Jr, DIETZLER F. K., BALDES E. J. Absorption of water from the upper part of the human gastrointestinal tract. Proc Staff Meet Mayo Clin. 1954 May 5;29(9):235–240. [PubMed] [Google Scholar]
- Cope O., Blatt H., Ball M. R. GASTRIC SECRETION. III. THE ABSORPTION OF HEAVY WATER FROM POUCHES OF THE BODY AND ANTRUM OF THE STOMACH OF THE DOG. J Clin Invest. 1943 Jan;22(1):111–115. doi: 10.1172/JCI101361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT H. W. SODIUM AND POTASSIUM IN CANINE GASTRIC MUCOSA AND SMOOTH MUSCLE. Am J Physiol. 1963 Sep;205:413–416. doi: 10.1152/ajplegacy.1963.205.3.413. [DOI] [PubMed] [Google Scholar]
- DURBIN R. P., FRANK H., SOLOMON A. K. Water flow through frog gastric mucosa. J Gen Physiol. 1956 Mar 20;39(4):535–551. doi: 10.1085/jgp.39.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN D. A., SOLOMON A. K. Determination of equivalent pore radius for human red cells by osmotic pressure measurement. J Gen Physiol. 1960 Sep;44:1–17. doi: 10.1085/jgp.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT J. N., MacDONALD I., SPURRELL W. R. The gastric response to pectin meals of high osmotic pressure. J Physiol. 1951 Oct 29;115(2):185–195. doi: 10.1113/jphysiol.1951.sp004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. A physical interpretation of the phenomenological coefficients of membrane permeability. J Gen Physiol. 1961 Sep;45:143–179. doi: 10.1085/jgp.45.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- MUIRHEAD H., PERUTZ M. F. STRUCTURE OF HAEMOGLOBIN. A THREE-DIMENSIONAL FOURIER SYNTHESIS OF REDUCED HUMAN HAEMOGLOBIN AT 5-5 A RESOLUTION. Nature. 1963 Aug 17;199:633–638. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Use of labelled plasma proteins in the study of nutritional problems. Prog Biophys Biophys Chem. 1957;7:115–163. [PubMed] [Google Scholar]
- OBRINK K. J. Water permeability of the isolated stomach of the mouse. Acta Physiol Scand. 1956 May 18;36(3):229–244. doi: 10.1111/j.1748-1716.1956.tb01320.x. [DOI] [PubMed] [Google Scholar]
- PAGANELLI C. V., SOLOMON A. K. The rate of exchange of tritiated water across the human red cell membrane. J Gen Physiol. 1957 Nov 20;41(2):259–277. doi: 10.1085/jgp.41.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REHM W. S., SCHLESINGER H., DENNIS W. H. Effect of osmotic gradients on water transport, hydrogen ion and chloride ion production in the resting and secreting stomach. Am J Physiol. 1953 Dec;175(3):473–486. doi: 10.1152/ajplegacy.1953.175.3.473. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- SCHOLER J. F., CODE C. F. Rate of absorption of water from stomach and small bowel of human beings. Gastroenterology. 1954 Nov;27(5):565–583. [PubMed] [Google Scholar]
- SCHULTZ S. G., SOLOMON A. K. Determination of the effective hydrodynamic radii of small molecules by viscometry. J Gen Physiol. 1961 Jul;44:1189–1199. doi: 10.1085/jgp.44.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]


