Abstract
1. Rings of rat jejunum incubated in vitro accumulate a mixture of amino acids at a rate of about 3 μmoles/cm. hr. The rate of incorporation of the accumulated amino acid into the tissue protein corresponds to a rate of synthesis of 50% of the protein of the whole wall in five days.
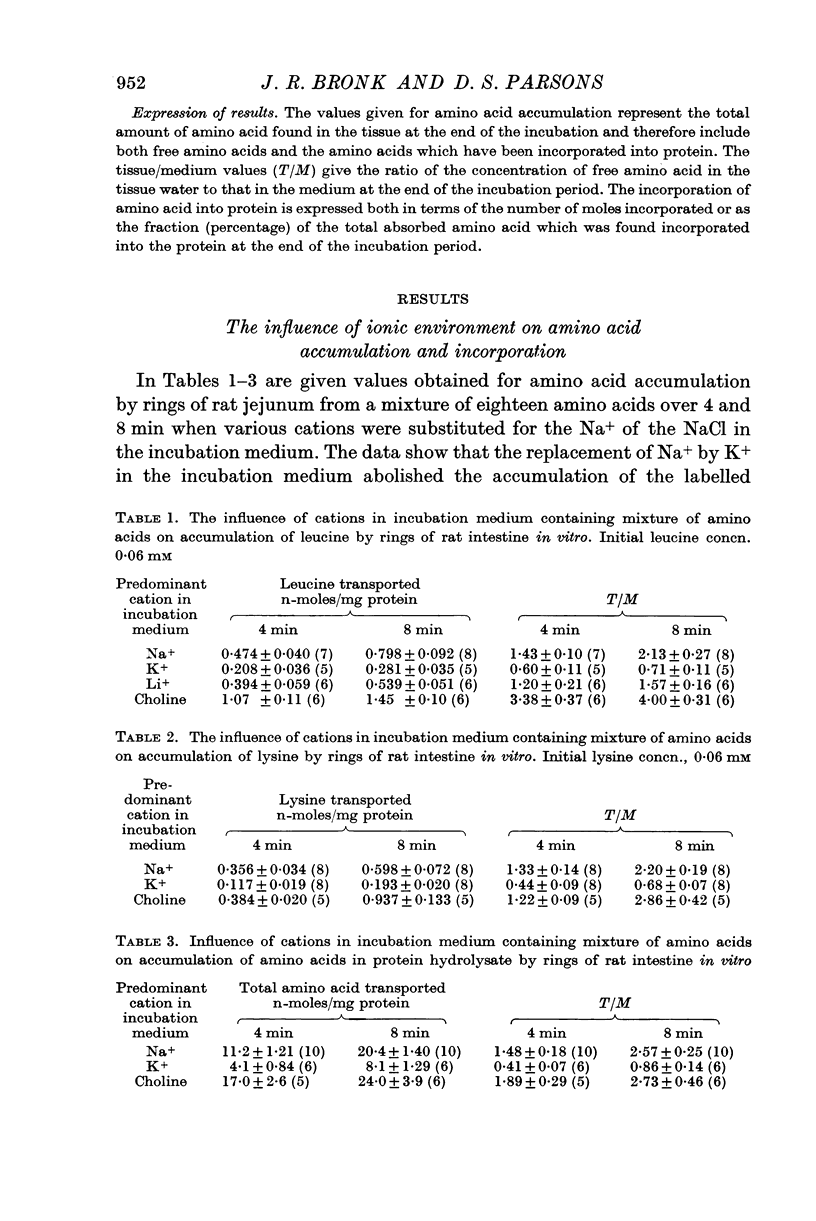
2. Replacement of the Na+ in the NaCl of the incubation medium by choline or by Li+ did not prevent amino acid accumulation by the tissue. However, replacement of the Na+ by K+ prevented the accumulation.
3. The accumulation of amino acids by rat jejunum in vitro proceeded at normal rates not only in the presence in the incubation medium of oxygen tensions below 10 Torr but also in the presence of 2,4-dinitrophenol. Reasons are given for supposing that the findings are compatible with the view that the energy upon which depend the processes of amino acid accumulation by the tissue could be derived from the movement of ions across cellular boundaries.
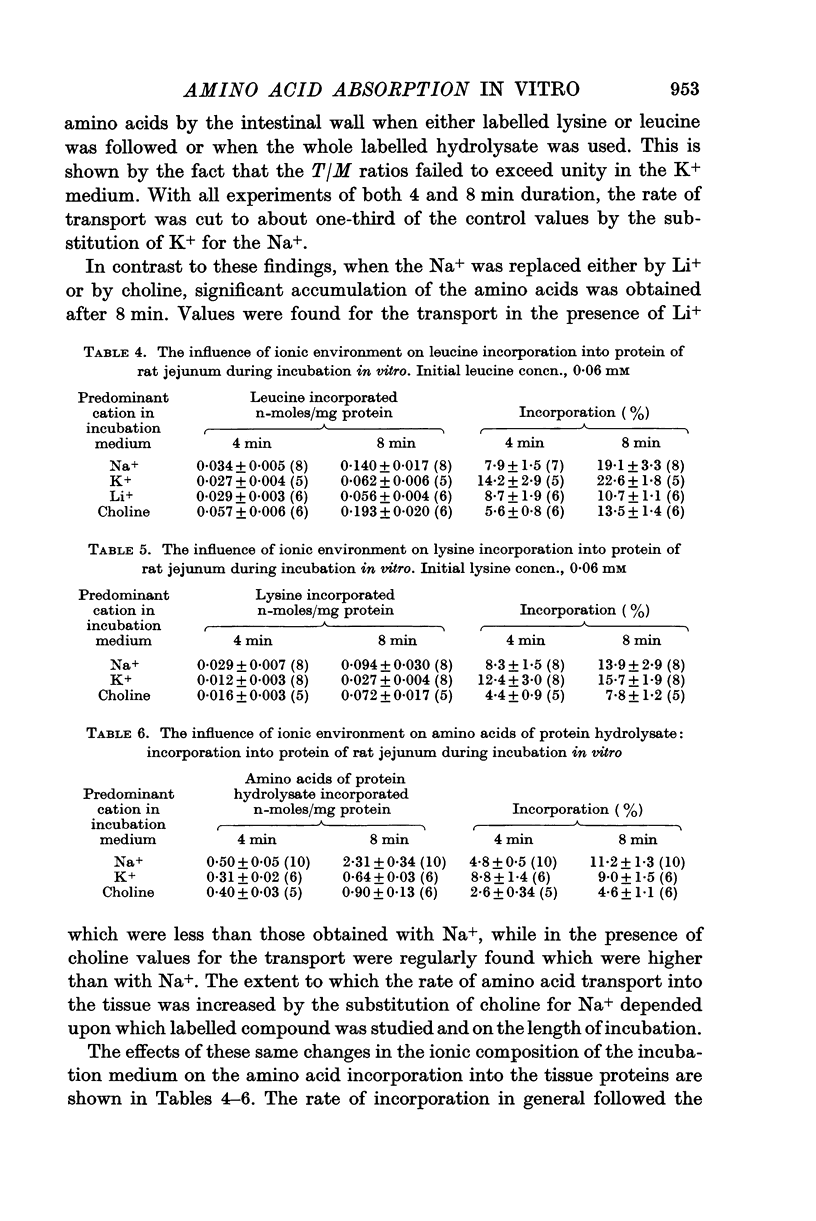
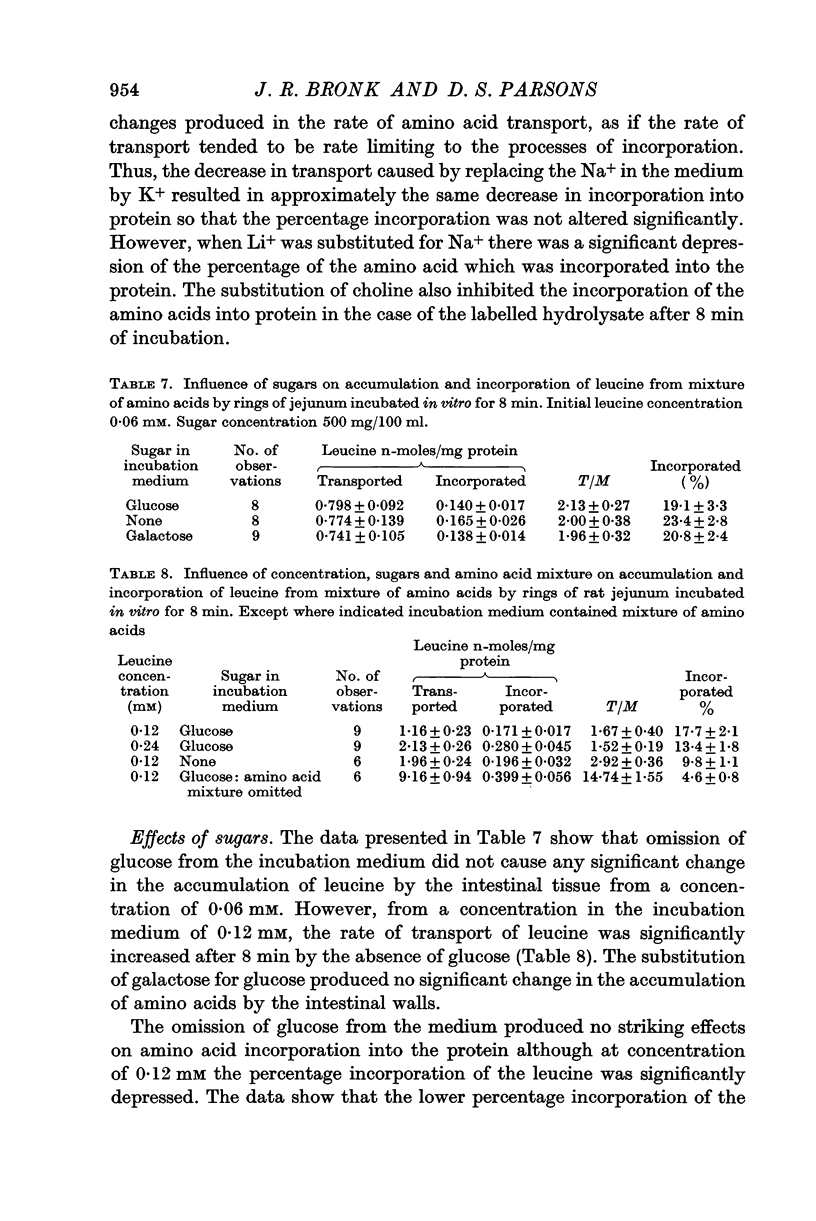
4. The amino acid incorporation into the tissue proteins was reduced to one tenth of the control rate in the presence of 2,4-dinitrophenol or by hypoxia so that the processes of incorporation depend upon energy derived from oxidative metabolism. In the presence of oligomycin the tissue respiration was depressed but the amino acid incorporation into the tissue protein was not inhibited. The view that the amino acid incorporation may be able to function with energy intermediates other than ATP is discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN M. M., PARSONS D. S. Observations on the changes in the potassium content of rat jejunal mucosa during absorption. Biochim Biophys Acta. 1962 May 7;59:249–251. doi: 10.1016/0006-3002(62)90728-x. [DOI] [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. The influence of thyroid gland on amino acid accumulation and protein synthesis by rat small intestine in vitro. J Physiol. 1966 Jun;184(4):942–949. doi: 10.1113/jphysiol.1966.sp007958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. The polarographic determination of the respiration of the small intestine of the rat. Biochim Biophys Acta. 1965 Oct 18;107(3):397–404. doi: 10.1016/0304-4165(65)90183-2. [DOI] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- DAWSON R., HOLDSWORTH E. S. An investigation into protein digestion with 14C-labelled protein. 1. The general pattern of 14C incorporation in body tissues and fluids of the rat up to 3 h after feeding. Br J Nutr. 1962;16:13–25. doi: 10.1079/bjn19620002. [DOI] [PubMed] [Google Scholar]
- Dickens F., Weil-Malherbe H. Metabolism of normal and tumour tissue: The metabolism of intestinal mucous membrane. Biochem J. 1941 Jan;35(1-2):7–15. doi: 10.1042/bj0350007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H., PIEZ K. A., FLEISCHMAN R., OYAMA V. I. Protein turnover in mammaliar cell cultures. J Biol Chem. 1959 Mar;234(3):592–597. [PubMed] [Google Scholar]
- HARRIS H., WATTS J. W. Turnover of protein in a non-multiplying animal cell. Nature. 1958 Jun 7;181(4623):1582–1584. doi: 10.1038/1811582b0. [DOI] [PubMed] [Google Scholar]
- Hendler R. W. Importance of membranes in protein biosynthesis. Nature. 1965 Sep 4;207(5001):1053–passim. doi: 10.1038/2071053a0. [DOI] [PubMed] [Google Scholar]
- KROON A. M. PROTEIN SYNTHESIS IN MITOCHONDRIA. II. A COMPARISON OF MITOCHONDRIA FROM LIVER AND HEART WITH SPECIAL REFERENCE TO THE ROLE OF OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1964 Sep 11;91:145–154. [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- NEWEY H., SMYTH D. H. EFFECTS OF SUGARS ON INTESTINAL TRANSFER OF AMINO-ACIDS. Nature. 1964 Apr 25;202:400–401. doi: 10.1038/202400b0. [DOI] [PubMed] [Google Scholar]
- RIGGS T. R., WALKER L. M., CHRISTENSEN H. N. Potassium migration and amino acid transport. J Biol Chem. 1958 Dec;233(6):1479–1484. [PubMed] [Google Scholar]
- Rapoport S., Ababei L., Wagenknecht C., Sarkar S. R. Enzyme regulatory mechanisms at the level of lactate-oxidoreductase in erythrocytes and ascites tumour cells. Nature. 1965 Oct 9;208(5006):185–187. doi: 10.1038/208185a0. [DOI] [PubMed] [Google Scholar]
- VIDAVER G. A. SOME TESTS OF THE HYPOTHESIS THAT THE SODIUM-ION GRADIENT FURNISHES THE ENERGY FOR GLYCINE-ACTIVE TRANSPORT BY PIGEON RED CELLS. Biochemistry. 1964 Jun;3:803–808. doi: 10.1021/bi00894a013. [DOI] [PubMed] [Google Scholar]


