Abstract
Objectives:
This prospective, randomized trial compared the safety and effectiveness of laparoscopic Roux-en-Y gastric bypass (LRYGBP) and laparoscopic mini-gastric bypass (LMGBP) in the treatment of morbid obesity.
Summary Background Data:
LRYGBP has been the gold standard for the treatment of morbid obesity. While LMGBP has been reported to be a simple and effective treatment, data from a randomized trial are lacking.
Methods:
Eighty patients who met the NIH criteria were recruited and randomized to receive either LRYGBP (n = 40) or LMGBP (n = 40). The minimum postoperative follow-up was 2 years (mean, 31.3 months). Perioperative data were assessed. Late complication, excess weight loss, BMI, quality of life, and comorbidities were determined. Changes in quality of life were assessed using the Gastro-Intestinal Quality of Life Index (GIQLI).
Results:
There was one conversion (2.5%) in the LRYGBP group. Operation time was shorter in LMGBP group (205 versus 148, P < 0.05). There was no mortality in each group. The operative morbidity rate was higher in the LRYGBP group (20% versus 7.5%, P < 0.05). The late complications rate was the same in the 2 groups (7.5%) with no reoperation. The percentage of excess weight loss was 58.7% and 60.0% at 1 and 2 years, respectively, in the LPYGBP group, and 64.9% and 64.4% in the LMGBP group. The residual excess weight <50% at 2 years postoperatively was achieved in 75% of patients in the LRYGBP group and 95% in the LMGBP group (P < 0.05). A significant improvement of obesity-related clinical parameters and complete resolution of metabolic syndrome in both groups were noted. Both gastrointestinal quality of life increased significantly without any significant difference between the groups.
Conclusion:
Both LRYGBP and LMGBP are effective for morbid obesity with similar results for resolution of metabolic syndrome and improvement of quality of life. LMGBP is a simpler and safer procedure that has no disadvantage compared with LRYGBP at 2 years of follow-up.
This randomized prospective study evaluated the surgical morbidity and results 2 years after laparoscopic Roux-en-Y gastric bypass (LRYGBP) and laparoscopic mini-gastric bypass (LMGBP) to treat morbid obesity. LMGBP was simpler and safer with lower surgical morbidity than LRYGBP (7.5% versus 20.0%, P < 0.05). Both procedures were effective in weight reduction and resolution of metabolic syndrome in patients with morbid obesity and provided similar quality of life.
Obesity is a pan-endemic health problem in both developed and developing countries. Obesity, and in particular morbid obesity (defined as a body mass index [BMI] > 40 kg/m2), leads to a high incidence of complications and a decrease in life expectancy, especially among younger adults.1,2 The results of medical treatment of obesity have been disappointing. According to the National Institutes of Health Consensus Conference in 1991, surgery, specifically open Roux-en-Y gastric bypass (RYGBP) and vertical banded gastroplasty (VBG), is the only recommended effective treatment of morbid obesity.3 Traditional RYGBP has been shown to be effective in achieving significant and durable long-term weight loss as well as improving medical comorbidities in morbidly obese patients.4–6 With advances in minimally invasive technology, laparoscopic Roux-en-Y bypass (LRYGBP) has been reported as a safe alternative to open RYGBP.7–10 However, it is a technically challenging procedure. The learning curve is very steep and associated with longer operating times and higher perioperative complication rates on the upward portion of the curve.11,12 Laparoscopic mini-gastric bypass (LMGBP), first reported by Rutledge, was proposed as a simple and effective treatment of morbid obesity.13 However, controversies about the relative safety of this procedure remain, mainly the incidence of marginal ulcer and reflux esophagitis.14 This randomized trial compared the operative morbidity and the results at the 2-year follow up after LRYGBP or LMGBP in patients with morbid obesity.
METHODS
Study Design and Participants
The study was conducted in the Department of Surgery of the En-Chu-Kong Hospital of National Taiwan University from October 2001 to March 2002. Prior approval for performance of the study was obtained from the ethics committee of the hospital. All patients were evaluated for surgical treatment of morbid obesity by a multidisciplinary and integrated medical unit, with the aid of a general physician, endocrinologist, psychiatrist, and dietician. A thorough assessment was performed of each patient's general condition and mental status, complications of obesity, risk factors, and motivations for surgery. Written informed consent was obtained from all patients who agreed to participate in the trial.
The inclusion criteria were: a history of obesity of >5 years’ duration; BMI > 40 kg/m2 or BMI > 35 kg/m2 with comorbidities; documented weight loss attempts in the past; and good motivation for surgery.3 The age was restricted to patients from 18 to 59 years of age. Exclusion criteria were previous obesity surgery, previous gastric surgery, large abdominal ventral hernia, pregnancy, psychiatric illness, or BMI > 60 kg/m2. After obtaining informed consent, patients were randomly assigned to LRYGBP (n = 40) or LMGBP (n = 40) by the use of sealed envelopes.
Interventions
Laparoscopic Roux-en-Y Gastric Bypass
The technique used for LRYGBP was a 5-port technique similar to that described by Shauer et al.9 The dissection began directly on the lesser curvature of the stomach, and a 15- to 20-mL gastric pouch was created using multiple Endo GIA 45 staplers (Tyco, United States Surgical Corporation, Norwalk, CT). Patients were then placed in the neutral position for creation of the jejunostomy. The jejunum was divided 50 cm distal to the ligament of Treitz. A stapled end-to-side jejunostomy anastomosis was performed with a 100-cm Roux limb for patients with BMI < 49 kg/m2, and a 150-cm Roux limb for patients with BMI > 50 kg/m2. The remaining enteroenterostomy defect was closed with continuous suture. All mesentery defects were closed with sutures. The Roux limb was tunneled via a retrocolic, retrogastric path and positioned near the transected gastric pouch. The CEEA-21 anvil (Tyco, United States Surgical Corporation) was pulled into the gastric pouch transorally following the technique described by Wittgrove and Clark.7 The CEEA stapler was then inserted through the Roux limb to perform the end-to-side gastroenterostomy (Fig. 1). After testing for air leak, the trocar fascial defects were closed. One hemovac drain was left in the lesser sac and a second hemovac drain was left in the subphrenic site.
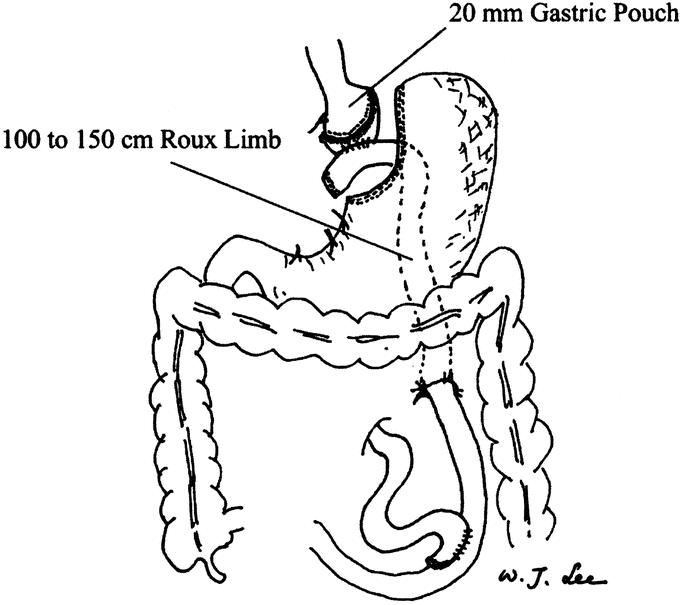
FIGURE 1. View of completed retrocolic, retrogastric laparoscopic Roux-en-Y gastric bypass. The gastric pouch is estimated to be 20 mL in volume. The Roux limb is 100 to 150 cm in length and is retrocolic and retrogastric in position. The mesentery defect is closed with interrupted sutures.
Laparoscopic Mini-Gastric Bypass
The technique used for LMGBP was a 5-port technique similar to that described by Rutledge.13 A long gastric tube was created using an EndoGIA stapler (Tyco, United States Surgical Corporation) approximately 1.5 cm to the left of the lesser curvature from the antrum to the angle of His. A loop gastroenterostomy was created with the small bowel about 200 cm distal to the ligament of Trietz with an Endo-GIA stapler. The gastroenterostomy was then closed with continuous suture (Fig. 2). One hemovac drain was left in the lesser sac before closure of the wound.
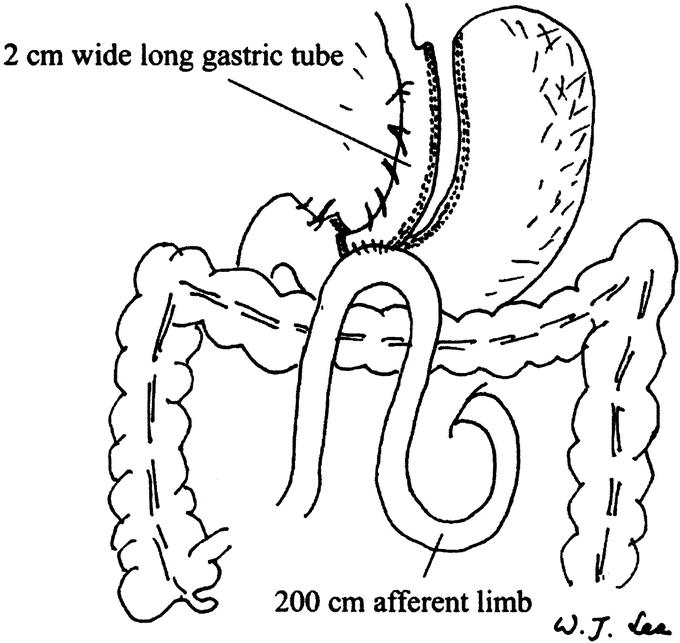
FIGURE 2. View of completed laparoscopic mini-gastric bypass. The narrow gastric tube roughly the diameter of esophagus (approximate 1.5 cm wide) is created parallel to the lesser curvature and up to the angle of His. Intraoperative endoscopy is used as a stent during the division of the stomach and assists in the anastomosis. The antecolic gastroenterostomy is created at the small bowel 200 cm distal to the Trietz ligament.
Postoperative Care
All of the patients received care under a standard clinical pathway. The nasogastric tube was removed on the first postoperative day in both groups, and patients were encouraged to ambulate as soon as they felt comfortable. Oral feeding was allowed starting on the third postoperative day provided the patient had flatus passage and a normal gastrografin contrast study. Patients were discharged on the fourth postoperative day if they felt able to return home, and the hemovac drains were removed at the outpatient clinic after the eighth postoperative day. Postoperatively, patients were followed up by the aforementioned multidisciplinary team, and outpatient clinic visits were scheduled once a month for the first 3 postoperative months and every 3 months thereafter. Patients were advised to take a daily multivitamin tablet as a supplement. Iron supplement, vitamin B12 injection, and blood transfusion were given only in symptomatic patients. Radiology or endoscopy examination was scheduled if clinically indicated.
A complication was defined as the occurrence of an unexpected medical event that made departure from clinical pathway necessary. An operative morbidity or early complication was defined as a complication that occurred within 30 days postoperatively. A major complication was defined as a complication that required interventional management and hospitalization for more than 14 days. Complications related to the operation that occurred more than 30 days postoperatively and required readmission were defined as late complications.
Baseline and Perioperative Measures
Demographic and laboratory data were collected at the preoperative evaluation. Metabolic syndrome was defined according to the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, ATPIII) definition.15 Briefly, metabolic syndrome was defined as having 3 or more of the following abnormalities: waist circumference >102 cm in men and 88 cm in women; serum triglyceride level of ≥150 mg/dL; high-density lipoprotein cholesterol (HDL-C) level of <40 mg/dL in men and 50 mg/dL in women; blood pressure ≥130/85 mm Hg; or serum glucose ≥110 mg/dL. Perioperative outcome measurement including operative time, blood loss, postoperative analgesic dosage requirement, hospital stay, and complications were recorded.
Follow-up Measures
Data on body weight and BMI as well as excess body weight reduction percentage were collected at 1, 3, 6, 12, and 24 months postoperatively. Blood chemistry and complete blood cell count were assessed at each follow-up visit.
Quality of Life Measures
Quality of life was assessed before and 1 year after operation using the Gastrointestinal Quality of Life Index (GIQLI), a 36-item questionnaire.16 The GIQLI is a well-validated tool to assess specific quality of life in patients with various gastrointestinal diseases.17–20 The questionnaire measures the following 4 domains: gastrointestinal symptoms (19 questions), physical function (7 questions), social function (5 questions), and emotional function (5 questions). The response to each question is scored from 0 to 4 (0 being the worst and 4 the best option). The maximum score is 144.
Statistical Analysis
The statistical software SPSS version 8.0 for Windows (SPSS, Chicago, IL) was used in the analysis. Prior to the study, we calculated that with a study group of 80 patients, the power to detect a mean operative difference of 0.6 SD (2-sided α = 0.05) would be 95%. The means of all continuous variables were compared using appropriate parametric or nonparametric tests. Categorical variables and proportions were compared using the χ2 test or the Fisher exact test. A value of P < 0.05 was considered statistically significant. All data are reported as percentage of patients, mean ± SD.
RESULTS
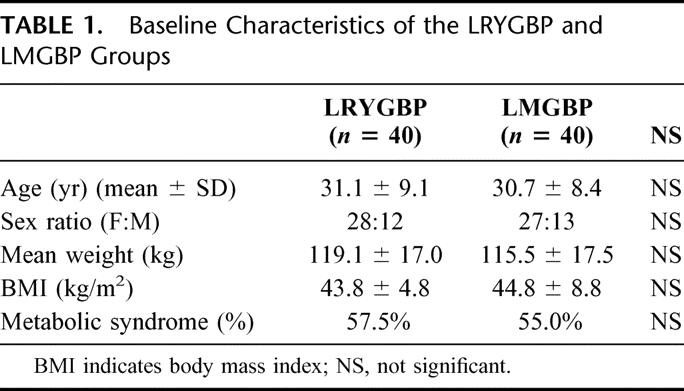
Between February 2001 and March 2003, 80 patients were randomized to either LRYGBP (n = 40) or LMGBP (n = 40). The 2 groups were comparable in sex, age, mean weight, BMI, and percentage of patients with metabolic syndrome (Table 1). Metabolic syndrome as defined by the ATPIII criteria affected 56% of the morbidly obese patients in this trial.
TABLE 1. Baseline Characteristics of the LRYGBP and LMGBP Groups
Operation
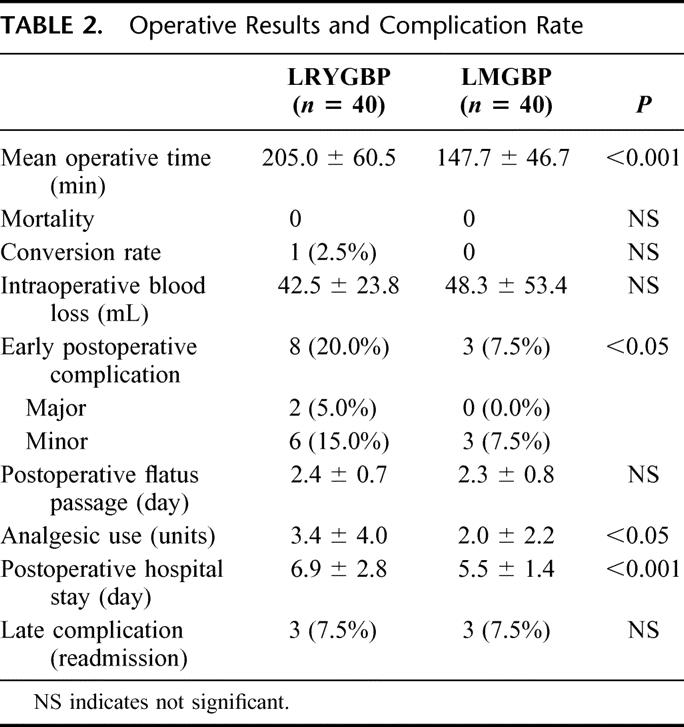
LMGBP was successfully completed in 100% of patients. One patient in the LRYGBP group required conversion to open surgery because of technique difficulty due to hypertrophy of the left hepatic lobe. This resulted in an overall successful completion rate of 97.5% for LRYGBP (Table 2). The mean duration of surgery was longer for LRYGBP than for LMGBP (205 versus 148 minutes; P < 0.05). The estimated blood loss was similar in the 2 groups. None of the patients died. Although there were a similar number of days until postoperative flatus passage in the 2 groups, the LRYGBP group had a longer hospital stay (6.9 versus 5.5; P < 0.05) and required a larger cumulative dose of analgesic medication (3.4 versus 2.0 doses; P < 0.05).
TABLE 2. Operative Results and Complication Rate
Operative Morbidities
The early postoperative complication rate was significantly higher in the LRYGBP group (20% versus 7.5%; P < 0.05). Two of the 8 complications in the LRYGBP group were major, and both were related to anastomotic leakage (5%). One of these patients required laparoscopic reoperation and the other required percutaneous drainage of the intra-abdominal abscess with total parental nutrition support. Six patients in the LRYGBP group developed minor complications, including upper gastrointestinal bleeding, ileus, and leakage from the drainage tube. All of them recovered after conservative treatment. There were no major but 3 (7.5%) minor complications in the LMGBP group. Wound infection related to minimal leakage occurred in 1 patient and upper gastrointestinal bleeding in 1 patient. Both of these patients recovered after conservative treatment. One patient had iatrogenic sutured nasogastric tube during the closure of gastroenterostomy and had the tube removed by gastroendoscopy 2 weeks postoperatively.
Follow-up
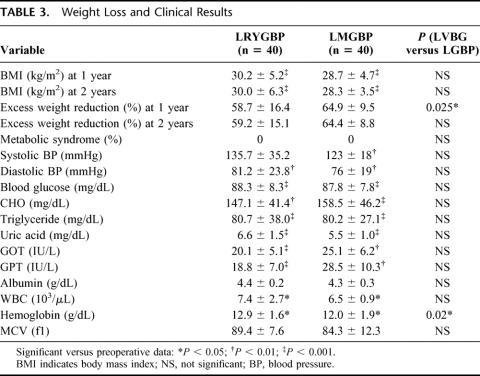
All patients had follow-up duration of at least 2 years during the study. The follow-up rate was 100% in both groups. Both groups had a significant reduction of BMI and percentage of excess weight after surgery with a significant improvement in obesity-related comorbidities including blood pressure, hyperglycemia, blood lipid, uric acid, and liver function. There was no difference in the clinical data after surgery between the 2 groups except for a significantly lower hemoglobin level and better loss of excess weight at 1 year in the LMGBP group (Table 3). The resolution rate of ATPIII defined metabolic syndrome was 100% in both groups. Late complications occurred in 3 patients (7.5%) in each group. In the LRYGBP group, ulcer bleeding developed in 1 patient, ileus in 1 patient, and pyothorax in 1 patient. In the LMGBP group, ulcer bleeding developed in 2 patients and ileus in 1 patient. All of the patients with late complications responded to medical treatment and none required surgery.
TABLE 3. Weight Loss and Clinical Results
Excess weight loss was significantly greater in the LMGBP group than the LRYGBP group at 1 year (64.9% versus 58.7%, P < 0.05) but was not significantly different at 2 years (64.4% versus 60.0%, P = 0.154). According to Reinhold's classification,21 a residual excess weight <50% was achieved at 2 years in 75% of patients in the LRYGBP group and 95% of patients in the LMGBP group (P < 0.05). One 40-year-old woman in the LRYGBP group with a preoperative BMI of 47.7 was scheduled for laparoscopic extralong limb revision surgery because of inadequate weight loss of 35.6% at 1 year and 29.9% at 2 years. She had received a 100-cm-long Roux limb, which was mark dilated on follow-up gastrointestinal barium study at 2 years postoperatively (Fig. 3).
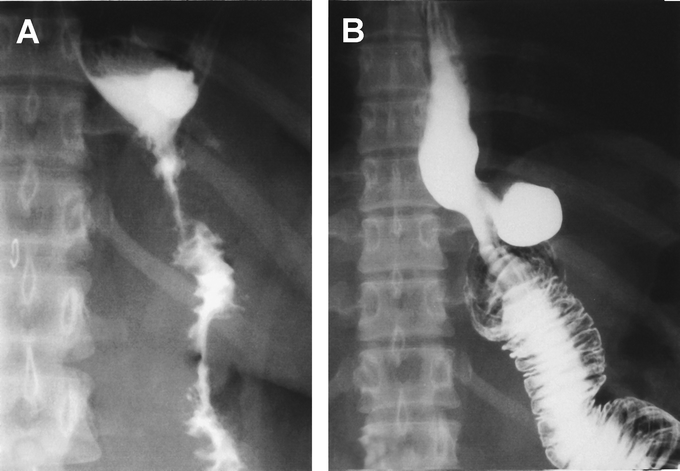
FIGURE 3. Upper gastrointestinal study immediately after LRYGBP (A) and 2 years later (B). A mark adaptation of R-Y limb was observed that resulted in inadequate weight loss in this patient.
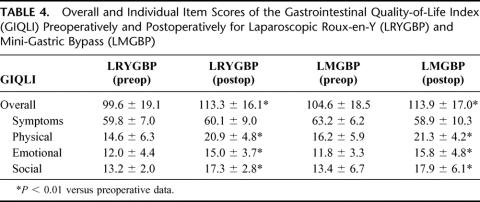
Quality of Life Assessment
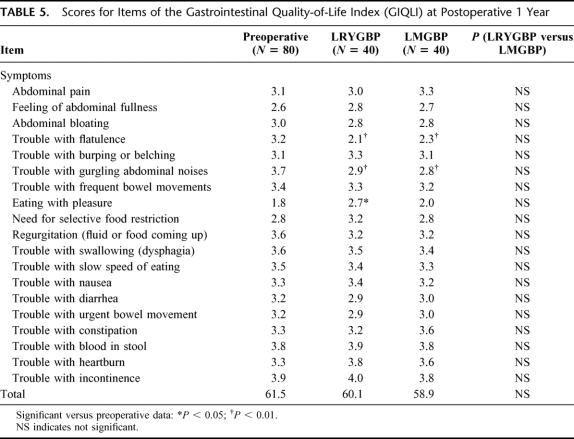
Preoperative GIQLI scores were similar in the 2 groups. The mean GIQLI score at 1 year after surgery was significantly higher than the preoperative score in both groups. Significantly higher subtotals were found in both groups in the domains of general life, including the physical, social, and emotional function (Table 4). For specific gastrointestinal symptoms, there was a slightly decreased overall score after surgery in both groups. LRYGBP or LMGBP or gastric bypass performed by other procedures may cause some specific gastrointestinal symptoms. In this study, both groups had flatulence and gurgling abdominal noises, but these patients also reported greater enjoyment of eating and relief of symptoms of acid regurgitation (Table 5). There was no significant difference in gastrointestinal symptoms before or after the procedure in the 2 groups.
TABLE 4. Overall and Individual Item Scores of the Gastrointestinal Quality-of-Life Index (GIQLI) Preoperatively and Postoperatively for Laparoscopic Roux-en-Y (LRYGBP) and Mini-Gastric Bypass (LMGBP)
TABLE 5. Scores for Items of the Gastrointestinal Quality-of-Life Index (GIQLI) at Postoperative 1 Year
DISCUSSION
Currently, VBG and Roux-en-Y gastric bypass are the only 2 bariatric operations approved by the NIH,3 although a growing number of adjustable gastric banding operations have been reported. Because gastric bypass has been demonstrated to result in better weight reduction than VBG in several randomized trials, RYGBP has been considered the gold standard of bariatric operations in the United States.22–25 According to a 1999 survey, RYGBP was performed in 70% of bariatric procedures.26 LRYGBP, however, has been rapidly gaining acceptance as a preferred bariatric procedure recently. Although perioperative complications appear to decrease with experience, the incidence of complication with this technically demanding operation remains high. The reported conversion rate of LRYGBP varied from 0.8% to 11.8%, the major complication rate from 3.3% to 15%, and the late complication rate from 2.2% to 27%.7–11 Leakage is the most frequent complication and ranged from 1.5% to 5.8%. The conversion rate of LRYGBP in this trial was 2.5% and the major complication rate was 5%, which is compatible with the findings of previous studies.22–25
The technical difficulty of LRYGBP is mainly related to the high anastomosis close to esophagogastric junction. Early in a surgeon's experience, a retro-colic Roux-en-Y limb is recommended to avoid tension on the mesentery. However, this approach poses high technical difficulty. Some surgeons are now performing an ante-colic Roux-en-Y limb, but usually with bivalving of the omentum to reduce tension on the mesentery. However, our trial predated these developments. Theoretically, LMGBP has the advantage of using a lower ante-colic gastrointestinal anastomosis, which is much easier to perform than the high retro-colic or ante-colic gastrointestinal anastomosis used in LRYGBP. Other advantages of the LMGBP include the requirement of using one less anastomosis and providing a better blood supply to the gastric tube, which may decrease the incidence of leakage. In this trial, LMGBP was demonstrated to be a simpler and safer procedure than LRYGBP. The operative time for LMGBP was 27.8% shorter than that for LRYGBP. The hospital stay and postoperative pain were also significantly less in LMGBP than in LRYGBP. Although the numbers of ports are similar, LRYGBP is more complex and more time-consuming than LMGBP due to need for a wider dissection area and more anastomosis required.
In this study, both groups were treated postoperatively under a standard clinical pathway. While the surgeons knew the arm in which the patient was enrolled, all the postoperation care was standardized and the patients were discharged based on the same criteria. This may have helped to limit any potential difference in discharge time, which may have arisen due to bias from surgeons participating in the study. In this study, patients with a BMI over 60 were excluded due to potential technical difficulty. Some studies have reported a higher complication rate for those patients with a BMI over 60 who underwent LRYGBP.7–11,27 Because the main study goal of this trial was to compare LRYGBP and LMGBP, we excluded patients with BMI over 60 to avoid the possible hazard and confounding factor from the technique difficulty when treating extremely obese patients.
There were no major complications resulting from LMGBP in this trial in contrast to a 5% complication rate for LRYGBP. The 7.5% minor complication rate for LMGBP was also lower than the 15% for LRYGBP. Although some authors have argued that the major complication rate of LRYGBP may be decreased to less than 1% in some excellent centers once the learning curve is over, the learning curve of LRYGBP is typically very steep.7–11,27 The learning curve of LRYGBP was previously estimated to be from 75 to 400 cases. During the first 100 cases of LRYGBP, a 3% to 5% major complication rate has been regarded as successful.7–12,27,28 In our experience, the learning curve for LMGBP is around 30 cases shorter than that for LRYGBP. Analysis of our accumulated data from performing more than 400 cases of LMGBP procedures revealed that the major complication rate was 1%. The most common cause of major complications in LMGBP is bleeding. Because the blood supply is profound in the gastric tube, gastroenterostomy staple line arterial bleeding may sometimes require reoperation. Therefore, it is recommended to carefully check the staple line for any bleeding before closure of the gastroenterostomy. Rutledge has also reported routine reinforcement of the staple line with continuous seromuscular suture.13 Leakage from LMGBP was very rare in our series and in a series of 1272 cases reported by Rutledge.13
Whether increased bile acid in the stomach might lead to chronic gastritis and possible carcinogenic effects after LMGBP has been controversial.14 A high incidence of annoying bilious vomiting and gastritis as high as 70% after Mason's old loop gastric bypass has been reported.29,30 The old loop GBP with its high transverse tiny pouch and loop adjacent to the esophagus was especially prone to these complications. The high incidence of symptoms related to alkaline reflux esophagitis has resulted in increased use of Roux-en-Y reconstruction in the performance of gastric bypass. However, bile reflux is rarely a problem when the anastomosis is placed low in the stomach as in LMGBP or BII gastrectomy. Although we didn't perform routine endoscopic examination, a previous study has found gastritis in 86% of patients who received loop gastric bypass and in 63% of patients who received RYGBP.31 The clinical importance of this gastritis is not clear because of the lack of follow-up data. In this study, we choose to evaluate the effects of the procedures on quality of life instead of using routine endoscopic examination to detect the possible adverse effects of LMGBP. The quality of life survey is patient oriented and with good patient's compliance. Our previous study found a significantly impaired gastrointestinal quality of life after VBG using the specific GIQLI scale.20 In this study, both procedures significantly improve the total GIQLI score to a similar extent. The GIQLI detected no difference in quality of life between LRYGBP and LMGBP. Although both procedures may result in the development of some specific gastrointestinal symptoms, the total score in the domain of symptoms remained stable. These findings suggested that LMGBP, which is similar to a lesser-curve tubed Billroth II gastrectomy, is not likely to result in the adverse effect of bile esophagitis or increased risk of gastric cancer,32–34 although further follow-up of patients is needed to test this hypothesis.
Another possible adverse effect of LMGBP is the development of marginal ulcer. In this trial, the incidence of marginal ulcer was 5% in the LMGBP group and 3% in the LRYGBP group. Marginal ulcer is usually transient and well controlled by the treatment with proton pump inhibitors. No specific ulcer-related symptoms were detected in the GIQLI results of this study. To avoid the development of marginal ulcer, it is mandatory to keep the gastric tube narrow during the performance of LMGBP. The development of marginal ulcer is usually related to the volume of the gastric tube and the usage of ulcerogenic drugs.
In terms of weight loss, LMGBP provided a small but significant advantage to LRYGBP in this study. This was attributed to the longer bypass foregut limb used in LMGBP compared with LRYGBP. In LMGBP, the intestinal loop is routinely bypassed at 200 cm. However, in LRYGBP, the length of the bypass limb in previous studies varied from 150 to 200 cm (50 cm afferent limb plus 100–150 RY limb) according to the BMI of the patient.8,9 This difference explains the lower hemoglobin level found in patients in the LMGBP group in comparison with the LRYGBP group in this study. Increased bypass limb of small intestine will increase weight loss but will also increase the incidence of late nutritional deficiencies, including iron deficiency anemia, vitamin B deficiency, folate deficiency, and other micronutrient deficiencies.4–6 In this study, the postoperative hemoglobin level was significantly lower than the preoperative level in both bypass procedures, but this difference was more severe in the LMGBP group. Most cases of anemia that develop as a result of gastric bypass can be resolved by adequate iron and vitamin supplementation. Blood transfusion is rarely indicated. However, determination of the impact of long-term squeal of micronutrient deficiencies, such as bone disease, requires further long-term follow-up. LRYGBP is very effective in weight reduction and resolution of the metabolic syndrome for morbidly obese patients. Tailoring of the bypass limb in LMGBP according to the BMI may allow the need for weight reduction to be balanced against the need to minimize the risk of resulting micronutrient deficiencies. The results suggest that use a bypass limb of 150 cm in those with BMI below 40, with a 10-cm increase in the bypass limb with the every BMI category related to obesity instead of using a fixed 200-cm limb for all patients may provide better results.
In this trial, we evaluated the cluster of metabolic abnormalities known as metabolic syndrome in morbidly obese patients rather than evaluating individual clinical items. The recently released ATPIII has drawn attention to the importance of metabolic syndrome and provides a working definition of this syndrome for the first time.15 Individuals with metabolic syndrome, a clustering of risk factors [triglycerides, glucose, high-density lipoprotein cholesterol, blood pressure (BP), abdominal obesity] are at high risk of coronary heart disease and type 2 diabetes mellitus.35 Recently, Isomaa et al showed that the mortality for cardiovascular pathologies increases by about 3-fold in subjects with metabolic syndrome compared with those without metabolic syndrome.36 In addition, the cluster of risk factors defining metabolic syndrome increased cardiovascular risk more than each single component.37 Thus, identification of these high-risk individuals is crucial to providing appropriate therapy with the available disease-modifying treatments. A more integrated strategy provides better outcomes than management of the individual abnormalities of the metabolic syndrome.38 In this trial, 56% of our patients had metabolic syndrome and 100% were cured at 1 year after gastric bypass. Obesity surgery should therefore be recommended as the definitive treatment of morbidly obese patients with metabolic syndrome. Recent advances in laparoscopic surgery have made laparoscopic bariatric surgery a minimally invasive procedure and have generated renewed interest in obesity surgery. The results of this study indicated that LMGBP has a better safety profile that LRYGBP and thus is the preferred gastric bypass treatment of patients with metabolic syndrome. Current indications for surgery in morbidly obese patients include BMI greater than 40 or greater than 35 if comorbidities are present.3 However, for patients with moderate obesity (BMI between 30 and 35) but complicated with metabolic syndrome, the low risk of laparoscopic gastric bypass surgery suggests that it might be included in the choices of treatments. Further cost-effectiveness study of laparoscopic gastric bypass surgery in the treatment of moderate obesity with metabolic syndrome is needed.
CONCLUSION
This study has demonstrated that both LRYGBP and LMGBP are effective treatments for morbid obesity. Both procedures can significantly resolve obesity-related metabolic complications and increase quality of life for morbidly obese patients. LMGBP was shown to be a simpler and safer procedure than LRYGBP with similar efficacy at the 1- and 2-year follow-up. LMGBP is thus an acceptable alternative treatment to standard LRYGBP for morbidly obese patients.
Footnotes
Reprints: Wei-Jei Lee, MD, PhD, Department of Surgery, En-Chu Kong Hospital, No 399, Fuhsing Road, San-shia Town, Taipei Hsien 237, Taiwan. E-mail: wjlee@km.eck.org.tw.
REFERENCES
- 1.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. [DOI] [PubMed] [Google Scholar]
- 2.Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289:187–193. [DOI] [PubMed] [Google Scholar]
- 3.NIH Conference: Gastrointestinal surgery for severe obesity. Ann Intern Med. 1991;115:959–961. [PubMed] [Google Scholar]
- 4.Brolin RE, Kenler HA, Gorman JH, et al. Long-limb gastric bypass in the superobese: a prospective randomized study. Ann Surg. 1992;215:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pories WJ, Swanson MS, MacDonld KG, et al. Who whould have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLean LD, Rhode BM, Nohr CW. Late outcome of isolated gastric bypass. Ann Surg. 2000;231:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittgrove A, Clark G. Laparoscopic gastric bypass, Roux-en-Y: 500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000;10:233–239. [DOI] [PubMed] [Google Scholar]
- 8.Higa K, Boone K, Ho T, et al. Laparoscopic Roux-en-Y gastric bypass for morbid obesity. Arch Surg. 2000;135:1029–1034. [DOI] [PubMed] [Google Scholar]
- 9.Schauer P, Ikranuddin S, Gourash W, et al. Outcomes after laparoscopic gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMaria EJ, Sugerman HJ, Kellum JM, et al. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westling A, Gustavsson S. Laparoscopic vs open Roux-en-Y gastric bypass: a prospective, randomized trial. Obes Surg. 2001;11:284–292. [DOI] [PubMed] [Google Scholar]
- 12.Reddy RM, Riker A, Marra D, et al. Open Roux-en-Y gastric bypass for the morbidly obese in the era of laparoscopy. Am J Surg. 2002;184:611–616. [DOI] [PubMed] [Google Scholar]
- 13.Rutledge R. The mini-gastric bypass: experience with the first 1272 cases. Obes Surg. 2001;11:276–280. [DOI] [PubMed] [Google Scholar]
- 14.Fisher BL, Buchwald H, Clark W, et al. Mini-gastric bypass controversy [Letter]. Obes Surg. 2001;11:773–777. [DOI] [PubMed] [Google Scholar]
- 15.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NECP) Expert Panel of Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 16.Espasch E, Williams JL, Wood-Dauphinee S, et al. Gastrointestinal quality of life index: development validation and application of new instrument. Br J Surg. 1995;82:216–222. [DOI] [PubMed] [Google Scholar]
- 17.Jentschura D, Winkler M, Strohmeier N, et al. Quality of life after curative surgery for gastric cancer: a comparison between total gastrectomy and subtotal gastric resection. Hepatogastroenterology. 1997;44:1137–1142. [PubMed] [Google Scholar]
- 18.Slim K, Bousquet J, Kwiatkowski F, et al. Quality of life before and after laparoscopic fundoplication. Am J Surg. 2001;180:41–45. [DOI] [PubMed] [Google Scholar]
- 19.Decker G, Borie F, Bouamrirene D, et al. Gastrointestinal quality of life before and after laparoscopic Heller myotomy with partial posterior fundoplication. Ann Surg. 2002;236:750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee WJ, Yu PJ, Wang W, et al. Gastrointestinal quality of life following laparoscopic vertical banded gastroplasty. Obes Surg. 2002;12:819–824. [DOI] [PubMed] [Google Scholar]
- 21.Reinhold RB. Critical analysis of long-term weight loss following gastric bypass. Surg Gynecol Obestet. 1982;155:385–394. [PubMed] [Google Scholar]
- 22.Freeman JB, Burchett HJ. A comparison of gastric bypass and gastroplasty for morbid obesity. Surgery. 1980;88:433–444. [PubMed] [Google Scholar]
- 23.Laws HL, Piantadosi S. Superior gastric reduction procedure for morbid obesity: a prospective, randomized prospective, randomized trial. Ann Surg. 1981;193:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechner GW, Callender AK. Subtotal gastric exclusion and gastric partitioning: a randomized prospective comparison of one hundred patients. Surgery. 1981;90:637–644. [PubMed] [Google Scholar]
- 25.Naslund I, Wickbom G, Christoffersson E, et al. A prospective randomized comparison of gastric bypass and gastroplasty: complications and early results. Acta Chir Scand. 1986;152:681–689. [PubMed] [Google Scholar]
- 26.American Society for Bariatric Surgery. Membership Survey. Gainesville, FL: American Society for Bariatric Surgery, 1999. [Google Scholar]
- 27.Dresel A, Kuhn JA, Westmoreland MV, et al. Establishing a laparoscopic gastric bypass program. Am J Surg. 2002;18:617–620. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen N, Goldman C, Rosenquist J, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am. 1967;47:1345–1352. [DOI] [PubMed] [Google Scholar]
- 30.Griffen WO, Young VL, Stevenson CC. A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg. 1977;186:500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy HB, Rucker RD Jr, Chan EK, et al. Gastritis after gastric bypass surgery. Surgery. 1985;98:68–71. [PubMed] [Google Scholar]
- 32.Welvart K, Warnsinck H. The incidence of carcinoma of the gastric remnant. J Surg Oncol. 1982;21:104–106. [DOI] [PubMed] [Google Scholar]
- 33.Schafer LW, Larson DE, Melton LJ III, et al. The risk of gastric carcinoma after surgical treatment for benign ulcer disease: a population-based study in Olmsted County, Minnesota. N Eng J Med. 1983;309:1210–1213. [DOI] [PubMed] [Google Scholar]
- 34.Hasson LE, Nyren O, Hsing AW, et al. The risk of stomach cancer in patients with gastric and duodenal ulcer disease. N Engl J Med. 1996;242–249. [DOI] [PubMed] [Google Scholar]
- 35.Tikkanen MS, Tuomilehto J. Multiple metabolic syndrome(syndrome X). Ann Med. 1992;24:461–489. [DOI] [PubMed] [Google Scholar]
- 36.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. [DOI] [PubMed] [Google Scholar]
- 37.Trevisan M, Liu J, Bahsas FB, et al. Syndrome X and mortality: a population-based study. Am J Epidemiol. 1998;148:958–966. [DOI] [PubMed] [Google Scholar]
- 38.Case CC, Jones PH, Nelson K, et al. Impact of weight loss on the metabolic syndrome. Diabetes Obes Metab. 2002;407–414. [DOI] [PubMed] [Google Scholar]