Abstract
Objective:
To evaluate risk factors for dysplasia and adenocarcinoma development in nondysplastic Barrett mucosa.
Summary Background Data:
The risk for patients with Barrett esophagus to develop esophageal adenocarcinoma is low, and most patients undergoing surveillance will not develop malignancy. Identification of risk factors may allow for more rational surveillance programs in which patients are stratified according to their individual risk of progressing to dysplasia and invasive adenocarcinoma.
Methods:
The development of dysplasia and esophageal adenocarcinoma was studied during long-term endoscopic and histologic surveillance in 140 patients with Barrett esophagus free from dysplasia. Risk factors for progression to dysplasia and adenocarcinoma were evaluated.
Results:
Median follow-up was 5.8 years. Forty-four patients (31.4%) developed low-grade dysplasia and 7 patients (5%) developed high-grade dysplasia or esophageal adenocarcinoma. Dysplasia development was significantly less common after antireflux surgery compared with conventional medical therapy. Low-grade dysplasia (relative risk = 5.5; 95% confidence interval, 1.1–28.6) and long duration of reflux symptoms (relative risk = 1.3; 95% confidence interval, 1.2–1.7) were independently associated with an increased risk of developing high-grade dysplasia or esophageal adenocarcinoma.
Conclusions:
Successful antireflux surgery protects the Barrett mucosa from developing high-grade dysplasia and esophageal adenocarcinoma, possibly by better control of reflux of gastric contents. Low-grade dysplasia is the only clinically useful risk factor that permits stratification of the surveillance intervals according to the risk of the individual patient.
The development of dysplasia was studied during long-term endoscopic surveillance in 140 patients with Barrett esophagus free from dysplasia. Successful antireflux surgery protects the metaplastic mucosa from progression to dysplasia and adenocarcinoma. Low-grade dysplasia is the only available, clinically useful risk factor that permits stratification of the surveillance intervals according to the risk of the individual patient.
Barrett esophagus is a premalignant condition in which there is thought to be a progression from metaplasia through low-grade dysplasia to high-grade dysplasia and subsequently to adenocarcinoma.1,2 Multiple studies have shown that most patients with Barrett esophagus do not progress to cancer.3–5 The estimated annual risk for adenocarcinoma ranges from 0.2% to 2.0% (1 in 441 to 1 in 52 patient-years), a risk that is 30 to 125 times that of an age-matched population.6–8 However, the risk may be overestimated as many of these reports were based on studies with a limited number of patients with Barrett esophagus.
Once symptomatic carcinoma is diagnosed, the prognosis is poor.9 This has led to a recommendation of regular endoscopic and histologic surveillance in patients with Barrett esophagus to detect malignancy at an early, potentially curable stage.10 However, it has been argued that surveillance is relatively ineffective and not very cost-effective5,11 because the risk of developing esophageal adenocarcinoma is low and most patients undergoing surveillance for Barrett esophagus will not progress to cancer. To develop more rational surveillance programs, patients need to be stratified according to their individual risk of developing invasive adenocarcinoma. Therefore, the aim of this study was to identify potential clinical risk factors for the development of dysplasia and adenocarcinoma in a large series of patients undergoing long-term endoscopic surveillance for Barrett esophagus free from dysplasia.
PATIENTS AND METHODS
From our registry of patients prospectively enrolled in a surveillance program for columnar lined esophagus 159 consecutive patients with histologically confirmed intestinal metaplasia and at least 3 surveillance endoscopies were evaluated for the presence of dysplasia. The patients were enrolled in the surveillance program between 1979 and 1999 and endoscopies were performed with an interval of 1 to 2 years.
The presence of dysplasia within the columnar lined esophagus was determined at each endoscopy. In an attempt to study the development of dysplasia in nondysplastic Barrett mucosa, patients with histologic evidence of dysplasia in any of the biopsy specimens of the first 2 endoscopies were excluded from the study. Nineteen such patients were found, resulting in a study population consisting of 140 patients. These patients were considered to be free from dysplasia, and the progression to dysplasia and adenocarcinoma was studied by evaluating the occurrence and the severity of dysplastic changes during the subsequent surveillance endoscopies.
Endoscopy
All patients had a complete systematic endoscopic examination of the duodenum, pylorus, stomach, and the esophagus. A hiatal hernia was diagnosed when the difference between the position of the crural impression and the gastroesophageal junction was 2 cm or more. A columnar-lined esophagus was suspected when the squamocolumnar junction or any part of its circumference extended above the gastric rugal folds. This included an irregular squamocolumnar junction with tongues of columnar mucosa extending into the esophagus. The presence of a columnar-lined esophagus was confirmed on histologic evaluation of the biopsy specimens. The extent of the columnar lined segment was defined as the distance from the gastroesophageal junction to the location of the highest point of the squamocolumnar junction. The histologic type of the metaplastic mucosa and the presence and severity of dysplastic changes were evaluated on multiple biopsies of the columnar-lined segment on each surveillance endoscopy. No specific biopsy protocol was used before 1984 when a policy of 4-quadrant biopsies every 2 cm along the length of the metaplastic mucosa was introduced. Between 1979 and 1984, 4 patients were included in the study.
Histology
The biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and mounted on slides by means of standard technique. Slides were stained with hematoxylin and eosin and analyzed for the histologic type of the epithelium. Intestinal metaplasia was identified by the presence of a columnar epithelium with a villiform surface, mucous glands, and well-defined goblet cells. Dysplasia was classified histologically as low-grade or high-grade according to the guidelines for inflammatory bowel disease described by Riddell et al,12 which has been modified for application to the esophagus.1 Biopsy specimens classified as indefinite for dysplasia were for the purpose of this study considered nondysplastic. Patients with a biopsy specimen positive for dysplasia had the specimens from all the endoscopies reexamined by an independent pathologist experienced in gastrointestinal histopathology (R.W.).
Postoperative pH Monitoring
All patients undergoing antireflux surgery had the effectiveness of the procedure evaluated by postoperative 24-hour pH monitoring. Abnormal esophageal acid exposure was diagnosed when more than 3.4% of the monitored time was spent below pH 4.0.
Statistics
Values are reported as medians and interquartile ranges unless otherwise stated. Proportions between 2 groups were compared using the Fisher exact test. The Mann-Whitney U test was used to compare continuous data between 2 groups. Survival curves were constructed using the Kaplan-Meier method, counting the cases of dysplasia detection as events and the remainder as censored as of the last day of endoscopic surveillance. Survival curves were compared by the log-rank method. Cox regression analyses were performed to estimate the relative risk for dysplasia and adenocarcinoma development. The factors evaluated in the Cox regression analysis were age, gender, body mass index (BMI), history of tobacco use, duration of surveillance, median surveillance interval, number of surveillance endoscopies, length of hiatal hernia, the presence of low-grade dysplasia, length of Barrett esophagus, and previous antireflux surgery.
RESULTS
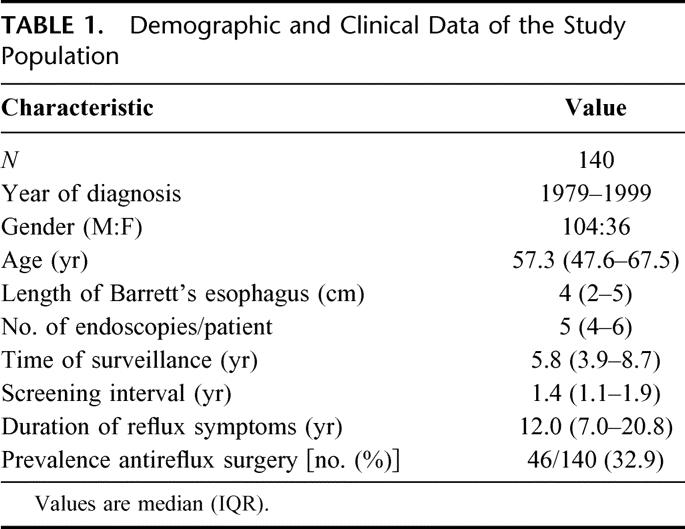
Demographic and clinical data of the study population are shown in Table 1. The patients underwent 770 surveillance endoscopies during a total of 945.6 patient-years. A median of 8 biopsy specimens was obtained per endoscopy. Low-grade dysplasia was detected in 44 (31.4%) of the 140 patients during the course of endoscopic surveillance. The median time from the diagnosis of uncomplicated Barrett esophagus to the finding of low-grade dysplasia was 4.0 years (range, 1.1–14.3 years). In 5 of these patients, low-grade dysplasia progressed to high-grade dysplasia (n = 3) or esophageal adenocarcinoma (n = 2) at a median time of 3.3 years (range, 1.0–5.4 years). One patient developed high-grade dysplasia and 1 patient an adenocarcinoma without a previous diagnosis of low-grade dysplasia after 3.0 and 12.5 years of surveillance, respectively. Thus, 7 patients developed high-grade dysplasia or esophageal adenocarcinoma, resulting in a high-grade dysplasia/adenocarcinoma incidence of 1 in 135.2 patient-years.
TABLE 1. Demographic and Clinical Data of the Study Population
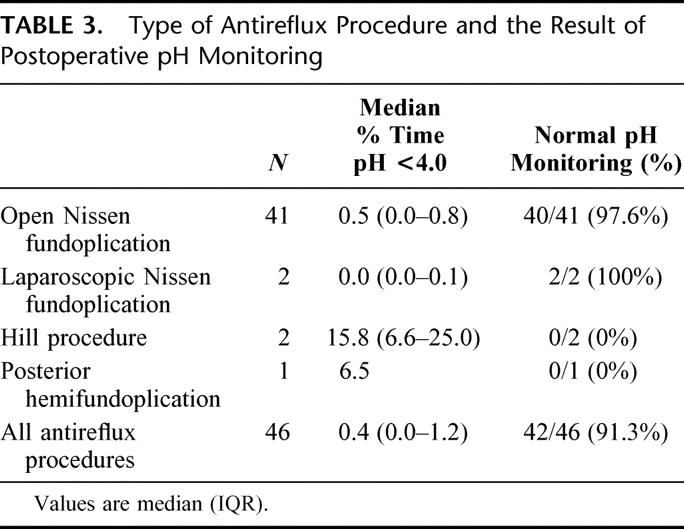
At the time of diagnosis, all patients were treated with acid suppression therapy. Subsequently, 46 of the patients underwent antireflux surgery (41 open and 2 laparoscopic Nissen fundoplications, 2 Hill procedures, and 1 posterior hemifundoplication), while the remaining 94 patients were treated with chronic acid suppression therapy. Demographic and clinical data of these patients are shown in Table 2. In the medically treated group, patients were treated with acid suppression therapy until they were free from reflux symptoms. Nine patients (9.6%) received H2 receptor antagonists and the remaining 85 patients (90.4%) proton pump inhibitors. In the latter group, 51 patients (60%) were treated with 1 daily dose and 34 (40%) with 2 daily doses. The surgically treated patients were significantly younger and had been under endoscopic surveillance for a longer time period compared with the patients receiving medical treatment. All patients who underwent antireflux surgery had the effectiveness of the procedure evaluated by 24-hour esophageal pH-monitoring 6 months postoperatively. The results of pH monitoring after the different types of antireflux procedures are shown in Table 3. The median time spent below pH 4.0 postoperatively was 0.4 and esophageal acid exposure was restored to normal in 42 of the patients (91.2%), while 4 patients continued to have abnormal acid reflux following surgery.
TABLE 2. Demographic and Clinical Data in Patients With Medically and Surgically Treated Reflux Disease
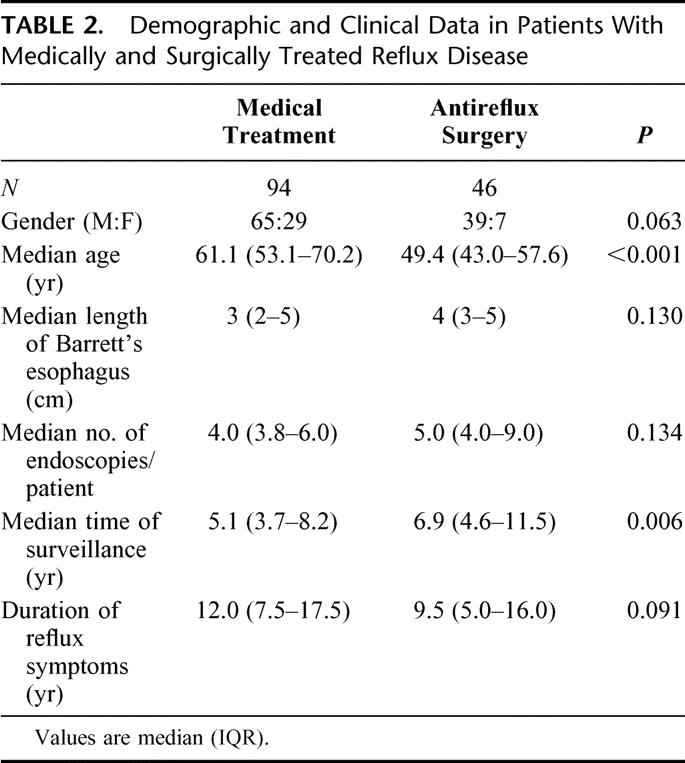
TABLE 3. Type of Antireflux Procedure and the Result of Postoperative pH Monitoring
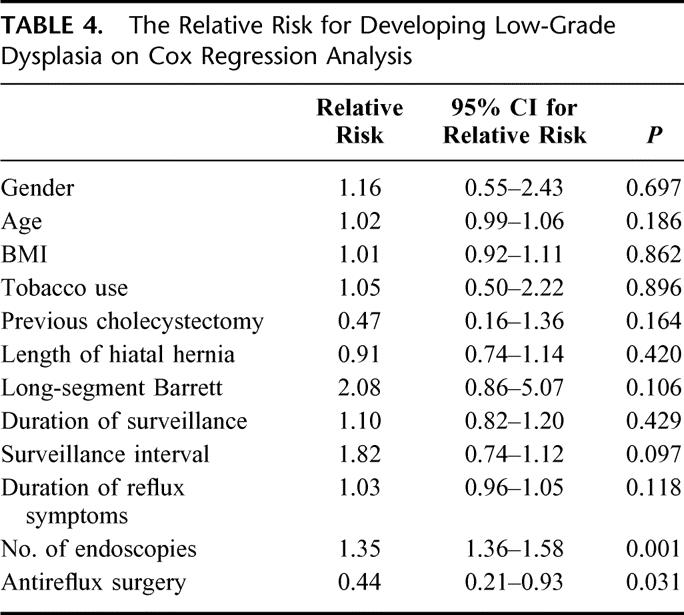
The risk of developing low-grade dysplasia was evaluated using a Cox regression analysis (Table 4). An increasing number of surveillance endoscopies were independently associated with a small but significantly increased risk of developing low-grade dysplasia. A previous antireflux procedure was the only factor associated with a decreased risk of low-grade dysplasia development. The relative risk was 0.44, which corresponds to a risk that is 2.3 times less than that found in patients receiving conventional medical treatment.
TABLE 4. The Relative Risk for Developing Low-Grade Dysplasia on Cox Regression Analysis
All cases of high-grade dysplasia and adenocarcinoma developed during treatment with proton pump inhibitors. The Cox regression analysis showed that 2 factors were independently associated with an increased risk of developing high-grade dysplasia or esophageal adenocarcinoma (Table 5). A long duration of reflux symptoms was associated with a small but statistically significant increased risk, and a previous diagnosis of low-grade dysplasia was associated with a 5 times increased risk of progressing to high-grade dysplasia or invasive cancer. The Cox regression analysis could not be used to evaluate the effects of medical and surgical therapy on high-grade dysplasia/adenocarcinoma development because there were no events in the surgical arm. Therefore, the effects of acid suppression therapy and antireflux surgery were plotted by means of Kaplan-Meier curves and compared using a log-rank analysis (Fig. 1). Patients receiving standard medical therapy developed high-grade dysplasia or adenocarcinoma significantly more often compared with patients treated with antireflux surgery.
TABLE 5. The Relative Risk for Developing High-Grade Dysplasia or Adenocarcinoma on Cox Regression Analysis
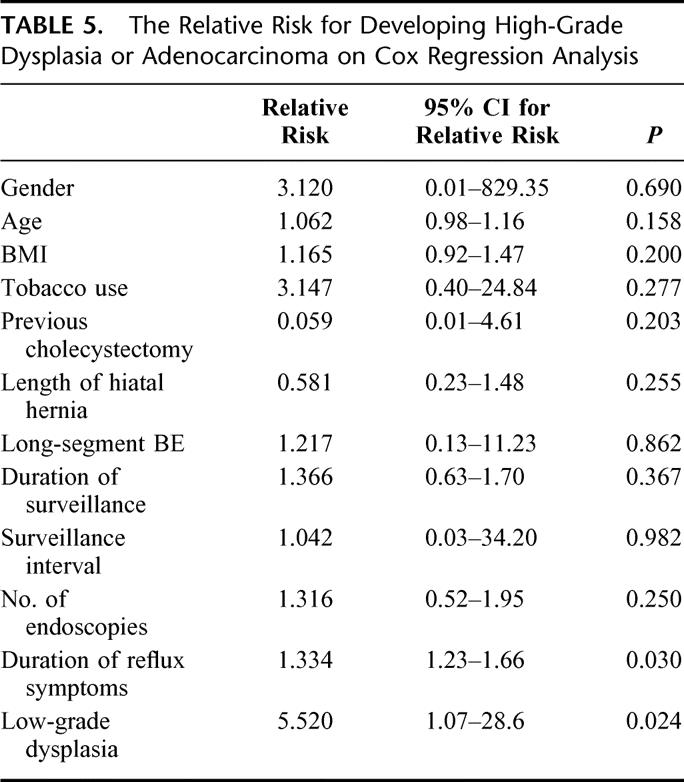
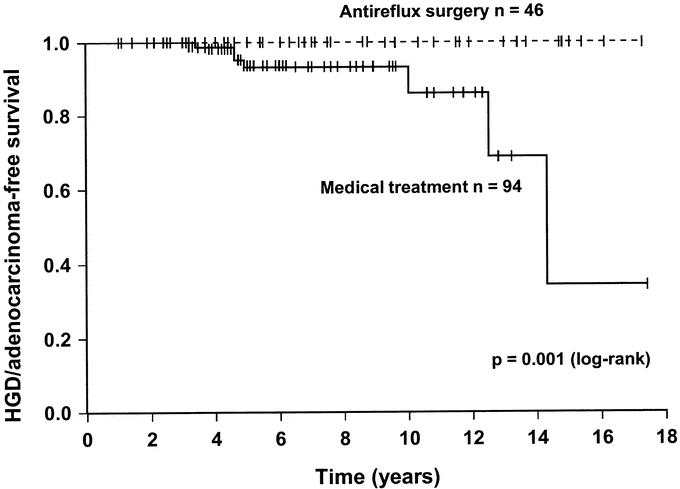
FIGURE 1. Kaplan-Meier curve comparing the high-grade dysplasia or adenocarcinoma-free survival during endoscopic surveillance of patients with medically and surgically treated reflux disease. The patients had no evidence of dysplasia on the first 2 endoscopies.
DISCUSSION
This study evaluated risk factors for the development of dysplasia and adenocarcinoma in a large series of patients undergoing long-term surveillance for Barrett esophagus. In surgically treated patients, the risk of developing low-grade dysplasia was 2.3 times reduced compared with patients receiving conventional acid suppression therapy. High-grade dysplasia or esophageal adenocarcinoma was not found in patients operated with an antireflux procedure but was detected in 7 patients in the medically treated group, a difference that was significantly different on log rank analysis.
The development of dysplasia and adenocarcinoma within nondysplastic Barrett mucosa was studied by excluding patients with histologic evidence of dysplasia in any of the biopsy specimens of the first 2 endoscopies. We recognize the concern of sampling error, ie, dysplasia was present but missed by the biopsies due to its patchy distribution. However, we think that a rigorous and systematic biopsy protocol with multiple biopsies of the Barrett mucosa on 2 subsequent occasions would find most cases of dysplasia. Also, it is unlikely that the difference in the detection of dysplasia in patients with surgically and medically treated reflux disease is explained by sampling error as the same biopsy protocol was applied in both groups.
Observations suggestive of a protective effect of successful antireflux surgery on the development of esophageal adenocarcinoma include the report Katz et al who reported on the dysplasia development in 102 patients with Barrett esophagus.13 Patients with medically treated reflux disease had a significantly higher prevalence of dysplasia compared with the surgically treated patients. As in the present study, none of the patients treated with an antireflux procedure developed high-grade dysplasia or adenocarcinoma. In a comprehensive review, Bammer et al calculated that the cancer risk for patients with Barrett esophagus treated by antireflux surgery (1 in 294.4 patient-years) was lower than the risk for Barrett's patients treated medically (1 in 114.7 patient-years).14 In a recent update of an ongoing prospective randomized study, Parrilla et al compared the results of pharmacologic and surgical treatment in 101 patients with Barrett esophagus.15 Although the development of dysplasia and adenocarcinoma was no different overall, the subgroup of surgical patients with normal postoperative pH studies developed significantly less dysplasia and had no adenocarcinoma. Thus, the results of our study and others suggest that complete elimination of reflux achieved by successful antireflux surgery protects the Barrett mucosa from progressing to dysplasia and adenocarcinoma, but the literature is conflicting with several reports of adenocarcinoma developing in Barrett esophagus following antireflux surgery.15–19 However, in many of these, no efforts were made to exclude dysplasia before surgery or to document the effectiveness of the repair postoperatively. In our study, 3 patients with an antireflux procedure who were excluded from the analysis for having dysplasia at entry of the study subsequently developed high-grade dysplasia or adenocarcinoma. It is thus important that these patients remain in endoscopic surveillance programs after surgery. Whether a competent antireflux repair can indeed reduce the rate of malignant progression in patients with Barrett esophagus is still unclear, and further studies are needed to clarify this issue.
In reports of patients developing adenocarcinoma early after the initiation of medical therapy or antireflux surgery, it is likely that dysplasia was present but undetected at the time of entry to the study or that the Barrett mucosa had already reached a point at which progression to malignancy was inevitable. It is unlikely that genetically normal and nondysplastic Barrett mucosa goes through the required sequence of genetic and morphologic steps to progress to an endoscopically visible adenocarcinoma within a year or 2. Therefore, it is important to allow for time for already genetically triggered and thus inevitable adenocarcinomas to develop before assessing the effect of different treatment modalities on the development of malignancy.
Dysplasia and adenocarcinoma usually develop after the Barrett's mucosa has been present for many years. It is plausible to hypothesize that the pathophysiology that leads to metaplastic columnar mucosa and intestinal metaplasia isthe same as the pathophysiology that leads to dysplasia. This hypothesis is controversial but supported by the results of this study and the report of Lagergren et al, in which patients with a history of longstanding reflux have the highest risk of developing esophageal adenocarcinoma.20 Further, studies have shown a relationship between the severity of reflux symptoms and the risk of developing malignancy.21 The association between the duration and the severity of reflux disease and the development of esophageal adenocarcinoma suggest that genetic changes necessary for the malignant progression may be triggered by long-lasting reflux-related injury to the Barrett mucosa. If this hypothesis holds true, complete control of reflux of acid and duodenal juice may be an important goal when treating patients with Barrett esophagus. Acid suppression therapy decreases pathologic acid reflux, but reflux of duodenal juice, although reduced, exceeds the normal range in a substantial proportion of patients.22,23 Generally, patients with Barrett esophagus have an alteration in pain perception, which allows repeated acid-reflux events and tissue injury that are not perceived by the patient.24 Thus, using symptom relief as the end point of medical therapy for patients with Barrett esophagus is unreliable. Antireflux surgery, however, has been shown to result in a more reliable elimination of reflux of both acid and duodenal contents23 and offers thus a theoretical advantage in the management of these patients. The importance of complete abolishment of reflux in patients with Barrett esophagus has been emphasized by Fitzgerald et al who suggested that the dynamic effects of acid exposure may effect columnar cell proliferation and differentiation.25 Using cultured human Barrett's specimens, they demonstrated a dramatic increase in proliferation when the tissue was exposed to short pulses of acid. Consequently, acid exposure may lead to altered growth properties and may contribute to the process of dysplasia development. In a recent report, Ouatu-Lascar et al confirmed the hypothesis that effective intraesophageal acid suppression favors differentiation and decreases proliferation in biopsy samples from Barrett's mucosa.26
Endoscopic surveillance of patients with Barrett esophagus is recommended to detect malignancy at an early, potentially curable stage.10 However, it has been argued that surveillance is relatively ineffective and not very cost-effective.5,11 This has led to a search for risk factors that allows for better stratification of the patients according to their individual risk of developing adenocarcinoma. In this study, the presence of low-grade dysplasia was associated with a risk that was 5 times higher than that of patients with no diagnosis of low-grade dysplasia. The results further suggest that low-grade dysplasia is the only useful clinical risk factor that indicates a need for shorter surveillance intervals in patients with Barrett esophagus. This is a policy that is practiced at most centers with surveillance programs for Barrett esophagus, and it is supported by the recent recommendations from the American College of Gastroenterology.10 The surveillance interval is a function, not only of the risk of progressing to cancer but of the time period over which cancer develops. The time course for the progression from low-grade dysplasia to high-grade dysplasia or esophageal adenocarcinoma is highly variable.8,27 In this study, 5 patients progressed from low-grade dysplasia to high-grade dysplasia or esophageal adenocarcinoma at a median time of 3.3 years, ranging from 1.2 to 5.4 years. The data on which the recommended surveillance intervals are based are limited. However, it is reasonable to project from available data that biannual endoscopies should be sufficient for patients with low-grade dysplasia. It is clear that conventional clinical risk factors for the development of Barrett's adenocarcinoma are neither sensitive nor specific enough for a reliable classification of individuals at high risk. In the future, more rationale surveillance programs may be available, as patients with Barrett esophagus will be further stratified according to their individual risk for progression to invasive carcinoma. Models that incorporate epidemiologic risk factors, reflux symptoms, and endoscopic and histologic findings will likely include panels of biomarkers for risk stratification. Therefore, the challenge over the next decade will be to define the role of molecular markers in endoscopic surveillance strategies.
CONCLUSION
The observations in this study suggest that low-grade dysplasia is the only available, clinically useful risk factor that permits stratification of the surveillance intervals according to the risk of the individual patient. Our results further suggest that successful antireflux surgery protects the nondysplastic Barrett mucosa from progression to dysplasia and adenocarcinoma, possibly by better control of reflux of gastric contents. Although controversial, these results are promising because they indicate that the natural history of Barrett esophagus can be affected by successful elimination of reflux. However, these data need cautious interpretation because recommending antireflux surgery for protection from esophageal adenocarcinoma has profound consequences for patients with Barrett esophagus, most of whom will never develop adenocarcinoma. It is important that these results are confirmed in large, well-designed prospective studies before a radical change in the indication for antireflux surgery is generally adopted.
Footnotes
Reprints: Stefan Öberg, MD, PhD, Department of Surgery, Lund University Hospital, 231 41 Lund, Sweden. E-mail: stefan.oberg@kir.lu.se.
REFERENCES
- 1.Schmidt HG, Riddell RH, Walther B, et al. Dysplasia in Barrett's esophagus. J Cancer Res Clin Oncol. 1985;110:145–152. [DOI] [PubMed] [Google Scholar]
- 2.Haggitt RC. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994;25:982–993. [DOI] [PubMed] [Google Scholar]
- 3.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 48 years. Am J Gastroenterol. 1997;92:212–215. [PubMed] [Google Scholar]
- 4.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. [DOI] [PubMed] [Google Scholar]
- 5.van der Burgh A, Dees J, Hop WC, et al. Oesophageal cancer is an uncommon cause of death in patients with Barrett's oesophagus. Gut. 1996;39:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron AJ, Ott BJ, Payne WS. The incidence of adenocarcinoma in columnar-lined (Barrett's) esophagus. N Engl J Med. 1985;313:857–859. [DOI] [PubMed] [Google Scholar]
- 7.Robertson CS, Mayberry JF, Nicholson DA, et al. Value of endoscopic surveillance in the detection of neoplastic change in Barrett's oesophagus. Br J Surg. 1988;75:760–763. [DOI] [PubMed] [Google Scholar]
- 8.Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256. [DOI] [PubMed] [Google Scholar]
- 9.Provenzale D, Schmitt C, Wong JB. Barrett's esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. [DOI] [PubMed] [Google Scholar]
- 10.Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol. 2002;97:1888–1895. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ. 2000;321:1252–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968. [DOI] [PubMed] [Google Scholar]
- 13.Katz D, Rothstein R, Schned A, et al. The development of dysplasia and adenocarcinoma during endoscopic surveillance of Barrett's esophagus. Am J Gastroenterol. 1998;93:536–541. [DOI] [PubMed] [Google Scholar]
- 14.Bammer T, Hinder RA, Klaus A, et al. Rationale for surgical therapy of Barrett esophagus. Mayo Clin Proc. 2001;76:335–342. [DOI] [PubMed] [Google Scholar]
- 15.Parrilla P, Martinez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett's esophagus. Ann Surg. 2003;237:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naef AP, Savary M, Ozzello L. Columnar lined lower esophagus: an acquired lesion with malignant predisposition. J Thorac Cardiovasc Surg. 1975;70:826–834. [PubMed] [Google Scholar]
- 17.Levine MS, Caroline D, Thompson JJ, et al. Adenocarcinoma of the esophagus: relationship to Barrett mucosa. Radiology. 1984;150:305–309. [DOI] [PubMed] [Google Scholar]
- 18.Haggitt RC, Tryzelaar J, Ellis FH. Adenocarcinoma complicating columnar epithelium-lined (Barrett's) esophagus. J Clin Pathol. 1979;70:1–5. [DOI] [PubMed] [Google Scholar]
- 19.Streitz JM Jr, Williamson WA, Ellis FH Jr. Current concepts concerning the nature and treatment of Barrett's esophagus and its complications. Ann Thorac Surg. 1992;54:586–591. [DOI] [PubMed] [Google Scholar]
- 20.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. [DOI] [PubMed] [Google Scholar]
- 21.Farrow DC, Vaughan TL, Sweeney C, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–238. [DOI] [PubMed] [Google Scholar]
- 22.Champion G, Richter JE, Vaezi MF, et al. Duodenogastroesophageal reflux: relationship to pH and importance in Barrett's esophagus. Gastroenterology. 1994;107:747–754. [DOI] [PubMed] [Google Scholar]
- 23.Stein HJ, Kauer WK, Feussner H, et al. Bile reflux in benign and malignant Barrett's esophagus: effect of medical acid suppression and nissen fundoplication. J Gastrointest Surg. 1998;2:333–341. [DOI] [PubMed] [Google Scholar]
- 24.Trimble KC, Pryde A, Heading RC. Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut. 1995;37:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett's esophagus: an ex vivo proliferation and differentiation model. J Clin Invest. 1996;98:2120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett's esophagus and the effects of acid suppression. Gastroenterology. 1999;117:327–335. [DOI] [PubMed] [Google Scholar]
- 27.Reid BJ, Blount PL, Rubin CE, et al. Flow-cytometric and histological progression to malignancy in Barrett's esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992;102:1212–1219. [PubMed] [Google Scholar]


