Abstract
Objective:
To analyze tumor biology and the outcome of differentiated treatment in relation to tumor subtype in patients with gastric carcinoid.
Background:
Gastric carcinoids may be subdivided into ECL cell carcinoids (type 1 associated with atrophic gastritis, type 2 associated with gastrinoma, type 3 without predisposing conditions) and miscellaneous types (type 4). The biologic behavior and prognosis vary considerably in relation to type.
Methods:
A total of 65 patients from 24 hospitals (51 type 1, 1 type 2, 4 type 3, and 9 type 4) were included. Management recommendations were issued for newly diagnosed cases, that is, endoscopic or surgical treatment of type 1 and 2 carcinoids (including antrectomy to abolish hypergastrinemia) and radical resection for type 3 and 4 carcinoids.
Results:
Infiltration beyond the submucosa occurred in 9 of 51 type 1, 4 of 4 type 3, and 7 of 9 type 4 carcinoids. Metastases occurred in 4 of 51 type 1 (3 regional lymph nodes, 1 liver), the single type 2 (regional lymph nodes), 3 of 4 type 3 (all liver), and 7 of 9 type 4 carcinoids (all liver). Of the patients with type 1 carcinoid, 3 had no specific treatment, 40 were treated with endoscopic or surgical excision (in 10 cases combined with antrectomy), 7 underwent total gastrectomy, and 1 underwent proximal gastric resection. Radical tumor removal was not possible in 2 of 4 patients with type 3 and 7 of 9 patients with type 4 carcinoid. Five- and 10-year crude survival rates were 96.1% and 73.9% for type 1 (not different from the general population), but only 33.3% and 22.2% for type 4 carcinoids.
Conclusion:
Subtyping of gastric carcinoids is helpful in the prediction of malignant potential and long-term survival and is a guide to management. Long-term survival did not differ from that of the general population regarding type 1 carcinoids but was poor regarding type 4 carcinoids.
Gastric carcinoids can be divided into different subtypes. Sixty-five prospectively followed cases were studied regarding biologic behavior, treatment, and prognosis. Tumor aggressiveness varied significantly in relation to type. Differentiated management strategy is recommended as used in the present study.
The incidence of gastric carcinoids may have increased over the last 50 years. In a large series from the United States, the percentage of these tumors among all gastric malignancies had increased from 0.3% to 1.8% and the proportion of these carcinoids among all enteric carcinoids from 2.4% to 8.7%.1 Although there may be a true increase, increasing use of endoscopy and improvements in histopathologic methods and evaluation during the last 50 years probably account for a large part of this increase, as well as the increase in the number of reports in the literature concerning these tumors.2
Regarding the pathogenesis of gastric carcinoids, it is fairly well understood considering tumors developing from the enterochromaffin-like (ECL) cells. These cells are unique for the gastric corpus (fundic) mucosa, where they constitute the largest endocrine cell population. One important function of these cells is secretion of histamine, which in turn stimulates acid secretion from adjacent parietal cells. The ECL cells express CCK-2 (gastrin) receptors, which can mediate histamine secretion and growth of these cells.3–9
Chronic hypergastrinemia is associated with ECL cell hyperplasia, which over time may lead to development of ECL cell carcinoid.4,6,10–12 Corpus predominant atrophic gastritis with or without intrinsic factor deficiency (pernicious anemia) as well as gastrinoma (Zollinger-Ellison syndrome) associated with multiple endocrine neoplasia (MEN) 1 syndrome are conditions associated with hypergastrinemia and an increased incidence of ECL cell carcinoid.13–17 ECL cell carcinoid may rarely occur in the absence of hypergastrinemia and ECL cell hyperplasia and is then described as sporadic ECL cell carcinoid. Furthermore, neoplasia may develop from other endocrine cells of the gastric mucosa, such as the serotonin-secreting enterochromaffin (EC) cells and the gastrin cells.18–21
Gastric carcinoids may accordingly be subdivided into different types.4,18–20,22 Types 1 to 3 are ECL cell carcinoids and type 4 other endocrine cell tumors, eg, serotonin-, gastrin-, or adrenocorticotrophic hormone (ACTH)-secreting tumors, poorly differentiated endocrine carcinomas, and mixed endocrine-exocrine tumors. ECL cell carcinoids are divided into those associated with corpus predominant atrophic gastritis (type 1), those associated with gastrinoma and MEN 1 (type 2), and the sporadic form (type 3). They are situated in the gastric corpus or fundus, while type 4 carcinoids may be located in any part of the stomach. Carcinoids associated with hypergastrinemia (types 1 and 2) are often multicentric, usually small (1 cm or less), without specific hormonal symptoms, and show a low malignant potential and rarely metastasise. Type 3 and type 4 carcinoids usually are solitary, larger, and often highly malignant.18–21,23–28
ECL cell hyperplasia may be reversible if the hypergastrinemia is abolished. In patients with type 1 carcinoid, this may be accomplished by surgical excision of the majority of the G cells, that is, antrectomy.29–31 However, in some cases, major reduction of the hypergastrinemia did not prevent development of ECL carcinoid,31 demonstrating that in addition to hypergastrinemia other pathogenic factors or genetic predisposition are involved in some cases. The somatostatin analogue octreotide may be used to inhibit gastrin release from the G cells and thus reduce the ECL cell hyperplasia.32–35 Morphometric studies showed that antrectomy caused a reduction of the ECL cell volume versus the total volume of endocrine cells, while octreotide reduced the volume of endocrine cells overall (unchanged proportions).32 Recommendations about management of ECL cell carcinoids have been presented20–23,28,36 but not evaluated in larger prospective studies.
The aims of this multicenter follow-up study were to determine tumor stage and the impact on long-term survival when differentiated treatment in relation to type of tumor was given. Secondary outcomes were concomittant other neoplasia, endocrine disease, and symptoms for each subtype of tumor.
PATIENTS AND METHODS
In 1993, the members of the Study Group for Endocrine Abdominal Tumors in Sweden had collected all 32 to them known living and prospectively followed cases with gastric carcinoid. After scrutinizing these cases as well as the relevant literature, all surgical and gastroenterology departments in Sweden were in 1994 asked to participate in a prospective study of patients with these tumors. Treatment and follow-up were done according to recommendations issued by the Study Group.37 During the following 5 years, 33 newly diagnosed cases were reported from 24 hospitals, so a total of 65 patients were prospectively studied.
The recommendations for treatment of type 1 carcinoids were radical endoscopic removal for up to 5 tumors with a maximum diameter of 10 mm and antrectomy for more than 5 tumors with a maximum diameter of 10 mm. For tumors larger than 10 mm (regardless of number), antrectomy was recommended with surgical excision of tumors larger than 10 mm. With signs of serosal involvement, or spread outside the stomach, total gastrectomy with proper lymph node dissection was advised. Besides management of the gastrinoma, it was adviced that type 2 carcinoids could be treated endoscopically when the maximum tumor size was 10 mm or less and by laparotomy with local excision of tumors with larger diameters. The recommendation regarding type 3 and type 4 carcinoids was laparotomy with local excision, orgastric resection with lymph node dissection, after tumor stage (judged by computerized tomography and at laparotomy).
The maximum diameter of the tumor (the largest, if multiple) and the number of tumors were recorded. Tumor tissue (collected at gastroscopy or surgery) and biopsies from nontumorous mucosa were stained with hematoxylin and eosin, Alcian blue-PAS (periodic acid Schiff), the Grimelius and Sevier-Munger argyrophil silver stains, and immunohistochemically with chromogranin A antiserum. Tumor tissue was also stained with the Masson argentaffin silver stain and immunohistochemically with serotonin antibodies. The immunohistochemical stainings included controls with excess antigen. Electron microscopy of glutaraldehyde fixed tumor tissue was performed in 10 cases with type 1 tumor.
The depth of tumor infiltration into the gastric wall was evaluated microscopically in sections from all removed tumors. Nontumorous corpus mucosa was examined for the presence of glandular atrophy, intestinal metaplasia, and endocrine cell hyperplasia. The latter was considered to be present with linear (“pearls on a string” around glands) or micronodular (“nests” of at least 5 endocrine cells in mucosa or muscularis mucosa) patterns.4,12,38
If pernicious anemia, or achlorhydria, had not been diagnosed, patients were examined with a Shilling test and/or pentagastrin test. Computerized tomography of the abdomen was performed in newly diagnosed cases. Pulmonary x-ray was done if the maximum tumor diameter exceeded 10 mm. It was recommended that gastroscopy with biopsy (antrum, corpus, and any remaining tumor) should be performed at 3, 6, and 12 months, and thereafter annually, after the initial diagnosis/treatment. At the time of diagnosis and follow-up visits, blood samples (fasting) were drawn for analysis of gastrin concentrations (radioimmunoassay, reference range <50 pmol/L, hypergastrinemia defined as concentration >100 pmol/L) and chromogranin A concentrations in plasma (radioimmunoassay, reference range <4.0 nmol/L). The principal histamine metabolite methyl-imidazole acetic acid (MeImAA) was analyzed in urine by HPLC with UV detection39 (reference range, 0.4–2.4 mmol/mol creatinine). Considering analysis of the serotonin metabolite 5-hydroxy-indolic acetic acid in urine (U-5-HIAA), there was no conformity between the hospitals, and results are presented as normal or increased. Patients diagnosed before chromogranin A or MeImAA analyses were available had no results or in some cases chromogranin A was analyzed in plasma stored at −70°C.
The tumor was classified as an ECL cell carcinoid, when the majority of tumor cells stained positive with the Sevier-Munger and Grimelius stains or with chromogranin A antiserum or with ultrastructural demonstration of secretory granules with typical electrolucent halo.40–42 It was classified as type 1 when there was more than mild atrophy of the corpus mucosa and concomitant endocrine cell hyperplasia and hypergastrinemia and as type 2 in the presence of extragastric gastrinoma, gastric endocrine cell hyperplasia, and hypergastrinemia. Solitary ECL cell carcinoids without hypergastrinemia and absence of endocrine cell hyperplasia in the corpus mucosa were classified as type 3. Tumors not fulfilling the criteria for an ECL cell carcinoid, or in which the majority of tumor cells stained positive for specific hormonal substances other than histamine, were classified astype 4.
The symptoms or reasons for the primary examination leading to diagnosis of the carcinoid were registered, as were other relevant diagnoses (other endocrine disease, other neoplasia, or pernicious anemia) established before or after the time of the carcinoid diagnosis. None of the patients had been on long-term treatment with proton pump inhibitors preceding the diagnosis of carcinoid.
Numerical data are summarized as median (range). The null hypothesis was tested with the Mann-Whitney U test (nonpaired data) or the Wilcoxon rank sum test (paired data). Fisher exact test, or χ2 analysis, was used for comparison of proportions. Kaplan-Meier analysis was used to calculate the crude cumulative survival rates from the time of diagnosis, and the cause and time of death were extracted from the files of the Swedish Central Bureau of Statistics (April 2003). The expected cumulative survival rate of age- and sex-matched persons in the general population during the same time period were extracted from the same files. Survival rates are given with 95% confidence limits and groups were compared with the log-rank test.
For type 1 tumors, Cox proportional hazard model was used to determine the prognostic value of the number of tumors (1, 2–5, 6–10, >10), depth of tumor infiltration (deeper than submucosa, or not) and presence of metastases, or not. In all tests, a 2-tailed P value of 0.05, or more, was considered as nonsignificant.
RESULTS
Demography and Symptomatology
Of the 65 patients, 51 had type 1, 1 type 2, 4 type 3, and 9 type 4 carcinoid. The median age at the time of diagnosis was 66 years (range, 39–86 years) in 38 women and 13 men with type 1 carcinoid, 71 years (range, 37–76 years) in 3 women and 1 man with type 3 carcinoid, and 72 years (range, 20–80 years) in 2 women and 7 men with type 4 carcinoid. The single patient (male) with type 2 carcinoid was 51 years old. Age did not differ significantly between the groups, but there was a significant difference in sex distribution between patients with type 1 and type 4 carcinoid (P = 0.004).
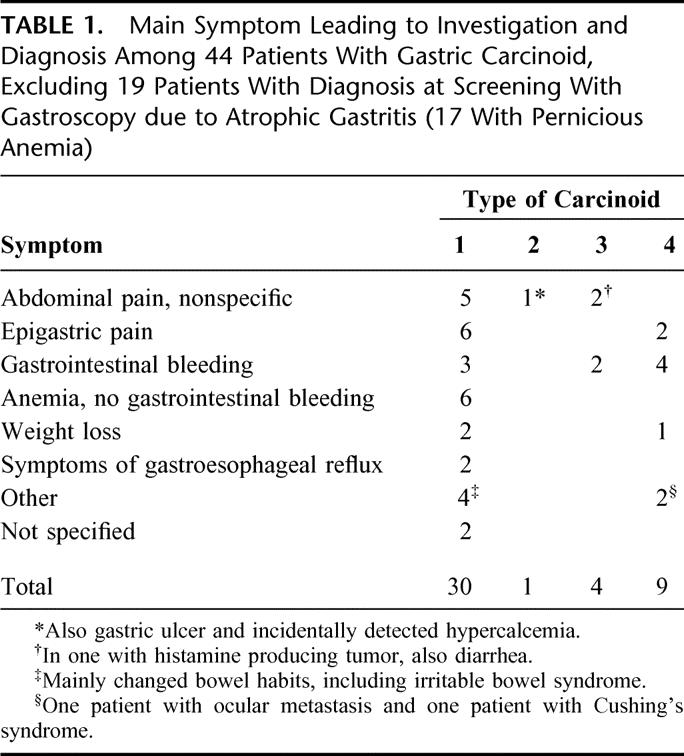
Nineteen patients had the diagnosis of type 1 carcinoid at screening with gastroscopy because of atrophic gastritis. The main symptoms among the remaining 44 patients are given in Table 1. The range of symptoms was wide among patients with type 1 carcinoid; only in 3 cases were the symptoms judged to be tumor-related (large tumor with pain and infiltration of the diaphragm; large tumor with bleeding; multiple tumors with abdominal pain and weight loss). The patient with type 2 carcinoid had recurrent gastric ulcer with chronic abdominal pain. In all patients with type 3 or type 4 carcinoid, the symptoms were tumor-related.
TABLE 1. Main Symptom Leading to Investigation and Diagnosis Among 44 Patients With Gastric Carcinoid, Excluding 19 Patients With Diagnosis at Screening With Gastroscopy due to Atrophic Gastritis (17 With Pernicious Anemia)
Concomitant Diseases
Of the 51 patients with type 1 carcinoid, 2 had a small leiomyoma and 1 a gastric adenoma at the time of diagnosis. Another patient had been treated endoscopically for gastric adenoma 3 years prior to the carcinoid diagnosis. Other tumors of digestive organs were diagnosed prior to or after diagnosis of a type 1 carcinoid in 6 patients (one squamous cell carcinoma of the esophagus, one adenocarcinoma of the pancreas, one adenocarcinoma of the jejunum, 2 adenocarcinomas of the colon, and one adenoma of the rectum). Of these 51 patients, 8 were also treated for thyroid disease, 4 for parathyroid adenoma and 7 had type 2 diabetes. The patient with type 2 carcinoid had duodenal and pancreatic gastrinoma, parathyroid adenoma, and prolactinoma (complete MEN 1 syndrome). None of the patients with type 3 carcinoid had any recognized neoplasia or endocrine disease. One of the patients with type 4 carcinoid had also been treated for adenoma of the colon and another one had type 2 diabetes.
Pathogenetic Background
All 51 patients with type 1 tumor had moderate to severe corpus predominant atrophic gastritis, in 33 cases (64.7%) associated with pernicious anemia. The diagnosis of pernicious anemia was made shortly before (less than 1 year) or at the time of diagnosis of the carcinoid in 8 patients and 1 to 32 years prior to the carcinoid diagnosis in the remaining 25 patients. Except for mild atrophy of the corpus mucosa in 1 patient with type 3 carcinoid, none of the other patients had atrophic gastritis.
At the time of diagnosis, hypergastrinemia (>100 pmol/L) was diagnosed in 50 of 50 examined cases with type 1 carcinoid (not analyzed prior to total gastrectomy in 1 case), the case with type 2 carcinoid, none of the cases with type 3 carcinoid, and 2 of 6 examined cases with type 4 carcinoid.
All patients with type 1 carcinoid and the patient with type 2 carcinoid had endocrine cell hyperplasia (usually both linear and micronodular) in the corpus mucosa. Although not quantified, simple (diffuse) endocrine cell hyperplasia was observed in the mucosa close to the tumor in 1 of the 4 cases with type 3 carcinoid and in 3 of 8 examined patients with type 4 carcinoid.
Endocrine Activity
P-chromogranin A levels were increased (>4.0 nmol/L) in 27 of 31 analyzed cases with type 1 carcinoid, the case with type 2 carcinoid, all 4 cases with type 3 carcinoid, and 3 of 4 analyzed cases with type 4 carcinoid. U-MeImAA was increased (>2.4 mmol/mol creatinine) only in 4 of 26 analyzed cases with type 1 carcinoid, the case with type 2 carcinoid, 3 of 3 analyzed cases with type 3 carcinoid, and 1 (borderline value) of 3 analyzed cases with type 4 carcinoid. U-5-HIAA was normal in 46 of 46 analyzed cases with type 1 carcinoid and all 4 cases with type 3 carcinoid. Increased U-5-HIAA was diagnosed in the case with type 2 carcinoid and 2 of 5 examined cases with type 4 carcinoid.
None of the 51 patients with type 1 carcinoid had specific hormonal symptoms. The median values (range) of P-gastrin, U-MeImAA, and P-chromogranin A among these patients were 515 pmol/L (range, 119–7500 pmol/L), 1.6 mmol/mol (range, 0.8–11.1 mmol/mol) creatinine, and 6.0 nmol/L (range, 2.3–250.0 nmol/L), respectively. The patient with type 2 carcinoid had gastric ulcer and abdominal pain and was previously diagnosed and treated for hypercalcemia and hyperprolactinemia. In this case, both the U-MeImAA and P-chromogranin A were markedly elevated (50.2 mmol/mol creatinine and 3651 nmol/L, respectively). Of 3 type 3 carcinoids, only one was associated with markedly increased U-MeImAA and P-chromogranin A concentrations (30.8 mmol/mol creatinine and 7300 nmol/L, respectively). This patient had abdominal pain and diarrhea. Of 9 patients with type 4 carcinoid, 1 had immunohistochemically an ACTH producing tumor with Cushing syndrome, 1 a gastrin cell tumor (P-gastrin 5360 pmol/L and P-chromogranin A 49.0 nmol/L) without hormonal symptoms, and 1 a serotonin-producing tumor (EC cell tumor) also without hormonal symptoms. Two other patients with type 4 carcinoid had increased U-5-HIAA, but serotonin staining was not prominent in the tumor.
Macroscopic Features and Stage
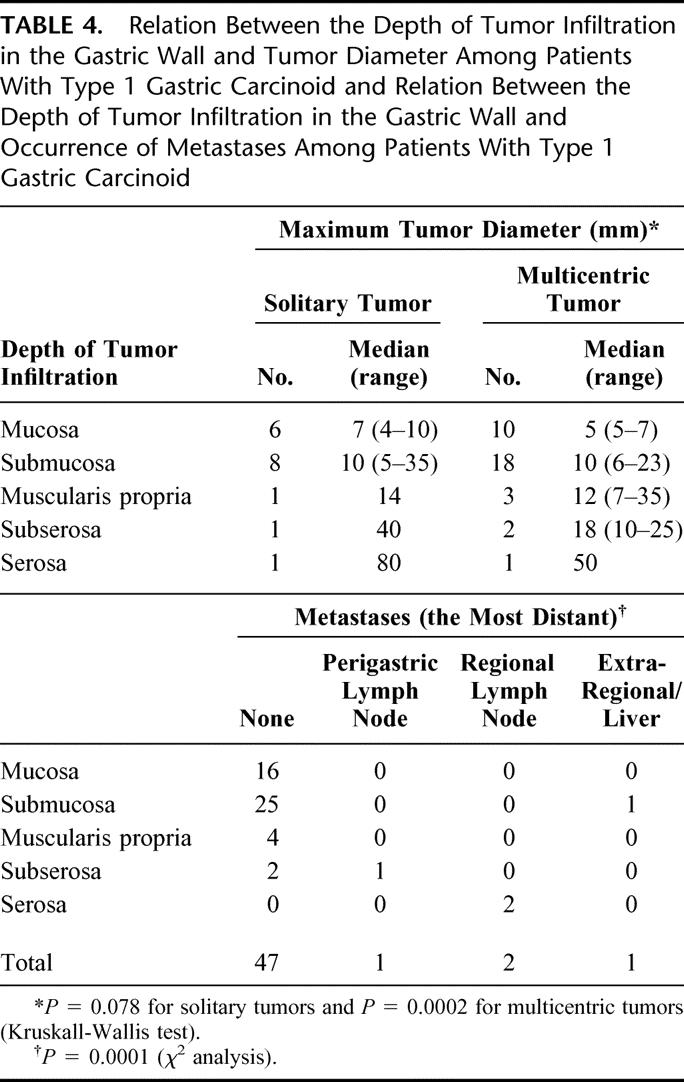
Of all 65 carcinoids, 29 were solitary and 36 multicentric. The number of tumors and their diameter in relation to type are presented in Table 2, which shows that type 3 and 4 carcinoids were significantly larger than type 1 carcinoids. Table 3 shows tumor stage in relation to tumor type. The rate of advanced stage at the time of diagnosis was significantly higher for type 3 and 4 carcinoids than for type 1 carcinoids. Considering type 1 carcinoids, there was a positive correlation between the maximum tumor diameter and the depth of tumor infiltration, as well as between the depth of tumor infiltration and the occurrence of metastases (Table 4). There was no correlation between the degree of multicentricity (1,2–5, 6–10, >10) of type 1 carcinoids and the occurrence of metastases.
TABLE 2. Numbers of Macroscopically Diagnosed Tumors in Patients With Different Types of Gastric Carcinoid and Maximum Tumor Diameter (the largest if multiple) in Relation to Type of Gastric Carcinoid
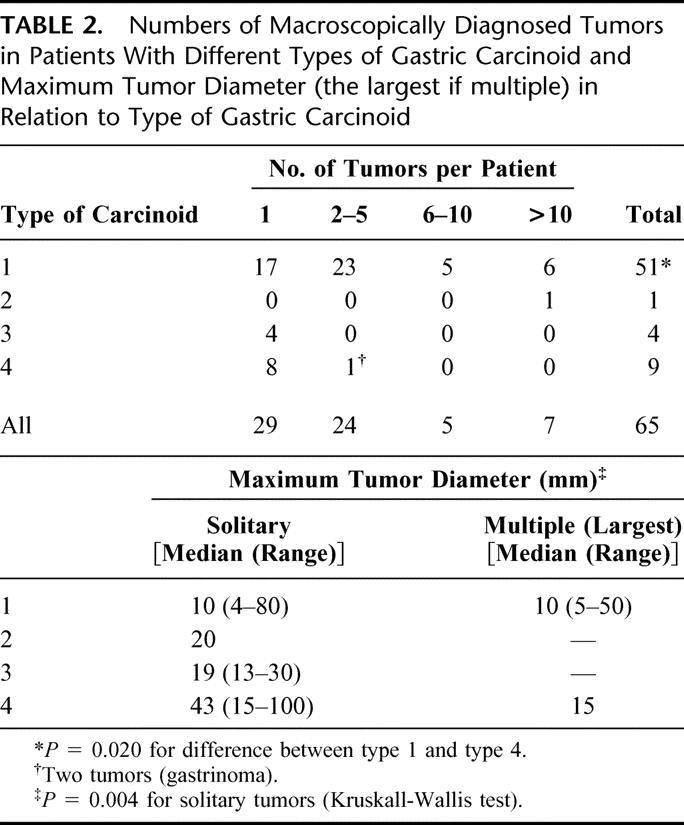
TABLE 3. Depth of Tumor Infiltration in the Gastric Wall in Relation to Type of Gastric Carinoid and Occurrence of Metastasis in Relation to Type of Gastric Carcinoid
TABLE 4. Relation Between the Depth of Tumor Infiltration in the Gastric Wall and Tumor Diameter Among Patients With Type 1 Gastric Carcinoid and Relation Between the Depth of Tumor Infiltration in the Gastric Wall and Occurrence of Metastases Among Patients With Type 1 Gastric Carcinoid
Treatment
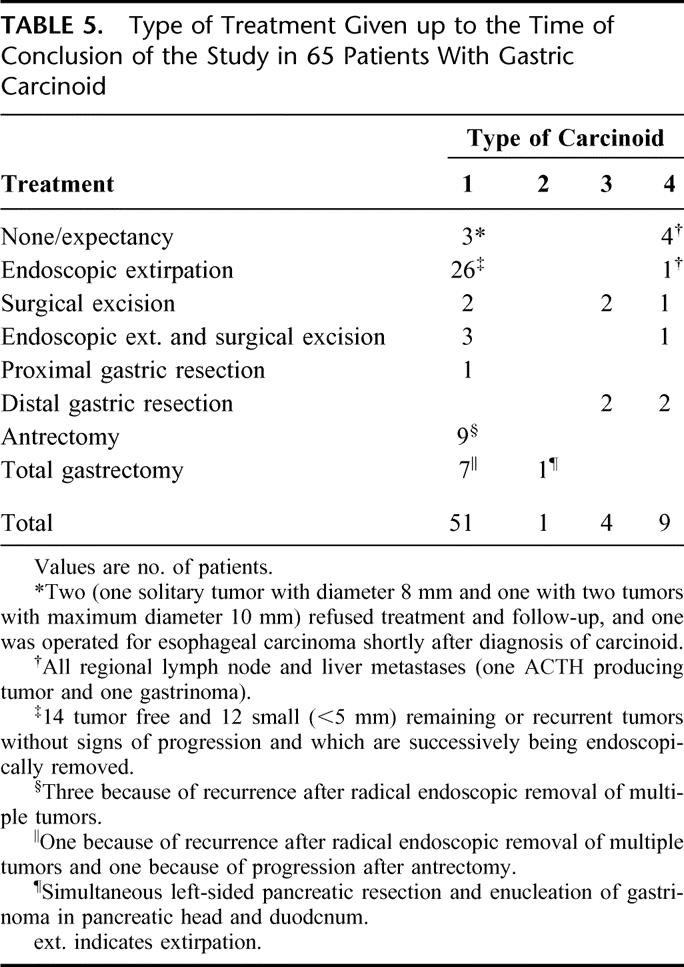
The endoscopic and surgical treatment performed until the conclusion of the study is presented in Table 5. Of 48 patients with type 1 carcinoid (3 had no treatment), 24 were treated according to the recommendations. Among patients not treated according to the recommendations, the treatment was often more extensive; of 48 treated patients, 34 had at least as extensive treatment as recommended.
TABLE 5. Type of Treatment Given up to the Time of Conclusion of the Study in 65 Patients With Gastric Carcinoid
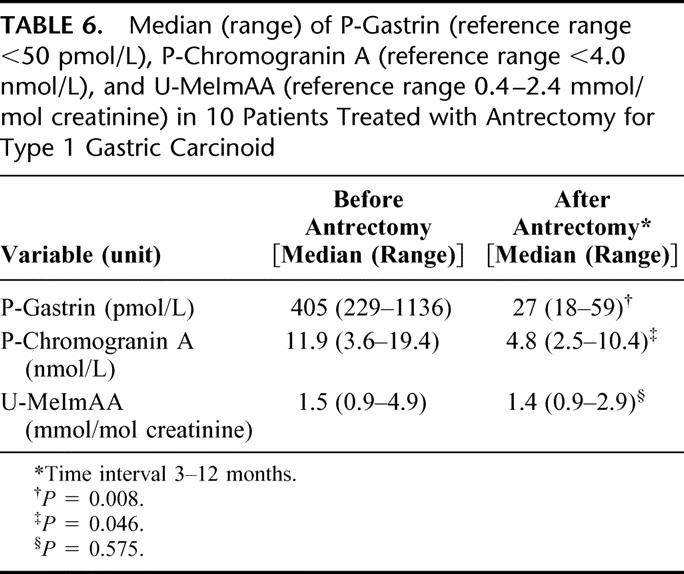
Among the 10 patients with type 1 carcinoid treated with antrectomy, additional treatment given was surgical/endoscopic removal of the largest tumor(s) in 8 and none in 2. Two of these 10 patients were cleared of macroscopically visible tumors at the time of antrectomy, and 8 had residual tumors with a diameter of less than 10 mm. Of the latter 8 patients, 7 became endoscopically tumor-free and 1 had tumor progression as shown by endoscopy and biopsy at a median of 36 months (range, 14–43 months) after antrectomy. The patient with tumor progression (37 months after antrectomy) underwent total gastrectomy. The other 9 patients treated with antrectomy remained tumor-free at regular gastroscopies 65 months (14–215 months) after antrectomy. At this time, the endocrine cell hyperplasia in the corpus mucosa (nodular hyperplasia present in all cases at diagnosis) had regressed in 5 cases and disappeared in 3 cases; 1 patient could not be evaluated. Concentrations of gastrin and chromogranin A in plasma and MeImAA in urine before and after antrectomy are presented in Table 6.
TABLE 6. Median (range) of P-Gastrin (reference range <50 pmol/L), P-Chromogranin A (reference range <4.0 nmol/L), and U-MeImAA (reference range 0.4–2.4 mmol/mol creatinine) in 10 Patients Treated with Antrectomy for Type 1 Gastric Carcinoid
Prognosis
Figure 1 shows the cumulative survival for patients with type 1, type 3, and type 4 carcinoid. Of the 51 patients with type 1 carcinoid, 20 had died: 1 (2.0%) because of metastases (clinical and radiologic diagnosis) 63 months after radical total gastrectomy and 19 of unrelated causes according to death certificates (very few postmortem examinations). For patients with type 1 carcinoid, the 5-year survival rate was 96.1% (range, 90.8%–100.0%) and the 10-year survival rate 73.9% (range, 60.9%–86.8%). These rates did not differ significantly from those in age- and sex-matched subjects in the general population (5-year survival rate 98.0% (range, 94.2%–100.0%) and 10-year survival rate 88.2% (range, 79.4%–97.1%). The patient with type 2 carcinoid (also radically treated for gastrinoma) is alive without recurrence 71 months after diagnosis. After a median follow-up time of 95 months (range, 26–234 months), 1 of 4 patients with type 3 carcinoid had died 26 months after diagnosis, the cause of death being gastric carcinoid with liver metastases. The cumulative crude survival rate for the 9 patients with type 4 carcinoid was only 33.3% (range, 2.5%–64.1%) at 5 years and 22.2% (range, 0.0%–49.4%) at 10 years after diagnosis.
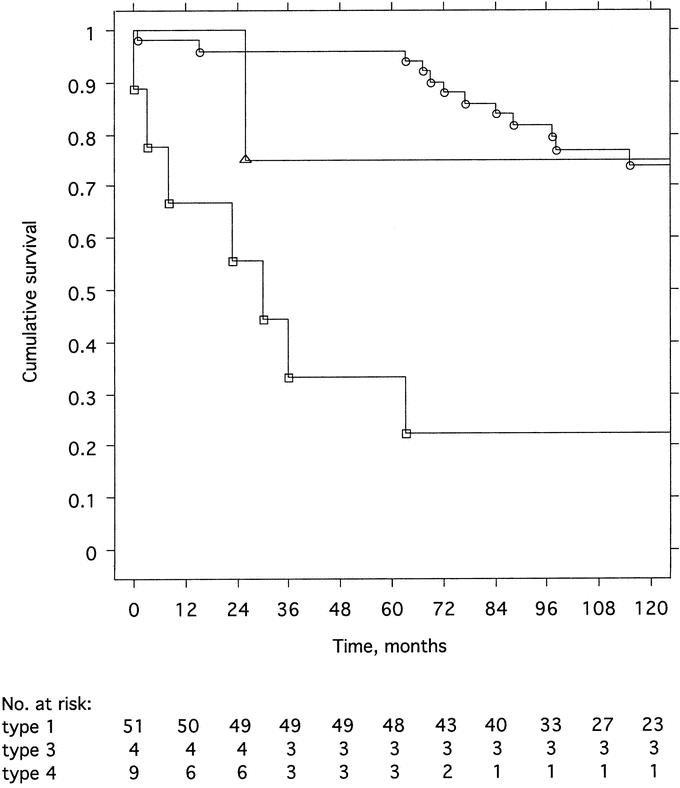
FIGURE 1. Cumulative crude survival after the time of diagnosis among 51 patients with type 1 (circles), 4 patients with type 3 (triangles), and 9 patients with type 4 (squares) gastric carcinoid. The difference between type 1 and type 4 was significant (P < 0.0001, log rank test).
Considering type 1 carcinoids, there was no significant difference in survival rates between patients with solitary and multicentric tumors, nor between patients treated with antrectomy or not. Regarding solitary type 1 carcinoids, survival rates did not differ between patients with a tumor diameter of 10 mm or less versus those with a tumor diameter of more than 10 mm. Also, survival did not differ significantly between patients with type 1 carcinoid and tumor infiltration into the submucosa, or not, nor between patients with tumor infiltration into the muscularis propria, or not. However, the survival rates were significantly lower in patients with type 1 carcinoid and metastases (5-year survival rate 75.0% [range, 32.6%–100.0%] and 10-year survival rate 25.0% [range, 0.0%–67.4%]) versus those without metastases (5-year survival rate 97.9% [range, 93.7%–100.0%] and 10-year survival rate 78.2% [range, 65.3%–91.0%]) (P = 0.008). Five- and 10-year survival rates did not differ significantly between patients with type 1 carcinoid treated less extensively than recommended (N = 17; 94.1% [range, 82.9%–100.0%] and 64.9% [range, 39.2%–90.6%], respectively), according to recommendations (N = 24; 95.8% [range, 87.8%–100.0%] and 77.2% [range, 59.4%–95.0%], respectively) or more extensively than recommended (N = 10; 100.0% and 80.0% [range, 55.2%–100.0%], respectively). There was a tendency (P = 0.091) toward a difference in 5- and 10-year survival between 34 patients with type 1 carcinoid, who were tumor-free (94.1% [range, 86.2%–100.0%] and 84.6% [range, 72.0%–97.1%], respectively), and 17 patients, who were not tumor-free (100.0% and 51.3% [range, 24.7%–77.8%], respectively) at the conclusion of the study. In the latter group, treatment was less extensive than recommended in 10 of 17 (58.8%) of the patients, while the corresponding rate for tumor-free patients was 7 of 34 (20.6%) (P = 0.011). Age, multicentricity of the carcinoids, tumor size (diameter larger than 10 mm), invasiveness (infiltration beyond the submucosa), and metastasis formation did not differ significantly between these 2 groups.
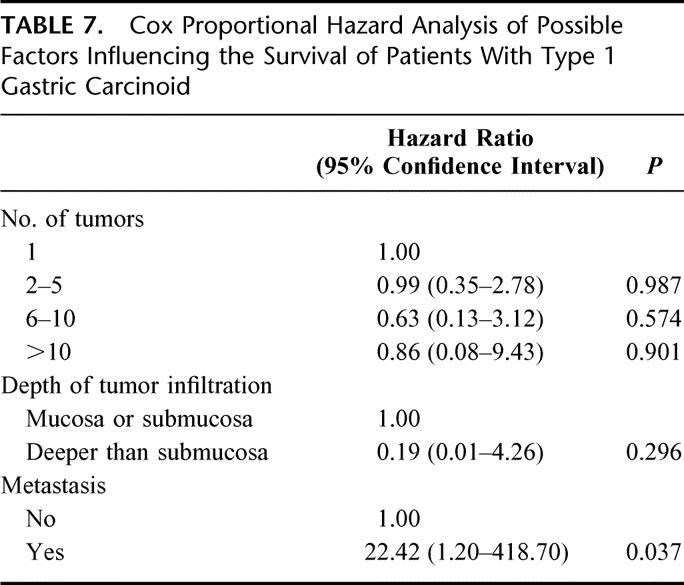
The results of Cox proportional hazard analysis regarding type 1 carcinoids are given in Table 7, which shows that the occurrence of metastases was the only factor with significant impact on survival.
TABLE 7. Cox Proportional Hazard Analysis of Possible Factors Influencing the Survival of Patients With Type 1 Gastric Carcinoid
DISCUSSION
Several authors have divided gastric carcinoids into 3 types: type 1 is associated with corpus predominant atrophic gastritis, type 2 is associated with gastrinoma as part of MEN-1, and type 3 is the sporadic type of ECL cell carcinoid as well as other types of neuroendocrine, or mixed tumors with or without hormone production.4,18,19 Other authors have defined 4 types of which type 1 is associated with atrophic gastritis, type 2 is the sporadic form, type 3 (classified as type 2 by others) is associated with gastrinoma and MEN-1 and type 4 are other types of endocrine carcinomas or mixed tumors.20 In this study, we preferred the classification presented by Modlin et al,22 which combines previous classifications so that predominantly ECL cell carcinoids (types 1–3) and non-ECL-carcinoids (type 4) are distinguished, that hypergastrinemic conditions are hold together (types 1 and 2) and that increasing classification number (types 1–4) indicates increasing degree of malignancy. However, it may be difficult and unnessessary to distinguish between type 3 and 4 carcinoids in clinical routine, which could justify the original classification of Rindi et al19 and Solcia et al.4
As shown previously,2,25 women were overrepresented (74.5%) among patients with type 1 carcinoid and, taking type 3 and 4 carcinoids together, men were overrepresented (8 of 13) among gastrin-independent carcinoids.2 Regarding age, we did not find any significant differences, although others have shown that patients with type 1 carcinoid are older than those with type 2, or 4, carcinoid.25
Not only the type 2 carcinoid is associated with other neoplasias (MEN-1). As also shown by others, endocrine diseases and other neoplasias were more common for patients with type 1 carcinoid, but there was no indication of such a relation for patients with type 3 and 4 carcinoid. A majority of the patients with type 1 carcinoid had pernicious anemia. This is not unexpected, since it represents the final stage of corpus predominant atrophic gastritis. Two patients with type 1 carcinoid had gastric adenoma, which is in line with the findings of others that gastric adenocarcinoma and carcinoid may occur simultaneously2,43 and that atrophic gastritis is also associated with increased risk of gastric adenocarcinoma.44 Awareness of this fact is important not only at the time of diagnosis, but also during follow-up of type 1 carcinoids.
We found that the majority of type 1 carcinoids were asymptomatic and often detected at endoscopic examination due to unrelated symptoms, or to screening of asymptomatic individuals with atrophic gastritis. Only advanced tumors of either type seem to cause symptoms and type 3 and 4 tumors may secrete excess of amines/peptides in advanced stage.45 Patients with type 1 carcinoid had no specific hormonal symptoms, although 4 (of 26) had slightly to moderately elevated U-MeImAA (range, 3.4–11.1 mmol/mol creatinine). Markedly elevated U-MeImAA was found in the patient with type 2 carcinoid (50.2 mmol/mol creatinine) and in 1 of the patients with type 3 carcinoid (30.8 mmol/mol creatinine). Except for diarrhea in the latter case, no specific symptoms related to hypersecretion of histamine (ie, atypical carcinoid syndrome: facial edema, flushing, lacrimation, headache, or bronchoconstriction)20,46,47 were evident. When markedly elevated U-MeImAA or 5-HIAA levels, or an atypical carcinoid syndrome are at hand, it is important to pretreat the patient prior to surgery.
Besides 1 patient with 2 gastric gastrinomas, only type 1 carcinoids were multicentric (66.7%). Considering solitary tumors (all types), the diameter increased significantly with classification number (types 1–4). A similar correlation was found between the depth of tumor infiltration and occurrence of metastases. These findings corroborate with previous findings2,18,20,21,24–28,45–48 and consolidate the classification system. Regarding type 1 carcinoids, there was also a positive correlation between tumor diameter and tumor invasiveness as well as between the invasiveness and presence of metastases. Although seemingly logical, these relations havenot previously been proven statistically. It is, however, wellrecognized that mestastases are rare in these patients.2,18,20,23–26,28,45,47,48
Several authors have suggested management strategies for gastric carcinoids, especially type 1.20–23,27,28,37 Regarding type 3 and 4 carcinoids, there is no doubt that they should be treated aggressively with radical resection, lymph node dissection and management of liver metastases and hormonal symptoms. Unfortunately, only palliative treatment of liver metastases can be offered to most of these patients. The management of the type 2 carcinoid is complex. As shown in our patient and other studies,25,26,47 these tumors are not infrequently associated with metastases, which should be localized and treated concomitantly with the gastrinoma. Type 1 carcinoids have probably been overtreated, which also was the case in the present study. It is generally agreed that it is safe to treat a limited number of small tumors endoscopically.20–23,27,28,37 Since such tumors may be stationary, or even regress over time,49,50 some authors have suggested a conservative approach without removal of the tumors.27 However, there are no prospective controlled studies on this approach, neither whether endoscopic surveillance is necessary. Regarding large or numerous type 1 tumors, the decision making is easier. As shown in the present and other studies, these tumors may, although rarely, be associated with regional lymph node2,20,23,29,51 or even liver metastases,51 the only factors that influence long-term survival.
This is the first study that has implemented antrectomy as a routine on certain indications in patients with type 1 carcinoid. A total of 10 patients underwent this treatment, which resulted in abolishment of hypergastrinemia (ie, antrectomy was complete) and a significant reduction of the P-chromogranin A concentrations in all patients. Of 8 patients with residual tumors after antrectomy, 7 became tumor-free and 1 had tumor progression after a median of 36 months. Including the 2 patients that were made tumor-free atthe time of antrectomy, a total of 9 patients were tumor-free at follow-up after a median of 65 months. Thus, as alsoshown in other studies, antrectomy is efficient in the majority of cases to prevent tumor recurrence. We found a total of 19 cases treated with antrectomy in the literature. In 3 of these, tumor regression did not occur or was only partial.20,29–33,52–55 Including the present series, antrectomy failed in 4 (13.8%) of 29 cases. These carcinoids may have been incorrectly classified, or as previously pointed out4,6,16 other factors than hypergastrinemia were involved in their pathogenesis. A suppression test with octreotide has been suggested asan examination to evaluate the expected effect of antrectomy.33
Despite the occurrence of metastases (perigastric lymph nodes in 3 and liver in 1) at the time of diagnosis in 4 patients with type 1 carcinoid (all treated with radical surgery), the overall 5- and 10-year survival rates did not differ from that in the general population. This corresponds well with the findings of others.24,25,28,43,48,56 With such low mortality, it is difficult to show differences between subgroups. Although there was no difference in long-term survival between patients with type 1 carcinoid treated as recommended, or not, there was a tendency toward longer survival among those without tumor versus those with residual tumor at the end of the study. Furthermore, survival rates were significantly lower among patients with type 1 carcinoid and metastases versus those without metastases. Cox proportional hazard analysis underlined this finding.
The treatment recommendations regarding type 1 carcinoids used in this study seem to be adequate, although the safety margin in case of a limited number of small tumors may be too wide. The recommendations for treatment of type 1 carcinoids were radical endoscopic removal for up to 5 tumors with a maximum diameter of 10 mm and antrectomy for more than 5 tumors with a maximum diameter of 10 mm. For tumors larger than 10 mm (regardless of number), antrectomy was recommended with surgical excision of tumors larger than 10 mm. With signs of serosal involvement, or spread outside the stomach, total gastrectomy with proper lymph node dissection was advised. Whether the safety margin in this recommendation is too wide can only be established in a long-term prospective randomized study (treatment and surveillance versus surveillance only), which is difficult to perform considering the low incidence and slow development of these tumors. Presently, we conclude that these patients should be examined endoscopically once a year with removal of any residual or recurrent tumors. The control interval can probably be prolonged, when all lesions have been removed.
CONCLUSION
Subtyping of gastric carcinoids is helpful in the prediction of tumor agressiveness and long-term survival and is a guide to management. Long-term survival after treatment does not differ from that in the general population regarding type 1 carcinoids and is poor regarding type 4 carcinoids.
ACKNOWLEDGMENTS
The authors thank the following colleagues for their contributions to the study: Stefan Ander, Leif Athlin, Björn Blomberg, Björn Cedermark, Stefan Dedorson, Kent Edin, Christer Eriksson, Philip Franklin, J Friestad, Hans Graffner, Hans-Olof Håkansson, Svante Jansson, Folke Jonsson, Pia Lindblom, Kjell Lundberg, Karsten Offenbartl, Kjell Öberg, Peter Öhman, Bo Olsson, Rein Seensalu, Arild Stubberöd, Erik Svartholm, René Tour, and Ola Wikman.
Footnotes
The study was initiated within the Swedish Research Council Study Group for Abdominal Endocrine Tumors.
Reprints: Kurt Borch, MD, Department of Surgery, University Hospital, S-58185 Linköping, Sweden. E-mail: Kurt.Borch@ibk.liu.se.
REFERENCES
- 1.Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004;99:23–32. [DOI] [PubMed] [Google Scholar]
- 2.Soga J. Gastric carcinoids: a statistical evaluation of 1,094 cases collected from the literature. Surg Today. 1997;27:892–901. [DOI] [PubMed] [Google Scholar]
- 3.Hakanson R, Owman C. Concomitant histochemical demonstration of histamine and catecholamines in enterochromaffin-like cells of gastric mucosa. Life Sci. 1967;6:759–766. [DOI] [PubMed] [Google Scholar]
- 4.Solcia E, Rindi G, Silini E, et al. Enterochromaffin-like (ECL) cells and their growths: relationships to gastrin, reduced acid secretion and gastritis. Baillieres Clin Gastroenterol. 1993;7:149–165. [DOI] [PubMed] [Google Scholar]
- 5.Hakanson R, Chen D, Andersson K, et al. The biology and physiology of the ECL cell. Yale J Biol Med. 1994;67:123–134. [PMC free article] [PubMed] [Google Scholar]
- 6.Bordi C, D'Adda T, Azzoni C, et al. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol 1995;19(suppl 1):8–19. [PubMed] [Google Scholar]
- 7.Andersson K, Chen D, Mattsson H, et al. Physiological significance of ECL cell histamine. Yale J Biol Med. 1998;71:183–193. [PMC free article] [PubMed] [Google Scholar]
- 8.Prinz C, Zanner R, Gerhard M, et al. The mechanism of histamine secretion from gastric enterochromaffin-like cells. Am J Physiol. 1999;277:C845–C855. [DOI] [PubMed] [Google Scholar]
- 9.Bakke I, Qvigstad G, Sandvik AK, et al. The CCK-2 receptor is located on the ECL cell, but not on the parietal cell. Scand J Gastroenterol. 2001;36:1128–1133. [DOI] [PubMed] [Google Scholar]
- 10.Hakanson R, Chen D, Lindstrom E, et al. Physiology of the ECL cells. Yale J Biol Med. 1998;71:163–171. [PMC free article] [PubMed] [Google Scholar]
- 11.Bordi C, D'Adda T, Azzoni C, et al. Pathogenesis of ECL cell tumors in humans. Yale J Biol Med. 1998;71:273–284. [PMC free article] [PubMed] [Google Scholar]
- 12.Borch K, Renvall H, Liedberg G, et al. Relations between circulating gastrin and endocrine cell proliferation in the atrophic gastric fundic mucosa. Scand J Gastroenterol. 1986;21:357–363. [DOI] [PubMed] [Google Scholar]
- 13.Borch K, Renvall H, Liedberg G. Gastric endocrine cell hyperplasia and carcinoid tumors in pernicious anemia. Gastroenterology. 1985;88:638–648. [DOI] [PubMed] [Google Scholar]
- 14.Sjoblom SM, Sipponen P, Miettinen M, et al. Gastroscopic screening for gastric carcinoids and carcinoma in pernicious anemia. Endoscopy. 1988;20:52–56. [DOI] [PubMed] [Google Scholar]
- 15.Solcia E, Capella C, Fiocca R, et al. Gastric argyrophil carcinoidosis in patients with Zollinger-Ellison syndrome due to type 1 multiple endocrine neoplasia: a newly recognized association. Am J Surg Pathol. 1990;14:503–513. [DOI] [PubMed] [Google Scholar]
- 16.Peghini PL, Annibale B, Azzoni C, et al. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68–85. [DOI] [PubMed] [Google Scholar]
- 17.Cadiot G, Lehy T, Mignon M. Gastric endocrine cell proliferation and fundic argyrophil carcinoid tumors in patients with the Zollinger-Ellison syndrome. Acta Oncol. 1993;32:135–140. [DOI] [PubMed] [Google Scholar]
- 18.Bordi C, Yu JY, Baggi MT, et al. Gastric carcinoids and their precursor lesions: a histologic and immunohistochemical study of 23 cases. Cancer. 1991;67:663–672. [DOI] [PubMed] [Google Scholar]
- 19.Rindi G, Luinetti O, Cornaggia M, et al. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. [DOI] [PubMed] [Google Scholar]
- 20.Ahlman H, Kolby L, Lundell L, et al. Clinical management of gastric carcinoid tumors. Digestion 1994;55(suppl 3):77–85. [DOI] [PubMed] [Google Scholar]
- 21.Gilligan CJ, Lawton GP, Tang LH, et al. Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol. 1995;90:338–352. [PubMed] [Google Scholar]
- 22.Modlin IM, Kida M, Lye KD. Biology and management of gastric carcinoid tumours: a review. Eur J Surg. 2003;168:669–683. [DOI] [PubMed] [Google Scholar]
- 23.Borch K. Atrophic gastritis and gastric carcinoid tumours. Ann Med. 1989;21:291–297. [DOI] [PubMed] [Google Scholar]
- 24.Rappel S, Altendorf-Hofmann A, Stolte M. Prognosis of gastric carcinoid tumours. Digestion. 1995;56:455–462. [DOI] [PubMed] [Google Scholar]
- 25.Rindi G, Bordi C, Rappel S, et al. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168–172. [DOI] [PubMed] [Google Scholar]
- 26.Solcia E, Rindi G, Paolotti D, et al. Natural history, clinicopathologic classification and prognosis of gastric ECL cell tumors. Yale J Biol Med. 1998;71:285–290. [PMC free article] [PubMed] [Google Scholar]
- 27.Hirschowitz BI. Clinical aspects of ECL cell abnormalities. Yale J Biol Med. 1998;71:303–310. [PMC free article] [PubMed] [Google Scholar]
- 28.Schindl M, Kaserer K, Niederle B. Treatment of gastric neuroendocrine tumors: the necessity of a type-adapted treatment. Arch Surg. 2001;136:49–54. [DOI] [PubMed] [Google Scholar]
- 29.Eckhauser FE, Lloyd RV, Thompson NW, et al. Antrectomy for multicentric, argyrophil gastric carcinoids: a preliminary report. Surgery. 1988;104:1046–1053. [PubMed] [Google Scholar]
- 30.Richards AT, Hinder RA, Harrison AC. Gastric carcinoid tumours associated with hypergastrinaemia and pernicious anaemia: regression of tumors by antrectomy: a case report. S Afr Med J. 1987;72:51–53. [PubMed] [Google Scholar]
- 31.Wangberg B, Grimelius L, Granerus G, et al. The role of gastric resection in the management of multicentric argyrophil gastric carcinoids. Surgery. 1990;108:851–857. [PubMed] [Google Scholar]
- 32.D'Adda T, Annibale B, Delle Fave G, et al. Oxyntic endocrine cells of hypergastrinaemic patients: differential response to antrectomy or octreotide. Gut. 1996;38:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higham AD, Dimaline R, Varro A, et al. Octreotide suppression test predicts beneficial outcome from antrectomy in a patient with gastric carcinoid tumor. Gastroenterology. 1998;114:817–822. [DOI] [PubMed] [Google Scholar]
- 34.Delle Fave G, Annibale B. Modulation of growth of human gastric enterochromaffin-like cells. Digestion 1996;57(suppl 1):15–16. [DOI] [PubMed] [Google Scholar]
- 35.Modlin IM, Lawton GP, Miu K, et al. Pathophysiology of the fundic enterochromaffin-like (ECL) cell and gastric carcinoid tumours. Ann R Coll Surg Engl. 1996;78:133–138. [PMC free article] [PubMed] [Google Scholar]
- 36.Ichikawa J, Tanabe S, Koizumi W, et al. Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy. 2003;35:203–206. [DOI] [PubMed] [Google Scholar]
- 37.Ahren B, Borch K. Multiple gastric carcinoid tumours. Eur J Surg. 1995;161:375. [PubMed] [Google Scholar]
- 38.Bordi C, Cocconi G, Togni R, et al. Gastric endocrine cell proliferation: association with Zollinger-Ellison syndrome. Arch Pathol. 1974;98:274–278. [PubMed] [Google Scholar]
- 39.Granerus G, Lonnqvist B, Wass U. Determination of the histamine metabolite tele-methylimidazoleacetic acid and of creatinine in urine by the same HPLC system. Inflamm Res. 1999;48:75–80. [DOI] [PubMed] [Google Scholar]
- 40.Rubin W, Schwartz B. Electron microscopic radioautographic identification of the ECL cell as the histamine-synthesizing endocrine cell in the rat stomach. Gastroenterology. 1979;77:458–467. [PubMed] [Google Scholar]
- 41.Solcia E, Capella C, Buffa R, et al. The diffuse endocrine-paracrine system of the gut in health and disease: ultrastructural features. Scand J Gastroenterol Suppl. 1981;70:25–36. [PubMed] [Google Scholar]
- 42.Borch K, Renvall H, Kullman E, et al. Gastric carcinoid associated with the syndrome of hypergastrinemic atrophic gastritis: a prospective analysis of 11 cases. Am J Surg Pathol. 1987;11:435–444. [DOI] [PubMed] [Google Scholar]
- 43.Modlin IM, Sandor A, Tang LH, et al. A 40-year analysis of 265 gastric carcinoids. Am J Gastroenterol. 1997;92:633–638. [PubMed] [Google Scholar]
- 44.Borch K, Kullman E, Hallhagen S, et al. Increased incidence of pancreatic neoplasia in pernicious anemia. World J Surg. 1988;12:866–870. [DOI] [PubMed] [Google Scholar]
- 45.Gough DB, Thompson GB, Crotty TB, et al. Diverse clinical and pathologic features of gastric carcinoid and the relevance of hypergastrinemia. World J Surg 1994;18:473–479; discussion 479–480. [DOI] [PubMed]
- 46.Kolby L, Wangberg B, Ahlman H, et al. Gastric carcinoid with histamine production, histamine transporter and expression of somatostatin receptors. Digestion. 1998;59:160–166. [DOI] [PubMed] [Google Scholar]
- 47.Modlin IM, Lye KD, Kidd M. Carcinoid tumors of the stomach. Surg Oncol. 2003;12:153–172. [DOI] [PubMed] [Google Scholar]
- 48.Thomas RM, Baybick JH, Elsayed AM, et al. Gastric carcinoids: an immunohistochemical and clinicopathologic study of 104 patients. Cancer. 1994;73:2053–2058. [DOI] [PubMed] [Google Scholar]
- 49.Harvey RF. Spontaneous resolution of multifocal gastric enterochromaffin-like cell carcinoid tumours. Lancet. 1988;8589:821. [DOI] [PubMed] [Google Scholar]
- 50.Brundler R, Gebbers JO, Criblez D. Multiple gastric carcinoid tumors in chronic atrophic gastritis: long-term follow-up under conservative management. Schweiz Med Wochenschr. 1999;129:957–960. [PubMed] [Google Scholar]
- 51.Granberg D, Wilander E, Stridsberg M, et al. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut. 1998;43:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guillem P, Vlaeminck-Guillem V, Leteurtre E, et al. Fundic endocrine tumors and atrophic gastritis: the value of antrectomy. Gastroenterol Clin Biol. 2002;26:782–785. [PubMed] [Google Scholar]
- 53.Hagarty S, Huttner I, Shibata H, et al. Gastric carcinoid tumours and pernicious anemia: case report and review of the literature. Can J Gastroenterol. 2000;14:241–245. [DOI] [PubMed] [Google Scholar]
- 54.Hirschowitz BI, Griffith J, Pellegrin D, et al. Rapid regression of enterochromaffinlike cell gastric carcinoids in pernicious anemia after antrectomy. Gastroenterology. 1992;102:1409–1418. [PubMed] [Google Scholar]
- 55.Kern SE, Yardley JH, Lazenby AJ, et al. Reversal by antrectomy of endocrine cell hyperplasia in the gastric body in pernicious anemia: a morphometric study. Mod Pathol. 1990;3:561–566. [PubMed] [Google Scholar]
- 56.Stolte M, Ebert D, Seifert E, et al. The prognosis of carcinoid tumors of the stomach. Leber Magen Darm. 1988;18:246–250, 253–256. [PubMed] [Google Scholar]